Abstract
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacterium that is a common cause of nosocomial infections. Here we describe investigation of a possible nosocomial transmission of M. chelonae at the Hospital of the University of Pennsylvania (HUP). M. chelonae strains with similar high-level antibiotic resistance patterns were isolated from two patients who developed post-operative infections at HUP in 2017, suggesting a possible point source infection. The isolates, along with other clinical isolates from other patients, were sequenced using the Illumina and Oxford Nanopore technologies. The resulting short and long reads were hybrid assembled into draft genomes. The genomes were compared by quantifying single nucleotide variants in the core genome and assessed using a control dataset to quantify error rates in comparisons of identical genomes. We show that all M. chelonae isolates tested were highly dissimilar, as indicated by high pairwise SNV values, consistent with environmental acquisition and not a nosocomial point source. Our control dataset determined a threshold for evaluating identity between strains while controlling for sequencing error. Finally, antibiotic resistance genes were predicted for our isolates, and several single nucleotide variants were identified that have the potential to modulated drug resistance.
Introduction
Mycobacterium chelonae, a rapidly growing nontuberculous mycobacterium (NTM), is ubiquitous in the environment and is a common source of opportunistic infection in humans. M. chelonae has caused several outbreaks from single point sources, but no human-to-human transmission has been observed to date [1–6]. M. chelonae is most commonly associated with nosocomial soft tissue infections of the skin and eye, but can also cause catheter-associated infections, disseminated and invasive infections, and pulmonary infections [1]. Mycobacterium can be highly resistant to many antibiotics because of their naturally impermeable cell walls as well as mutation of bacterial genes that encode antibiotic targets [7–9].
Among the M. chelonae isolates collected in 2017 from the Hospital of the University of Pennsylvania (HUP) clinical microbiology laboratory were two isolates from female patients who underwent surgery within the same year and developed post-operative surgical site infections in breast tissue. Culture of the inflammatory lesions yielded M. chelonae isolates with similar drug resistance patterns, raising suspicion of a nosocomial point source infection. To investigate this possibility, we performed whole genome sequencing on every M. chelonae isolate collected by the HUP clinical microbiology laboratory over a one-year period (n = 7) and examined differences in the single nucleotide variants (SNVs) of each isolate’s core genome. As a control, we created a dataset from a single Vibrio campbellii strain that was sequenced 39 times independently, allowing us to determine a threshold of SNVs that would distinguish different strains while controlling for sequencing error. Finally, we investigated genes and SNVs related to drug resistance in each isolate.
Methods
Samples were collected as part of routine clinical practice. Penn IRB approved collection of human samples by the clinical Microbiology Laboratory at the Hospital of the University of Pennsylvania under the IRB protocol #829497. Patients had written consent from their providers. The IRB protocol also included a waiver of consent. Respiratory samples were decontaminated with NaOH and N-acetylcysteine. Tissue specimens were pulverized in a tissue grinder. Prepared specimens were inoculated on 7H11 selective and non-selective solid media and a Mycobacterial Growth Indicator Tube (MGIT) broth. All cultures were incubated at 35–37°C for 6 weeks. Positive cultures for Mycobacterium were identified at the species level using hsp65 gene sequencing with primers TB11 (ACCAACGATGGTGTGTCCAT) and TB12 (CTTGTCGAACCGCATACCCT) [10]. Susceptibility testing and MIC determination were performed using the RAPMYCO microbroth dilution plate (ThermoFisher, catalog number RAPMYCO) and susceptibility was determined using the CLSI M24-A2 [11]. Tigecycline susceptibility breakpoints for the MIC have not been established for Mycobacterium [12]. For our analysis, we based our tigecycline thresholds for resistance of ≤0.25 on the methods in Wallace et al. [13]. For antibiotic resistance gene investigation, we categorized susceptibility into susceptible, intermediate, and resistant.
All Mycobacterium samples had been previously frozen for routine clinical purposes. Isolates were re-isolated on chocolate agar and propagated by growth in Middlebrook 7H9 media for 5 days. Multiple DNA purification methods were compared to identify one producing high molecular weight DNA in good yield. Ultimately, DNA was purified from each sample using a phenol-chloroform DNA extraction designed for high molecular weight DNA [14]. Long-read libraries were prepared using the Rapid Barcoding Kit, version SQK-RBK004 (Oxford Nanopore, Oxford, UK) and sequenced on the MinION using a R9.4.1 flow cell. Short-read libraries were prepared using the TruSeq DNA Nano Library Prep Kit (Illumina, San Diego, CA), and sequenced on the HiSeq 2500 using 2x125 bp chemistry.
V. campbellii was grown in Difco Marine broth 2216 culture media (BD) overnight and DNA was extracted using DNeasy Blood & Tissue Kits (Qiagen). Short read libraries were prepared using the Nextera XT Library Prep Kit (Illumina, San Diego, CA) and sequenced on the HiSeq 2500 using 2x125bp chemistry.
We used the Sunbeam v1.3 [15] pipeline to process the short reads and a custom snakemake v.5.6.0 pipeline, Nanoflow (https://github.com/zhoac1/nanoflow), to process the long reads and perform the hybrid assembly. Short read processing included trimming adapters off reads using Trimmomatic (parameters: leading: 3; trailing: 3; slidingwindow: [4, 15]; minlen:36), filtering out low quality reads using fastqc and removing low complexity reads using Komplexity (parameters: kz_threshold: 0.55) [15]. Long read processing included base calling using Albacore v2.3.4 (https://community.nanoporetech.com), trimming adaptors using Porechop v0.2.3_seqan2.1.1 (https://github.com/rrwick/Porechop), and filtering using Filtlong v0.2.0 (parameters:—min_length 1000—keep_percent 90—target_bases 1000000000) (https://github.com/rrwick/Filtlong). Hybrid assembly was performed by two methods: 1) using Canu v1.9, polishing the genome using Nanopolish v0.11.1 and correction with short reads using Pilon or 2) using Unicycler v0.4.7, a program that uses the short read assembler Spades v3.12 guided by long reads as scaffolds, which is further polished using Pilon [16, 17]. CheckM v1.1.2 and alignment to reference genome (M. chelonae strain CCUG 47445) were used to check the quality of the draft genomes to select the best one [18].
We used another custom snakemake pipeline, CoreSNPs (https://github.com/chrgu/coreSNPs) to investigate how related the clinical isolates were to each other and to other whole genome sequenced references collected from GenBank. CoreSNPs uses Prokka v1.14.5 for genome annotation, and Roary v3.12.0 for investigating the pangenome and a hierarchical cluster based on the presence and absence of accessory genes [19, 20]. Custom R and shell scripts were used to extract the core genes from the isolates to compare SNVs by hamming distance. SNVs per Mbp core genome were calculated dividing the hamming distances by length of core genome between the sequence sets for identical DNAs. The SNV analysis used SamTools v1.9 and SNP-sites v2.4.1 [21, 22]. Approximately maximum-likelihood phylogenetic trees for the core genes were generated by Fasttree 2 v2.1.10 [23]. Accession numbers for all M. chelonae isolates and references can be found in S1 Table.
The threshold for calling strain identity between genomes was determined using a dataset of a single isolate of V. campbellii sequenced 39 times (Illumina only) and examining the number of SNVs between sequencing runs. The dataset was analyzed using the same in-house pipelines as above. Roary defined core genes as genes present in 100% of the samples.
We used Resistance Gene Identifier (RGI) on the output of Prokka to examine resistance genes within the genome [24]. We searched for both high identity and low identity homologous hits to identify previously known genes, such as gyrA, and potentially novel resistance genes, respectively. We also manually aligned known genes that can harbor resistance mutations with Muscle to identify and compare mutations within the gene that corresponded with drug susceptibility [25].
Nanoflow is available at www.github.com/zhaoc1/nanflow while all other computer code used in this study is available at www.github.com/chrgu. M. chelonae and V. campbellii assembled genomes are available in GenBank under project PRJNA594977.
Results
M. chelonae was isolated from seven patients at the Hospital of the University of Pennsylvania in 2017 (Table 1), including the two breast tissue cases. Ages ranged from 45 to 64. Sites of infection included skin (n = 2), breast tissue (n = 2), and respiratory tract (sampled as bronchial alveolar lavage; n = 1) and sputum (n = 2).
Table 1. Patient demographics and the suspected transmission pair.
| ID | SUSPECTED NOSOCOMIAL INFECTION PAIR* | AGE | SEX | SITE OF COLLECTION |
|---|---|---|---|---|
| MYCO1 | X | 45 | Female | breast |
| MYCO2 | X | 47 | Female | breast |
| MYCO3 | 61 | Female | skin | |
| MYCO4 | 64 | Male | bronchiolar lavage | |
| MYCO5 | 61 | Male | sputum | |
| MYCO6 | 61 | Male | sputum | |
| MYCO7 | 55 | Male | leg skin |
Drug susceptibility testing was performed on each isolate using 11 different antibiotics to determine the minimum inhibitory concentration (MIC) (Table 2). As expected, most of the isolates of M. chelonae were highly resistant to antibiotics. All strains (n = 7) were either resistant or intermediately resistant to TMP-SMX, ciprofloxacin, imipenem, moxifloxacin, cefoxitin, and minocycline. Only a few drugs such as clarithromycin, tigecycline, and tobramycin, were effective against all the strains. Some variation in susceptibility and resistance was sporadic (e. g. Myco6 alone was sensitive to doxycycline).
Table 2. Antibiotic resistance profile of M. chelonae isolates against 11 antibiotics.
| ID | TMP* | LINE† | CIPRO§ | IMI¶ | MOXI# | CEFOX** | DOXY†† | MINO§§ | TIGE¶¶ | TOBRA## | CLAR*** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MYCO1 | >8/152 (R) | 32 (R) | 4 (R) | 16 (I) | 8 (R) | >128 (R) | >16 (R) | 4 (I) | 0.5 (S) | 4 (I) | 0.5 (S) |
| MYCO2A/B | >8/152 (R) | 32 (R) | >4 (R) | >64 (R) | 8 (R) | >128 (R) | >16 (R) | >8 (R) | 0.5 (S) | 2 (S) | 0.5 (S) |
| MYCO3A/B | >8/152 (R) | 32 (R) | >4 (R) | 16 (I) | 8 (R) | 128 (R) | >16 (R) | >8 (R) | 0.5 (S) | 2 (S) | 0.5 (S) |
| MYCO4 | 4/76 (R) | 8 (S) | 4 (R) | 16 (I) | 4 (R) | >128 (R) | >16 (R) | >8 (R) | 0.5 (S) | <1 (S) | 0.25 (S) |
| MYCO5 | 8/152 (R) | 16 (I) | 4 (R) | 32 (R) | 8 (R) | >128 (R) | >16 (R) | >8 (R) | 0.25 (S) | 2 (S) | 0.5 (S) |
| MYCO6 | >8/152 (R) | 16 (I) | 2 (I) | 32 (R) | 4 (R) | 64 (I) | 1 (S) | 2 (I) | 1 (S) | 4 (I) | 0.5 (S) |
| MYCO7 | >8/152 (R) | >32 (R) | 2 (I) | 32 (R) | 4 (R) | >128 (R) | >16 (R) | >8 (R) | 0.5 (S) | 2 (S) | 2 (S) |
Footnote: R = Resistant, I = Intermediate, S = Susceptible
*TMP-SMX
†Linezolid
§Ciprofloxacin
¶Imipenem
#Moxifloxacin
**Cefoxitin
††Doxycycline
§§Minocycline
¶¶Tigecycline
##Tobramycin
***Clarithromycin
The M. chelonae strains were isolated from seven patients at HUP during routine clinical treatment. Frozen stocks of the M. chelonae isolates were cultured and DNA was extracted. Extensive optimization was required to allow lysis of the tough Mycobacterium cell wall while preserving long DNA chains (see methods). We purified high molecular weight DNA for most strains but we were unable to do so for Myco5. DNA sequencing data was acquired using the Illumina HiSeq 2500 to generate short reads and the Oxford Nanopore MinION to generate long reads. Two assembly methods were compared for each isolate and the best draft genome was chosen based on completeness (by checkM), number and length of contigs, and alignment to a reference genome (described in detail in the methods). For Myco5 only short read assembly was carried out.
In one case, two isolates were cultured from the same patient at different time points and analyzed separately (Myco3a/3b). In another case, a single genomic DNA preparation was sequenced and assembled twice (Myco2a/b). Both pairs provide further empirical data on the sources of error in library preparation and DNA sequencing.
The whole genome sequencing resulted in a range of contig numbers (n = 1 to 76) comprising the main chromosome. For those that were hybrid assembled, the range of contigs was one to four. Three of the nine assemblies yielded complete circular contigs for the main chromosome. The genomes ranged in size from 4.95 to 5.20 Mbp. No clearly defined episomes were found, as judged by detection of extrachromosomal circles (S1 Table).
We assessed the phylogenetic relationships by comparing the number of single nucleotide variants (SNVs) between cores genes (genes found in every isolate), which allowed us to interrogate potential transmission chains. We used all 43 whole genome sequences of M. chelonae present in GenBank (retrieved June 2018) as reference to construct a maximum-likelihood phylogenetic tree. The genomes were annotated by Prokka. CheckM analysis were performed to ensure completion and quality of the reference genomes prior to analysis. Analysis of our set of M. chelonae genomes returned a total of 17,582 genes in the pan-genome, of which only 3,368 were considered core genes. The length of the total concatenated core genes per genome was 3,296,947 bases. Of the 3,368 core genes, 25 genes did not contain any SNVs. A list of core genes along with the number of SNVs and SNVs per Mbp can be found in S2 Table. There were no obvious genes related with resistance among the top genes with SNVs. Within the core genes, the number of SNVs between unique isolates ranged from 3,383 to 62,854.
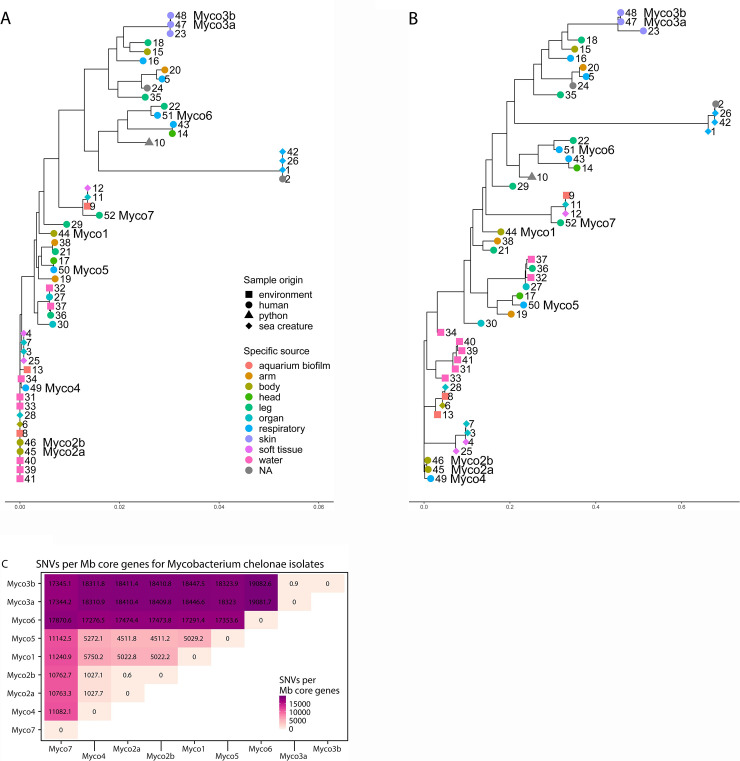
Our two samples from the same individual (Myco3a and Myco3b) differed by 3 SNVs while our technical replicates (Myco2a and Myco2b) differed by 2 SNVs. The potential transmission pair, Myco1 and Myco2a/b differed by 16544/16542 SNVs in the core genes (SNVs are indicated for Myco2 replicates a and b, respectively). A maximum likelihood phylogenetic tree based on the SNVs data is shown in Fig 1A. There was no obvious clustering of our clinical isolates compared with database samples. There was some clustering between human samples, and some of our samples fell into those clusters (e. g. Myco3a/3b and Myco5). Other strains clustered with environmental isolates (e. g. Myco2a/2b and Myco4). Likewise, the tree based on presence or absence of accessory genes (Fig 1B) also showed a lack of obvious clustering of the Phildelphia strains.
Fig 1. Relationship of M. chelonae genome sequences.
A. A maximum-likelihood phylogenetic tree showing relationships among M. chelonae isolates based on SNVs in the core genes. Numbers next to branch tips correspond with genomes found in S1 Table. Isolates from our study are indicated with “Myco” and the isolate number. The sampling site and host of the isolate is coded by color and shape, respectively, at branch tips. The scale at the bottom represents the number of substitutions per sequence site based on length of the tree. B: Amaximum-likelihood tree showing the relationship among M. chelonae isolates based on presence or absence of accessory genes. C: SNVs per Mbp core genome between M. chelonae isolates. SNVs, calculated as hamming distance between the core genes of all isolates divided by the total length of core genes. Myco2a/2b and Myco3a/3b are technical and biological replicates, respectively.
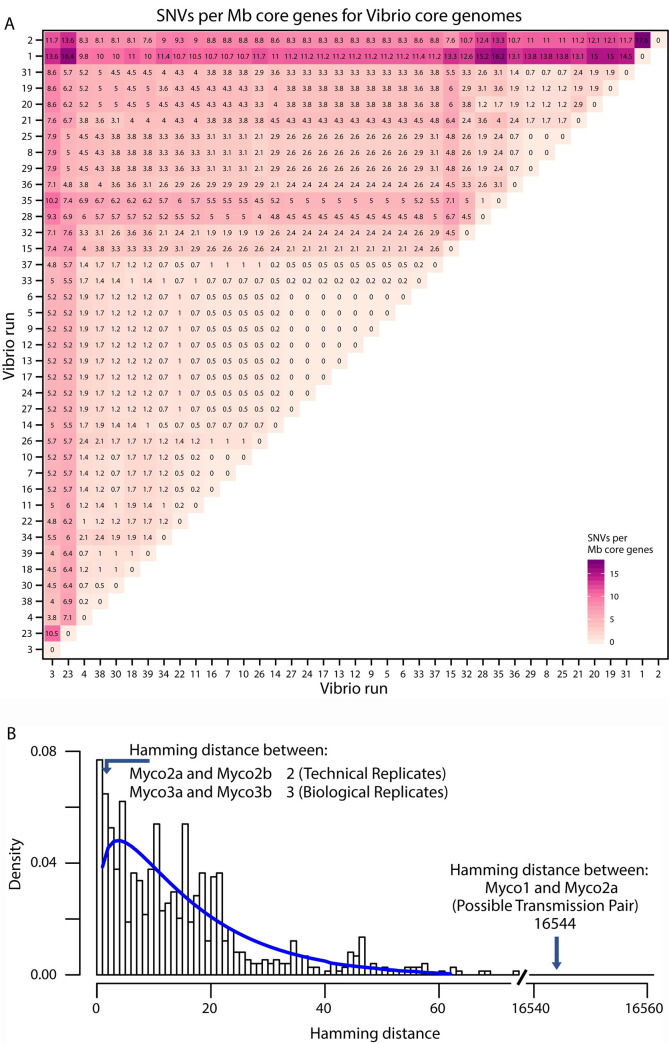
To assess the likelihood of infection from a common point source, we next empirically assessed the numbers of SNVs expected due to sequencing error in a larger set of genomes. As a positive control in shotgun metagenomic studies, we repeatedly sequenced a single bacterium, Vibrio campbellii, a luciferase-encoding marine bacterium, that was divergent from strains likely present in our samples. We recovered an average of 9,072,182 reads over 39 replicates, allowing generation of 39 full genome sequences from the same isolate. Analysis of the V. campbellii genomes using Roary disclosed 4495 core genes in our samples; 643 genes, or ~12.5%, were not 100% conserved in the V. campbellii genome, likely due to errors in genome sequence determination. Within the core genes, we found a range of SNVs from 0 to 74, with mean of 15 SNVs (Fig 2A). The total length for the concatenated core genes was 4,209,934 bases. The two most divergent V. campbellii assemblies also had low sequence coverage (S3 Table). These data provide a rough upper bound on the number of SNV errors associated with suboptimal sequence acquisition.
Fig 2. Comparison of Vibrio campbellii genomes by SNVs per Mbp core genome to develop statistics for calling isolate identity.
A. The set of SNVs per Mbp core genome, calculated by hamming distances divided by length of core genome between the sequence sets for identical DNAs. B. Graph showing the Hamming distances (x-axis) and the frequencies of distances between pairs (y-axis). The distances between the technical and biological replicates are marked (Myco2a and 2b, and Myco 3a and 3b), as is the distance between the candidate transmission pair (Myco1 and 2a).
This comparison takes advantage of Illumina sequence reads only, whereas our M. chelonae isolates were sequenced using hybrid assembly of short and long reads. We thus generated short read only assemblies for the M. chelonae isolates for a more direct comparison to the V. campbellii dataset. Our short-read-only genomes contained slightly fewer core genes (3,143 vs 3,368) compared to our hybrid assemblies. The short-read genomes also had more SNVs per Mbp core genes. Both of our technical replicates showed slightly higher SNV counts (3 and 64), but were lower than the maximum number of SNVs for identical strains in our control V. campbellii dataset (the maximum number of SNVs for any pair of isolates was 74). Phylogeny of the short-read-only core genomes maintained similar placement as with the hybrid assembled core genome tree.
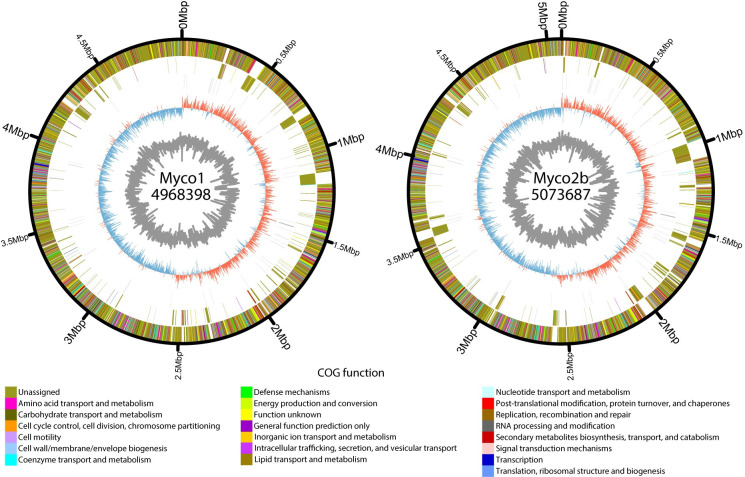
The possible transmission pair, isolates Myco1 and Myco2a/b (Fig 3), differed by 16,542/16,544 SNVs (Fig 2B), having 99.5% nucleotide identity in the core genes. There were 1107 genes that were not shared between them. Together, this provides strong evidence that they are different strains and not related by direct person-to-person transmission, or acquisition from a common nosocomial point source. This corresponds to a difference of 5,237.58 SNVs per Mbp core genes. The smallest difference between our isolated M. chelonae strains was 3,426 SNVs in the core genes or 1,038.18 SNVs per Mbp core genes. Our technical replicates Myco3a and Myco3b differed by 3 SNVs (0.91 SNVs per Mbp core genes), and Myco2a and Myco2b differed by 2 SNVs (0.61 SNVs per Mbp core genes) (Fig 1C). For comparison, the mean number of SNVs in pairwise comparisons of V. campbelli control assemblies was 5.71 SNVs per Mbp core genes; the maximum number was 17.62 SNVs per Mbp core genes (Fig 2B). The number of SNVs in the candidate transmission pair thus far exceeds the number of SNVs that could be generated by sequencing error per Mbp as seen from the V. campbellii controls and exceeds the SNVs generated in our M. chelonae replicates (p-value < 0.001 by binomial test).
Fig 3. Comparison of genomes from the candidate transmission pair.
Circos plots are shown of assemblies of Myco1 (left) and Myco2b (right). Each ring represents, from inner to outer ring GC content; GC skew; RNA genes; genes unique to the isolate, colored by COG function; and genes shared between the two isolates, colored by COG function. Each genome’s start positionwas rotated to the origin of replication and genomes with multiple contigs were merged to form a single contig for visualization.
The molecular determinants of antibiotic resistance in M. chelonae are not well studied, so we sought to assess possible antimicrobial resistance mechanisms disclosed in our sequence data. Analysis using Resistance Gene Identifier (RGI) searching for high identity hits to previously known genes related to resistance [24], yielded two partial hits to all the isolates, and a third in three of seven isolates. The two partial hits in all isolates were LRA-3 (100% identity) and erm(38) (80% identity); the third gene was mtrA (95.17% identity). LRA-3 is a gene coding for a beta-lactamase originally identified in soil samples and could possibly contribute to imipenem resistance [26]. The gene, erm(38), encodes for a 23S dimethyltransferase found in Mycobacterium smegmatis and can provide resistance to macrolides and lincosomides [27]. It has been shown to increase resistance to Clarithromycin [28]. mtrA is a gene from Mycobacterium tuberculosis whose expression has been shown to influence cell morphology and drug resistance in Mycobacterium smegmatis [29, 30].
We also used RGI to identify genes with lower homology to known resistance genes, providing possible starting points for follow up studies. We found a list of candidate genes that may contribute to resistance and further narrowed down the list using the susceptibility testing data by filtering out genes that were present in susceptible populations and genes that were not present in intermediate or fully resistant populations. Only 6 of the 11 drugs had low identity matches associated with their resistance pattern: TMP-SMX (sulfonamide), ciprofloxacin (fluoroquinolone), moxifloxacin (fluoroquinolone), imipenem (carbapenem), cefoxitin (cephalosporin), and Minocycline (tetracycline). Genes are listed in S4 Table.
We further examined genes reported to be targets of mutations that may cause resistance, such as mutations in 23S rRNA for linezolid resistance and gyrA for fluoroquinolone resistance. There were no known or novel mutations present in the 23S rRNA genes in our isolates that correlated with drug susceptibility or resistance. For gyrA, we compared the two intermediate resistant isolates (Myco6 and Myco7) to five resistant isolates. We found that there were no consistent mutations that resulted in resistance, but Myco6 had two mutations (S710R and E773D) not present in the other isolates and Myco7 had one mutation not present in the other isolates (T832A).
Discussion
Here we investigated the possibility of a nosocomial M. chelonae transmission at the HUP in 2017 and found that there is no evidence to support a point source outbreak. Our analysis found each isolate of M. chelonae was no more similar to each other than to the strains collected from the NCBI database. This supports that these infections were instead likely acquired from the environment and not from a single point source. Our analysis supports the use of next generation sequencing to assess possible nosocomial transmissions within the hospital environment.
Our data provide perspective on the amount of genetic variation in the population of M. chelonae in Philadelphia. The number of SNVs found in our isolates of M. chelonae was up to 62,854 SNVs (19,082.6 SNVs per Mpb). This is similar to Mycobacterium marinum, a species that targets fish, and Mycobacterium avium, a species that can cause respiratory illness in humans, which showed up to 89,000 SNVs (24,054.1 SNVs per Mbp) and 26,871 SNVs (6249.1 SNVs per Mbp), respectively. Both show more variation than Mycobacterium tuberculosis, which showed a maximum 1800 SNVs between strains [31–33].
To provide a control for sequencing error, we developed a dataset to establish an empirical threshold for calling identity between two isolates that takes account of error in DNA sequence determination. We sequenced a strain of V. campbellii using the Illumina method 39 times independently. The number of SNVs ranged from 0 to 74 SNVs depending on which pair of sequencing runs was compared, despite the same input DNA. There were several runs that, though following the same protocol, had higher numbers of SNVs on average in pairwise comparisons, and these correlated with low sequence coverage (S3 Table). Upon considering the length of the core genome, the average SNVs per Mbp was 3.6 which could be used for a general threshold for comparing microbial genomes considering sequencing error. Going forward, the data presented here provides useful background for comparing microbial genomes.
Our analysis also included two samples collected from the same individual and a single isolate sequenced twice, again to help evaluate thresholds for calling identity despite sequencing error. We found that our two samples from the same patient only differed by 0.91 SNVs per Mbp core genes after our hybrid assembly; our single isolate sequenced twice differed by 0.61 SNVs per Mbp core genes. These control M. chelonae genomes show SNV numbers well within the range expected for identical sequences based on our V. campbellii dataset. This indicates that the sequencing and hybrid assembly pipeline works as expected to generate high quality genomes and allows identification of identical organisms sequenced twice independently.
Our analysis also shed light on the host preferences of sequenced M. chelonae strains. Some of our isolates were closely related to environmental samples annotated as from water and sea creatures, while others clustered with human isolates. Our data did not provide evidence for a strongly human-associated clade.
Examination of SNVs in particular genes and RGI provided a list of possible mechanisms of resistance. For the SNVs that were identified in gyrA, the resulting amino acid changes might potentially modulate the resistance phenotype, though functional confirmation is needed. As for the genes from the RGI, while there were a few high identity resistance gene hits in our isolates, the presence of these genes did not correspond with the drug susceptibility phenotypes. For example, every isolate had an 80% identity hit to erm(38), a gene found in M. smegmatis to mediate resistance to macrolides, such as clarithromycin, but all of the isolates were susceptible to clarithromycin. Thus, it is possible that the erm(38) homolog in M. chelonae performs a different role. For LRA-3, a metallo-beta-lactamase gene, all isolates were intermediately or fully resistant to the carbapenem tested. Since there are two phenotypes in the presence of the gene, this suggests that it may play a role in resistance, but other mechanisms may be involved as well. We also assessed low homology hits to resistance genes and mutations in genes that are known to influence drug susceptibility. For both, we have a list of possible genes or mutations, but given our low sample size, it is likely that many are false positives, and all require further validation to confirm. Resistance in M. chelonae may in part be due to blocking entry of antibiotics by the tough cell wall documented previously [34]. Data presented here may help guide future studies of mechanisms of resistance in M. chelonae.
In conclusion, we investigated a possible nosocomial outbreak of M. chelonae at HUP. Our analysis concluded that no point source transmission occurred and that each case of M. chelonae involved clearly distinct strains, likely acquired from the environment. Our analysis also includes a dataset to help determine thresholds for evaluating identity between different strains while controlling for sequencing error. Finally, we queried potential antibiotic resistance mechanisms by genomic analysis, providing candidate genes and mutations for potential follow up.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We are grateful to members of the Bushman laboratory for help and suggestions; and Laurie Zimmerman for help with figures.
Data Availability
Nanoflow is available at www.github.com/zhaoc1/nanoflow while all other computer code used in this study is available at www.github.com/chrgu. M. chelonae and V. campbellii assembled genomes available at GenBank under project PRJNA594977.
Funding Statement
This work was supported by the Penn Center for AIDS Research P30 AI 045008 (https://www.med.upenn.edu/cfar)under FDB; and the PennCHOP Microbiome Program (https://pennchopmicrobiome.chop.edu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akram SM, Saleh D. Mycobacterium Chelonae. Treasure Island (FL); 2019. [Google Scholar]
- 2.Donohue MJ, Wymer L. Increasing prevalence rate of nontuberculous mycobacteria infections in five states, 2008–2013. Ann Am Thorac Soc. 2016. 10.1513/AnnalsATS.201605-353OC [DOI] [PubMed] [Google Scholar]
- 3.Wang HX, Yue J, Han M, Yang JH, Gao RL, Jing LJ, et al. Nontuberculous mycobacteria: Susceptibility pattern and prevalence rate in Shanghai from 2005 to 2008. Chin Med J (Engl). 2010. 10.3760/cma.j.issn.0366-6999.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy BS, Bedard B, Younge M, Tuttle D, Ammerman E, Ricci J, et al. Outbreak of Mycobacterium chelonae Infection Associated with Tattoo Ink. N Engl J Med. 2012. 10.1056/NEJMoa1205114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers H, Brown‐Elliott BA, Moore D, Curry J, Truong C, Zhang Y, et al. An Outbreak of Mycobacterium chelonae Infection Following Liposuction. Clin Infect Dis. 2002. 10.1086/340399 [DOI] [PubMed] [Google Scholar]
- 6.Freitas D, Alvarenga L, Sampaio J, Mannis M, Sato E, Sousa L, et al. An outbreak of Mycobacterium chelonae infection after LASIK. Ophthalmology. 2003. 10.1016/S0161-6420(02)01643-3 [DOI] [PubMed] [Google Scholar]
- 7.Monego F, Duarte RS, Biondo AW. gyrA and gyrB Gene Mutation in Ciprofloxacin-Resistant Mycobacterium massiliense Clinical Isolates from Southern Brazil. Microb Drug Resist. 2012;18: 1–6. 10.1089/mdr.2011.0047 [DOI] [PubMed] [Google Scholar]
- 8.Hillemann D, Rüsch-Gerdes S, Richter E. In vitro-selected linezolid-resistant Mycobacterium tuberculosis mutants [4]. Antimicrobial Agents and Chemotherapy. 2008. 10.1128/AAC.01189-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nessar R, Reyrat JM, Murray A, Gicquel B. Genetic analysis of new 16s rRNA mutations conferring aminoglycoside resistance in Mycobacterium abscessus. J Antimicrob Chemother. 2011. 10.1093/jac/dkr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNabb A, Eisler D, Adie K, Amos M, Rodrigues M, Stephens G, et al. Assessment of partial sequencing of the 65-kilodalton heat shock protein gene (hsp65) for routine identification of Mycobacterium species isolated from clinical sources. J Clin Microbiol. 2004. 10.1128/JCM.42.7.3000-3011.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard—Second Edition. CLSI document M24-A2. Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes. 2011. [PubMed] [Google Scholar]
- 12.Hatakeyama S, Ohama Y, Okazaki M, Nukui Y, Moriya K. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect Dis. 2017. 10.1186/s12879-017-2298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace RJ, Brown-Elliott BA, Crist CJ, Mann L, Wilson RW. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob Agents Chemother. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Soolingen D, Hermans PWM, De Haas PEW, Soll DR, Van Embden JDA. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: Evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991. 10.1128/JCM.29.11.2578-2586.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke EL, Taylor LJ, Zhao C, Connell A, Lee JJ, Fett B, et al. Sunbeam: An extensible pipeline for analyzing metagenomic sequencing experiments. Microbiome. 2019. 10.1186/s40168-019-0658-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13: 1–22. 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19: 455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015. 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 20.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, et al. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015. 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genomics. 2016. 10.1099/mgen.0.000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price MN, Dehal PS, Arkin AP. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS One. 2010. 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017. 10.1093/nar/gkw1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen HK, Moe LA, Rodbumrer J, Gaarder A, Handelsman J. Functional metagenomics reveals diverse Β-lactamases in a remote Alaskan soil. ISME J. 2009. 10.1038/ismej.2008.86 [DOI] [PubMed] [Google Scholar]
- 27.Madsen CT, Jakobsen L, Douthwaite S. Mycobacterium smegmatis Erm(38) is a reluctant dimethyltransferase. Antimicrob Agents Chemother. 2005. 10.1128/AAC.49.9.3803-3809.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nash KA. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38). Antimicrob Agents Chemother. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouquette C, Harmon JB, Shafer WM. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol Microbiol. 1999. 10.1046/j.1365-2958.1999.01517.x [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Zeng J, Zhang H, He ZG. The characterization of conserved binding motifs and potential target genes for M. tuberculosis MtrAB reveals a link between the two-component system and the drug resistance of M. smegmatis. BMC Microbiol. 2010. 10.1186/1471-2180-10-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S, Pettersson BMF, Behra PRK, Mallick A, Cheramie M, Ramesh M, et al. Extensive genomic diversity among Mycobacterium marinum strains revealed by whole genome sequencing. Sci Rep. 2018. 10.1038/s41598-018-30152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuite AR, Guthrie JL, Alexander DC, Whelan MS, Lee B, Lam K, et al. Epidemiological evaluation of spatiotemporal and genotypic clustering of mycobacterium tuberculosis in Ontario, Canada. Int J Tuberc Lung Dis. 2013. 10.5588/ijtld.13.0145 [DOI] [PubMed] [Google Scholar]
- 33.Lande L, Alexander DC, Wallace RJ, Kwait R, Iakhiaeva E, Williams M, et al. Mycobacterium avium in community and household water, suburban Philadelphia, Pennsylvania, USA, 2010–2012. Emerg Infect Dis. 2019. 10.3201/eid2503.180336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarlier V, Nikaido H. Mycobacterial cell wall: Structure and role in natural resistance to antibiotics. FEMS Microbiology Letters. 1994. 10.1111/j.1574-6968.1994.tb07194.x [DOI] [PubMed] [Google Scholar]