Abstract
Background
Leishmaniasis is a neglected disease, and the current therapeutic arsenal for its treatment is seriously limited by high cost and toxicity. Nanostructured lipid carriers (NLCs) represent a promising approach due to high drug loading capacity, controlled drug release profiles and superior stability. Here, we explore the efficacy of a unique pH-sensitive amphotericin B-loaded NLC (AmB-NLC) in Leishmania braziliensis infection in vitro and in vivo.
Methods and Results
AmB-NLC was assessed by dynamic light scattering and atomic force microscopy assays. The carrier showed a spherical shape with a nanometric size of 242.0 ± 18.3 nm. Zeta potential was suggestive of high carrier stability (−42.5 ± 1.5 mV), and the NLC showed ~99% drug encapsulation efficiency (EE%). In biological assays, AmB-NLC presented a similar IC50 as free AmB and conventional AmB deoxycholate (AmB-D) (11.7 ± 1.73; 5.3 ± 0.55 and 13 ± 0.57 ng/mL, respectively), while also presenting higher selectivity index and lower toxicity to host cells, with no observed production of nitric oxide or TNF-α by in vitro assay. Confocal microscopy revealed the rapid uptake of AmB-NLC by infected macrophages after 1h, which, in association with more rapid disruption of AmB-NLC at acidic pH levels, may directly affect intracellular parasites. Leishmanicidal effects were evaluated in vivo in BALB/c mice infected in the ear dermis with L. braziliensis and treated with a pentavalent antimonial (Sb5+), liposomal AmB (AmB-L) or AmB-NLC. After 6 weeks of infection, AmB-NLC treatment resulted in smaller ear lesion size in all treated mice, indicating the efficacy of the novel formulation.
Conclusion
Here, we preliminarily demonstrate the effectiveness of an innovative and cost-effective AmB-NLC formulation in promoting the killing of intracellular L. braziliensis. This novel carrier system could be a promising alternative for the future treatment of cutaneous leishmaniasis.
Keywords: leishmaniasis, neglected disease, nanoparticles, drug delivery
Introduction
Leishmaniasis is a widespread group of neglected vector-borne tropical diseases caused by Leishmania spp. (protozoa: Trypanosomatidae). The disease mainly manifests in two forms: visceral leishmaniasis (VL), which is potentially lethal, and the more common form, cutaneous leishmaniasis (CL). It is estimated that between 700,000 and 1,000,000 new cases are reported annually worldwide; about 95% of CL cases occur in Afghanistan, Algeria, Bolivia, Brazil, Colombia, Iran, Iraq, Pakistan, the Syrian Arab Republic and Tunisia.1 In Brazil, CL mainly arises from Leishmania braziliensis, which can induce ulcerating lesions often characterized by crusted papules in the human host.2,3
Pentavalent antimonials (Sb5+) have been employed as the first-line of treatment against leishmaniasis since the 1940s.4 However, Sb5+ therapy presents several limitations, such as systemic side effects, cardiac, renal and hepatic toxicity and a prolonged course of therapy (20–30 days). In addition, increasing evidence of variable efficacy and higher numbers of resistant cases has prompted the development of novel therapies.5–10
Amphotericin B (AmB) is the second-line drug of choice for leishmaniasis treatment in Brazil. Despite being considered the most effective antileishmanial commercially available, AmB is also one of the most toxic compounds utilized in clinical practice.11,12 Among the lipid formulations developed to mitigate the toxic effects caused by conventional AmB (AmB-deoxycholate), the liposomal formulation of AmB (Ambisome®) presents the lowest toxicity, and has therefore been recommended by the Brazilian Ministry of Health as a first-line drug for VL treatment in priority groups.13 However, its wide clinical use in the treatment of CL has been restricted by high cost, which also limits its application in large-scale clinical trials designed to test safety and efficacy.
The present study developed and tested Nanostructured Lipid Carriers (NLC) loaded with AmB (AmB-NLC) to evaluate efficacy as candidates for CL treatment. NLCs are spherical lipid particles, sized 10–1000 nm, with a lipid nanoparticle formulation consisting of a solid lipid core matrix mixed with liquid lipids. NLCs have gained prominence due to increased bioavailability, improvements in controlled drug release at specific sites, the non-use of organic solvents or high concentrations of surfactants, physical stability over longer periods and feasibility of production on an industrial scale.14,15
The development and characterization of AmB-NLC has been previously demonstrated by our group, with outstanding results achieved in terms of sustained release and reduced AmB cytotoxicity, probably related to the polyaggregate drug form.16 The particle presents a sustained controlled release profile of up 46 h at pH 7.4, with faster disruption in acidic environments (pH 5), and presents a target-driven effect due to the phospholipid nanoparticle composition employed. In this context, AmB-NLC represents an innovative new approach to AmB treatment in leishmaniasis and fungal infections.
In clinical use, despite recent advances in the literature employing new pharmaceutical forms of AmB, this drug is conventionally administered by intravenous route. The development of lipid carrier delivery systems has made new routes of AmB administration possible, including oral, topic and intraperitoneal.17–19 Ammar et al employed AmB-loaded PLGA to treat cutaneous leishmaniasis by topical therapy, with controlled drug release demonstrated by this carrier system.20 Few studies have intraperitoneally administered AmB via lipid carrier to treat leishmaniasis.17,18 The AmB lipid carrier developed by De Carvalho et al to treat cutaneous leishmaniasis demonstrated efficacy and significantly greater reduction in parasitic burden compared to AmB-deoxycholate.17
Here we show that AmB-NLC, with a pH-sensitive design, was able to significantly reduce the rate of L. braziliensis infection in vitro, as well as lesion development in vivo in a BALB/c mouse model. Accordingly, our data suggest that AmB-NLC constitutes a promising new and potentially safer and cheaper formulation of AmB for CL treatment.
Materials and Methods
Material and Reagents
To produce AmB-NLC, the liquid lipid extract of MCT (medium-chain triacylglycerides) and a surfactant were acquired from Lipoid GMBH (Ludwigshafen, Germany), while solid stearic acid lipid nanoparticles, the co-surfactant of sodium cholate hydrate and Triton™ X-100 were all acquired from Sigma Aldrich (St. Louis, MO, USA). Polyethylene glycol 400 co-surfactant was acquired from Cosmo Química (São Paulo, SP, Brazil), and antioxidant vitamin E was obtained from Tovani Ltda (São Paulo, SP, Brazil). The active compound of amphotericin B was acquired from North China Pharmaceutical Huasheng (Hong Kong, China), and Amphotericin B reference medicine was acquired from União Farmacêutica Nacional S/A- Unianf® (Embu-Guaçu, SP, Brazil). The liposomal formulation of Amphotericin B (Ambisome®) was acquired from United Medical Ltda (São Paulo, SP, Brazil). The other reagents employed herein were Schneider’s insect medium, lipopolysaccharide (LPS), IFN-γ and Resazurin sodium salt, all obtained from SIGMA-Aldrich (St Louis, MO, USA). Lactate Dehydrogenase (LDH) Cytotoxicity Detection Kit and Nutridoma™-SP were obtained from Roche Diagnostics GmbH (Sandhofer Strasse, Mannheim, Germany). Inactive Fetal bovine serum (FBS), RPMI 1640 medium and RPMI 1640 medium without phenol red, Geneticin® antibiotic and penicillin were purchased from GIBCO (Carlsbad, CA, USA). Streptomycin, L-glutamine and DAPI were obtained from Invitrogen (Carlsbad, CA, USA). Lumogen Red F300® (0.02%w/v) was purchased from BASF (Ludwigshafen, Germany). A Human TNF-α DuoSet Elisa kit was purchased from R&D Systems (Minneapolis, MN, USA).
NLC Production
A previously established proportion of ingredients was used to formulate AmB-NLC.16 Initially, NLC was produced by hot microemulsion (65 ± 5°C), consisting of a blend of liquid and solid lipids, a surfactant and co-surfactants, using the method described by Tadini et al.16,21 To obtain AmB-NLC, amphotericin B (0.1%) and the antioxidant (0.005%) were added to a hot microemulsion under stirring at 150 rpm. The microemulsion containing the drug was then diluted in cold water (1–4°C) under homogenization (IKA - TURRAX T25). Empty NLC (Ø-NLC) was prepared in the absence of amphotericin B. The above-described procedure resulted in the formation of nanostructured lipid carriers suspended in water, which were subsequently physicochemically characterized. NLC-AmB and Ø-NLC containing Lumogem Red F300 (employed only in the Uptake assessment) were prepared by adding the fluorophore (0.02% w Lumogem/w lipid phase) by hot microemulsion.22 In sequence, the same steps were performed for the NLC preparation. All resulting suspensions were employed in biological testing.
AmB-NLC Physicochemical Characterization
Dynamic Light Scattering
An aliquot of 100 µL of AmB-NLC was diluted 1000-fold in deionized water. The diluted suspension was analyzed by Zetasizer Nano ZS dynamic light scattering (DLS) equipment (Malvern Panalytical). Z-average particle size, polydispersity index and Zeta Potential were measured by optical path. All measurements were taken at room temperature (~24°C) and performed in triplicate.
Atomic Force Microscopy
To perform microscopic characterization using an atomic force microscope (Shimadzu, SPM-9600), AmB-NLC was first diluted 100-fold in deionized water and the diluted suspension was placed on mica plates (200 µL). Analysis was performed after the sample dried.
Drug Content
The analysis employed herein was performed using a HPLC-DAD (Shimadzu, Kyoto, Japan) in accordance with the method previously described by Tadini et al, which was previously validated by our group in accordance with the European Medicines Agency (EMA) guideline on Bioanalytical Method Validation.16,23
The guard column (3.0 mm x 4.6mm, 2.7 µm) was coupled to a Ascentis Express Fused Core C18 chromatographic column (100 mm x 4.6 mm, 2.7 µm), both acquired from Supelco (St. Louis, MO, USA). Analyses were carried out at 30°C under ambient yellow light. The flow rate was set to 1 mL/min in isocratic mode, with 70% of methanol and 30% of an aqueous solution containing 0.1% of formic acid. The injection volume was 10 µL. Carbamazepine was used as the internal standard (25 µg mL−1). Amphotericin B and carbamazepine were detected at wavelengths of 407 nm and 285 nm, respectively. The analytical curve range was 5.0–60.0 µg mL−1, applying a weighting factor of 1/x.
Entrapment Efficiency
Aliquots of 1.00 mL of AmB-NLC suspension were transferred to a vial and centrifuged at 14,000 × g for 30 min using a Hitachi RX II (Hitachi) centrifuge at a controlled temperature of 4°C. Sample aliquots (100 μL) were diluted 10-fold in methanol, mixed and submitted to HPLC-DAD analysis. Concentrations of free Amphotericin B (CFreeAmB) were determined using the previously generated analytical curve. CTotalAmb, referring to the total mass of amphotericin B in the suspension of AmB-NLC, was quantified using the analytical curve. Entrapment efficiency was calculated using the equation below:16
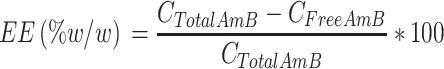 |
Antileishmanial Positive Controls
For comparison purposes, we tested the effects of the following first- and second-line antileishmanials recommended by the Brazilian Ministry of Health: Glucantime®, a meglumine antimonate (Sb5+), Unianf®, a micellar formulation of amphotericin B deoxycholate (AmB-D) and Ambisome®, a liposomal formulation of amphotericin B (AmB-L).24 In addition, the active compound of amphotericin B (AmB) was used as control in its free state.
Ethics Statement
Male BALB/c mice aged 6–8 weeks were obtained from the animal care facility at the Gonçalo Moniz Institute (IGM-FIOCRUZ), located in the city of Salvador, Bahia-Brazil. All animal experimentation was conducted in accordance with the Guidelines for Animal Experimentation established by the Brazilian Council for the Control of Animal Experimentation (CONCEA). The present study received approval from the local institutional review board (CEUA protocol no.: 018/2017, IGM/FIOCRUZ).
Parasites
Wild-type Leishmania braziliensis (MHOM/BR/01/BA788) (1 x 106) were cultured in Schneider’s insect medium supplemented with 10% inactive Fetal Bovine Serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin and 2 mM L-glutamine in 25 cm2 flasks at 24°C for 6 days. Promastigotes of Leishmania braziliensis (MHOM/BR/01/BA788) (1 x 106) modified to express green fluorescent protein (GFP) were cultured at 24°C in Schneider’s Insect medium supplemented with 10% FBS, 100 U/mL penicillin-streptomycin-glutamine and 50μg/mL of Geneticin® antibiotic (G418).25 Leishmania braziliensis GFP was exclusively employed in uptake assessment assays.
Macrophage Toxicity Assay
Bone-marrow derived macrophages (BMDM) were obtained from BALB/c mice femurs and cultured at 37°C under 5% CO2 for 7 days in RPMI medium supplemented with 20% FBS, 100U/mL penicillin, 100mg/mL streptomycin, 2mM L-glutamine and 20% L929 cell culture supernatant (a source of macrophage colony-stimulating factor). Next, differentiated BMDM were harvested using cold saline solution. BMDM (2×105/well) were plated on 96-well plates and cultured at 37°C under 5% CO2 in RPMI-supplemented medium for 24 hours. BMDM were then treated with either AmB, AmB-D, AmB-L or AmB-NLC at varying concentrations (8–0.0078 μg/mL) at 37°C for 48h. Next, cells were reincubated for another 4h with supplemented RPMI medium containing 10% Resazurin sodium salt. Absorbance was read at 570 nm and 600 nm using a spectrophotometer (SPECTRA Max 190). Uninfected BMDM cultures supplemented RPMI medium (Medium) and empty Nanostructured Lipid Carriers (Ø-NLC) were as used as controls.
Measurements of cytoplasmic lactate dehydrogenase (LDH) enzyme activity were performed by plating BMDM (2×105/well) on 96-well plates, followed by culturing at 37°C under 5% CO2 in RPMI medium without phenol red, supplemented with 1% Nutridoma instead of FBS. BMDM were then treated as described above. Next, cells were centrifuged at 250×g and the cell-free culture supernatant was used to spectrophotometrically measure LDH activity using a commercial LDH Cytotoxicity Detection Kit. According to the manufacturer’s instructions, absorbance was recorded at 490 nm using a spectrophotometer (SPECTRA Max 190). Total LDH activity was determined by lysing the cells with 1% Triton X-100. The percentage of LDH release was calculated as follows: [(LDH sample – Blank LDH) × 100]/total LDH.
Determination of AmB-NLC IC50 by Assessing L. braziliensis Intracellular Viability
BMDM were isolated as described above and 105 cells/well were seeded on 96-well plates in supplemented RPMI medium. Macrophages were then infected (10∶1) with stationary-phase Leishmania braziliensis (MHOM/BR/01/BA788) promastigotes for 24h. Next, macrophage cultures were washed 2x with saline to remove non-internalized parasites and treated with varying concentrations (250–3.9 ng/mL) of AmB, AmB-D or AmB-NLC for 48h. After the medium was replaced with 0.2 mL of supplemented Schneider’s insect medium, cells were cultured at 24°C for an additional 6 days, after which the number of viable parasites was determined by direct counting. The obtained IC50 corresponding to each of the treatments was validated as follows: BMDM were plated at a density of either 105/well on 96-well plates (parasite viability) or 2x105/well on 24-well plates with glass coverslips (parasite counts via microscopy). Cells were then infected and treated with the corresponding IC50 concentrations of each of the compounds as described above. Coverslips were stained with hematoxylin and eosin to calculate infection index (percentage of infected cells x mean number of amastigotes per cell), as determined by random counts of 200 cells/field on each glass coverslip under optical light microscopy.
Uptake Assessment of AmB-NLC by Macrophages
BMDM (105/well) were seeded on glass coverslips and cultured overnight at 37°C under 5% CO2. Next, cells were infected with stationary-phase Leishmania braziliensis GFP promastigotes for 24h. After washing 2x with saline, cells were treated at a dilution of 1:200 (~4 mg/mL) of AmB-NLC or Ø-NLC containing the fluorochrome Lumogen Red F300. Plates were then incubated at 37° C under 5% CO2 for 1, 2, 24 or 48h. After each treatment time, cells were washed 2x with 1x PBS and fixed with a 4% paraformaldehyde solution. Coverslips were then labeled with DAPI (Invitrogen) and kept protected from light at −20°C until analysis by spectral confocal microscopy (SPS TC8, Leica Microsystems, Germany).
Nitric Oxide and TNF-α Level Quantification
BMDM (106/well) were stimulated with IFN-γ (100 UI/mL) for 24h and infected with stationary-phase Leishmania braziliensis promastigotes for an additional 24h. Macrophages were then washed twice to remove any non-internalized parasites, the RPMI cell medium was replaced and IFN-γ stimulation was reapplied along with the obtained IC50 concentrations of AmB, AmB-D and AmB-NLC, or Ø-NLC, for 48h (Table 1). After collecting culture supernatants, the Griess reaction was used to measure nitric oxide (NO) production. Production levels were measured in culture supernatants using a Human TNF-α DuoSet Elisa kit (R&D), in accordance with the manufacturer’s instructions.
Table 1.
AmB-NLC Characterization
| Diameter | PDIª | Zeta Potential | Amphotericin B Amount |
EEb |
|---|---|---|---|---|
| 242.0 ± 18.3 nm | 0.44 ± 0.02 | −42.5 ± 1.5 mV | 1.02 ± 0.05 mg mL−1 | ~ 99% |
Abbreviations: aPDI, polydispersity index; bEE, entrapment efficiency.
In vivo Evaluation
BALB/c mice were inoculated with stationary-phase L. braziliensis promastigotes (105 parasites in 10µL of saline) in the left ear dermis using a 30G needle, according to the model described by Moura et al.26 The animals were divided into four experimental groups (n = 12) at 3 weeks post-infection and treated intraperitoneally with seven doses (6mg/kg/day) of: 1) Ø-NLC; 2) AmB-NLC or 3) AmB-L (Ambisome®) on alternating days (14-day total treatment period). In parallel, 4) another experimental group of mice received Sb5+ (Glucantime® at 100mg/Sb5+/kg/day) intraperitoneally five times a week for 5 weeks.27 Lesion development was monitored weekly for 10 weeks using an analog caliper (Kroeplin, Schlüchtern, GER). Mouse ear lesion size development was considered successful when ~1 mm, with a nodular and ulcerated presentation, in accordance with the model previously described by Moura et al.26 At six and 10 weeks post-infection, half of the mice in each group were euthanized with a lethal dose of sodium thiopental (100 mg/kg, intraperitoneally), after which the ears and draining lymph nodes (dLN) near the site of infection were aseptically removed and homogenized in supplemented Schneider’s medium. These homogenates were serially diluted and seeded onto 96-well plates. Parasite load was determined using a limiting-dilution assay, as described by Titus et al.28 The number of viable parasites was determined using the lowest concentration at which promastigotes were able to grow after 2 weeks of incubation in a BOD incubator at 24°C.
Histological Analysis
The postmortem aseptic removal of mouse ears was performed at six or 10 weeks post-infection, followed by fixing in 10% formaldehyde. Tissues were then embedded in paraffin, 5-µm-thick sections were stained with hematoxylin and eosin and subsequently analyzed by light microscopy.
Statistical Analysis
Data are represented as the average of 3–4 independent experiments (mean ± SEM) performed at least in triplicate, or a representative experiment performed in vivo with 12 animals/group (mean ± SD) (Figure legends indicate the respective number of experiments performed). The Kruskal–Wallis non-parametric test was used for multiple comparisons and comparisons between two groups were performed by Mann–Whitney non-parametric test. Sigmoidal dose–response curves were used to determine mean inhibition concentration (IC50) values relative to intracellular parasite viability, as well as cytotoxicity concentration implying 50% cell viability (CC50). Values representative of selectivity index (SI), which indicates how selective a compound is relative to parasite versus macrophages, were calculated using CC50: IC50 ratios. Analyses were performed using GraphPad Prism v 5.00 for Windows (GraphPad Software, San Diego California). Results were considered statistically significant when p <0.05.
Results
AmB-NLC Physicochemical Characterization
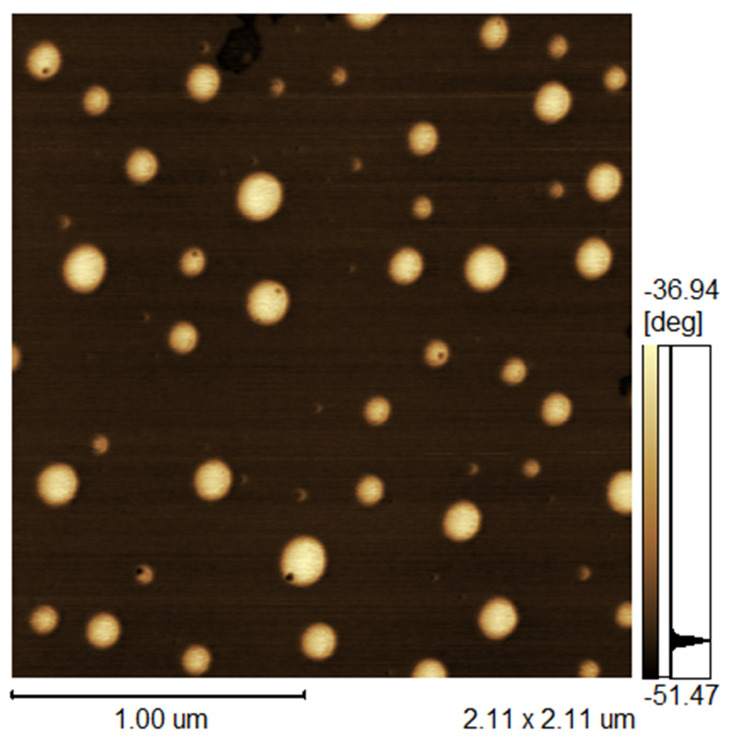
AFM images of AmB-NLC (Figure 1) illustrate the morphological characteristics of the obtained nanoparticle. The particle was spherical in shape and the observed nanometric particle Z-average size of 242.0 ± 18.3 nm corroborated DLS analysis. AmB-NLC exhibited a characteristic polydispersity index (PDI) of 0.44 ± 0.02 and a Zeta potential of −42.5 ± 1.5 mV (Table 1).
Figure 1.
Atomic force microscopy of AmB-NLC.
Abbreviation: AmB-NLC, amphotericin B-loaded nanostructured lipid carrier.
The HPLC-DAD analysis revealed an AmB content of 1.02 ± 0.05 mg mL−1 in AmB-NLC, which corresponded to the amount of active compound added to the formulation. The resulting entrapment efficiency was ~99%, indicating that the lipid carrier was capable of encapsulating all available AmB (Table 1).
AmB-NLC Presents Low Toxicity to Uninfected Macrophages
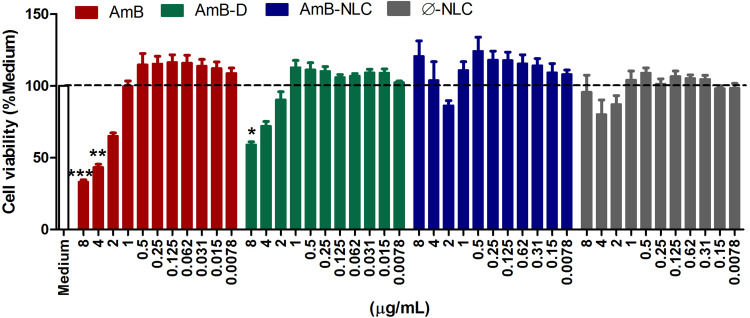
The cytotoxicity of amphotericin B formulations to uninfected macrophages was assessed by resazurin reduction assay (Figure 2) and by Lactate dehydrogenase activity (Supplementary Figure 1) in the supernatants of macrophages treated at concentrations ranging from 0.0078 to 8 µg/mL. After 48h of treatment, the highest tested concentrations of AmB and AmB-D were found to reduce cell viability by 66% and 40%, respectively, compared to Medium (p< 0.0001 and p< 0.01, respectively). By contrast, no cytotoxic effects were observed in cells treated at identical concentrations with AmB-NLC and Ø-NLC (Figure 2). In addition, a significant increase in LDH levels in AmB-treated macrophages at the highest concentrations tested (p< 0.001 and p< 0.01) indicated cell damage as evidenced by loss of plasma membrane integrity (Supplementary Figure 1).
Figure 2.
AmB-NLC cytocompatibility in vitro. Macrophages were treated with AmB, AmB-D or AmB-NLC (8–0.0078 µg/mL) for 48h. Cell viability was determined by Resazurin sodium salt reduction percentage. Supplemented RPMI Medium (Medium) and empty carriers (Ø-NLC) were used as controls. Bars represent ± SEM of two experiments. Kruskal–Wallis nonparametric testing, followed by Dunn’s multiple comparison post-test, were used for comparisons between experimental groups (*p< 0.05, **p< 0.01 and ***p< 0.0001).
Abbreviations: AmB, active compound of amphotericin B; AmB-D, deoxycholate amphotericin B; AmB-NLC, amphotericin B-loaded nanostructured lipid carrier; RPMI, cell culture medium developed at Roswell Park Memorial Institute; SEM, standard error of the mean.
Accordingly, the highest CC50 value for uninfected macrophages was obtained using AmB-NLC (12.34 ± 2.67 µg/mL), compared to the other tested formulations: 4.31 ± 2.66 and 10.79 ± 1.54 µg/mL for AmB and AmB-D, respectively (Table 2).
Table 2.
IC50, CC50 and Selective Index (SI) of Different Amphotericin B Formulations
| Compound | Amastigote IC50a ± SEMb (ng/mL) |
Macrophage CC50c ± SEM (µg/mL) |
SId |
|---|---|---|---|
| AmB | 5.3 ± 0.55 * | 4.31 ± 2.66 | 813 |
| AmB-D | 13 ± 0.57 | 10.79 ± 1.54 | 830 |
| AmB-NLC | 11.7 ± 1.73 | 12.34 ± 2.67 | 1046 |
Note: *Indicate statistically significant differences (p = 0.0210) in relation to AmB-D (deoxycholate amphotericin B).
Abbreviations: aIC50, half-inhibitory concentration. Concentration needed to inhibit a given biological process by half; bSEM, standard error of the mean; cCC50, 50% cytotoxic concentration, the concentration needed reduce cell viability by 50%; dSI, selectivity index. Macrophage CC50/amastigote intracellular IC50
AmB-NLC Reduces Intracellular L. braziliensis Viability in a Dose-Dependent Manner
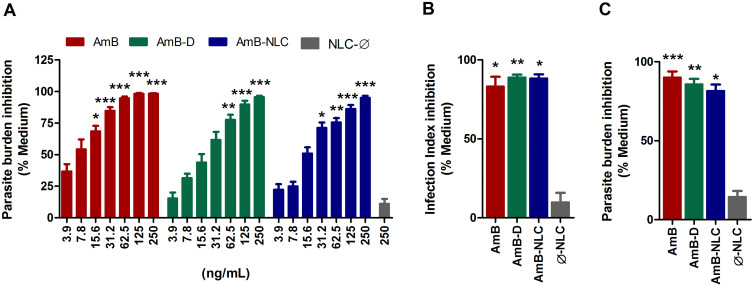
Next, we evaluated the efficacy of AmB-NLC in reducing intracellular L. braziliensis viability. After 48h of treatment, both AmB and AmB-NLC significantly reduced the number of viable parasites by 84% and 70%, respectively, beginning at a concentration of 31.2 ng/mL (p < 0.0001 and p < 0.05, respectively) (Figure 3A). AmB-D was also shown to significantly reduce the number of viable parasites recovered in a similarly dose-dependent manner (≥62.5 ng/mL, p< 0.01). No significant antileishmanial effect was detected using Ø-NLC.
Figure 3.
Leishmanicidal effect of AmB-NLC on intracellular L. braziliensis viability. (A) Dose-dependent effect of AmB-NLC on intracellular L. braziliensis viability: macrophages were infected with L. braziliensis and treated with either AmB, AmB-D or AmB-NLC (250–3.9 ng/mL) for 48h. Leishmanicidal effects at IC50 concentrations of different amphotericin B formulations: (B) Parasite burden; (C) Infection Index. Supplemented RPMI Medium (Medium) and empty carriers (Ø-NLC) were used as controls. Bars represent ± SEM of two or four experiments. Kruskal–Wallis nonparametric testing, followed by Dunn’s multiple comparison post-test, were used for comparisons between experimental groups, while the Mann–Whitney non-parametric t-test was used for comparisons between two groups (*p< 0.05, **p< 0.01 and ***p< 0.0001).
Abbreviations: AmB, active compound of amphotericin B; AmB-D, deoxycholate amphotericin B; AmB-NLC, amphotericin B-loaded nanostructured lipid carrier; IC50, half-inhibitory concentration; RPMI, cell culture medium developed at Roswell Park Memorial Institute; SEM, standard error of the mean.
The IC50 values found for AmB, AmB-D and AmB-NLC were 5.3 ± 0.55; 13 ± 0.57 and 11.7 ± 1.73 ng/mL, respectively (Table 2). We then validated the obtained IC50 values by analyzing the leishmanicidal effect on infected AmB-treated macrophages using the infection index described above in Materials and Methods. Our data showed similar infection rates across all three tested AmB treatments, with statistically significant results compared to the control (Medium) (p< 0.01 and p< 0.05) (Figure 3B). Treatment with AmB-D and AmB-NLC similarly affected promastigote viability in infected macrophages (85.9% and 81.6% growth inhibition, respectively), with the highest efficacy observed for AmB (90.2% inhibition) (p< 0.001 and p <0.05) (Figure 3C).
AmB-NLC was not only found to present the highest CC50 value in our comparisons, but also had the highest SI value (1046) in comparison to AmB (813) and AmB-D (830) (Table 2). In sum, AmB-NLC appears to effectively reduce parasite load and presents low toxicity to macrophages, the main host cell of Leishmania.
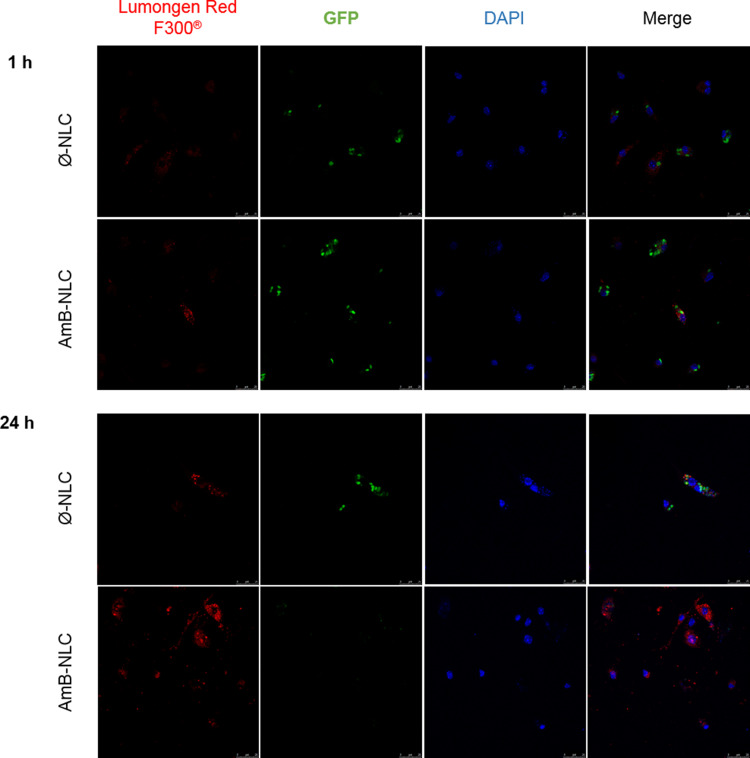
Rapid Uptake of NLCs by L. braziliensis-Infected Macrophages
The ability of NLCs to cross the membrane of the GFP mutant L. braziliensis-infected macrophage was evaluated by treatment kinetics at 1, 2, 24 and 48 h. After 1 h of treatment at 4µg/mL, NLCs were detectable in the macrophage cytoplasm (Figure 4). Greater fluorescence intensity of NLCs (shown in red) was seen at the highest tested concentration, which increased over time. The colocalization of nanoparticles with amastigote forms was also evident (shown in green). Reduced parasite fluorescence was observed in the presence of AmB-NLC over time, which further reinforces our previous finding that AmB-NLC effectively reduces intracellular parasite viability. The intracellular fluorescence intensity of both NLCs was similar at 2 h and 48 h of treatment, compared to 1 h and 24 h, respectively (Supplementary Figure 2).
Figure 4.
Intracellular delivery of NLC. Confocal microscopy of GFP mutant L. braziliensis-infected macrophages treated with Ø-NLC or AmB-NLC at 4 µg/mL for 1 or 24 h. Blue: nuclei stained with DAPI; Green: GFP mutant L. braziliensis; Red: Lumogen Red F300® fluorochrome-containing NLCs.
Abbreviations: AmB-NLC, amphotericin B-loaded nanostructured lipid carrier; DAPI, 4ʹ,6-diamidino-2-phenylindole, dihydrochloride; GFP, green fluorescent protein; RPMI, cell culture medium developed at Roswell Park Memorial Institute; Ø-NLC, empty nanostructured lipid carrier.
Modulation of Nitric Oxide and TNF-α Production by Different Amphotericin B Formulations
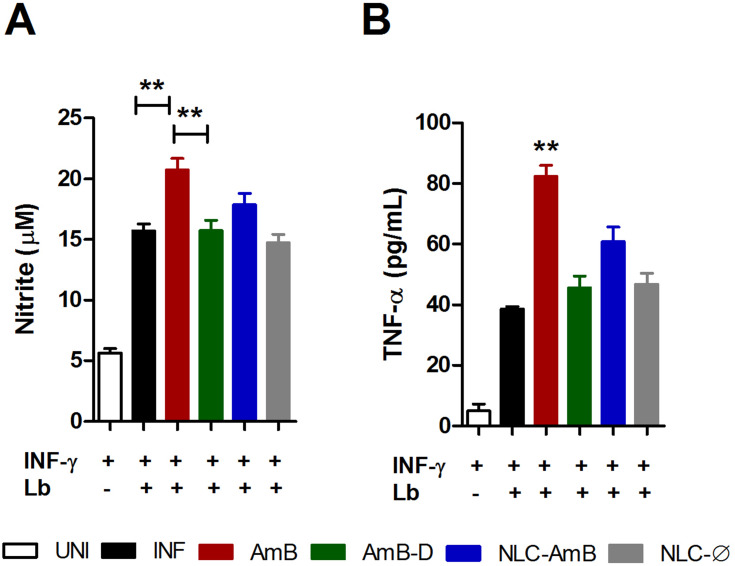
The leishmanicidal effects of amphotericin B on nitric oxide (NO) and TNF-α production by INF-γ-activated uninfected or infected murine macrophages were assessed in vitro (Figure 5). Among all tested amphotericin B formulations at IC50 concentrations, only AmB was found to significantly increase both NO and TNF-α production in activated L. braziliensis-infected macrophages compared to the infected control (INF) (p< 0.01) (Figure 5A and B, respectively). As expected, no nitric oxide or TNF-α production was detected following treatment with Ø-NLC.
Figure 5.
Modulation of nitric oxide and TNF-α production by different amphotericin B formulations. L. braziliensis-infected macrophages were treated with respective IC50 concentrations of AmB, AmB-D or AmB-NLC in the presence of IFN-γ for 48 h. (A) TNF-α levels measured by ELISA (B) Nitric oxide production determined by nitrite levels through the Griess reaction. Bars represent means ± SD of one representative experiment for TNF-α and means ± SEM of four experiments for NO. Kruskal–Wallis nonparametric testing, followed by Dunn’s multiple comparison post-test (**p<0.01).
Abbreviations: AmB, active compound of amphotericin B; AmB-D, deoxycholate amphotericin B; AmB-NLC, amphotericin B-loaded nanostructured lipid carrier; ELISA, enzyme-linked immunosorbent assay; INF, infected; NO, nitric oxide; SD, standard deviation; SEM, standard error of the mean; TNF-α, tumor necrosis factor-alpha; UNI, uninfected.
AmB-NLC Treatment Reduces Lesion Size and Parasite Load in vivo
The effects of AmB-NLC on L. braziliensis infection were evaluated in vivo. Mice were inoculated in the left ear dermis with L. braziliensis promastigotes and treated intraperitoneally 3 weeks later with Sb5+ (Glucantime®), AmB-L (Ambisome®), AmB-NLC or Ø-NLC, as described in Materials and Methods.
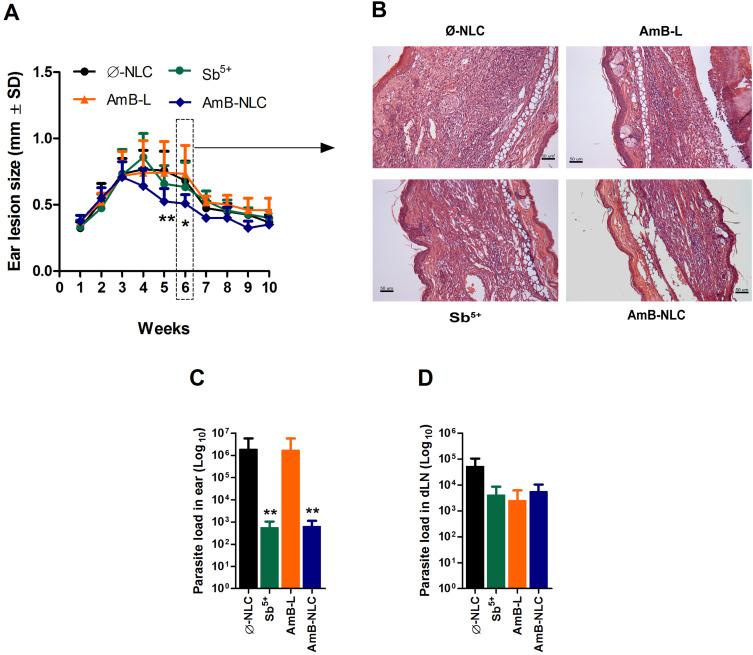
The animals treated with AmB-NLC had the smallest ear lesion size of all treated mice (p< 0.05 and p< 0.01) (Figure 6A). Histopathological analysis showed an intense inflammatory cell infiltrate consisting mainly of lymphocytes and macrophages, characteristic of chronic inflammation, in the ears of the animals in both the Ø-NLC and AmB-L groups (Figure 6B). On the other hand, the animals that received Sb5+ or AmB-NLC treatment showed milder inflammatory cell infiltrate (Figure 6B). Lower parasitic loads were seen in the ears of mice that received either AmB-NLC or Sb5+ (p< 0.05) (Figure 6C). Surprisingly, AmB-L treatment did not effectively reduce L. braziliensis parasite burden or lesion size. None of the treatments evaluated reduced parasitic load in the dLNs near the site of infection (Figure 6D). A previous pilot study using Ø-NLC as well as the dilution vehicle (Dextrose 5%) to treat animals (n = 5 animals/group) revealed no differences between these groups, as evidenced by nodular and ulcerated ear lesions measuring approximately 1.0 mm in size (Supplementary Figure 3). Thus, in the above in vivo experiment (n = 12 animals/group), the dilution vehicle control group was replaced by the Ø-NLC treated group, simply in an effort to reduce the number of animals used in the study.
Figure 6.
Intraperitoneal AmB-NLC treatment controls L. brasiliensis infection. BALB/c mice infected with L. braziliensis were treated intraperitoneally three weeks later with Sb5+ (Glucantime® 100 mg/Sb5+/kg/day, 5x a week), or AmB-L (Ambisome®), AmB-NLC or Ø-NLC (6 mg/kg/day, on alternating days over 2 weeks). (A) Lesion size development was monitored weekly; (B) Ear sections, obtained six weeks after infection, were analyzed by optical microscopy (200X magnification). Parasitic load in (C) infected ears and (D) draining lymph nodes. Bars represent mean ± SD of an experiment performed with 12 animals per group. Kruskal–Wallis nonparametric testing, followed by Dunn’s multiple comparison post-test (*p< 0.05 and **p< 0.01).
Abbreviations: AmB-L, liposomal amphotericin B; AmB-NLC, amphotericin B-loaded nanostructured lipid carrier; Sb5+, meglumine antimonate; SD, standard deviation; Ø-NLC, empty nanostructured lipid carrier.
Discussion
Nanotechnology-based stable drug delivery systems represent a key strategy in the search for more effective and less toxic leishmanicidal drugs. Here we investigated for the first time the leishmanicidal potential of nanostructured lipid carriers loaded with Amphotericin B (AmB-NLC), the most potent leishmanicidal drug currently commercially available, in L. braziliensis infection in vitro and in vivo.11 Besides demonstrating promising effects in controlling infection both in vitro and in vivo, the manufacture of AmB-NLC in Brazil would also be more cost-effective than currently available technological formulations, eg, liposomal, which can cost as much as US$ 400/ampoule (Ambisome®), thereby limiting treatment in poor countries.29
Physical analysis indicated that AmB-NLC presents a spherical shape, with a homogenous size and distribution in solution despite the obtained polydispersity index (PDI) value of 0.44 ± 0.02 (results closer to 0 indicate greater homogeneity).30 Since no filtration system was used to segregate particles of larger size, we considered the resulting PDI value to be satisfactory. In addition, other approaches on an industrial scale could be employed to decrease NLC-AmB PDI values. Particles with a non-spherical shape can suffer from low drug encapsulation efficiency, as well as deficiencies in particle transport and drug release, which can affect the biodistribution, pharmacokinetic and target-driven delivery properties.31 Moreover, the small average size of AmB-NLC (242.0 ± 18.3 nm) is also pharmacokinetically advantageous, since this facilitates the transport of small particles through biological barriers and provides an increased microenvironment contact surface.32 The Zeta potential, a measure of the electric potential of particles in a diffuse layer, of AmB-NLC (−42.5 ± 1.5 mV) was predictive of its stability in aqueous suspension, and indicates a reduced tendency to form agglomerates over the long term; ideal Zeta Potential values range between | 30 | and | 60 | mV.33 Furthermore, AmB-NLC was designed with a unique and innovative phospholipid composition in order to be pH-sensitive, thereby conferring the ability to target infectious tissues. A previous study by our group showed that AmB-NLC presents controlled sustained release profile (up to 46 hours) at physiological pH (7.4), as well as faster disruption in acidic environments (pH 5), lending a target-driven release characteristic to this AmB delivery system. Moreover, the developed nanoformulation more efficiently protected AmB from degradation in acidic environments compared to a micellar formulation (AmB-D).16 Considering that parasitophorous vacuoles, structures that allow parasites to survive and develop inside the host cell, are acidic compartments (pH 4.74 to 5.26), nanocarriers offering a stable, controlled and target drug release, such as AmB-NLC, hold promising potential for intracellular Leishmania control in acidified pH environments.34
Our evaluation of cytotoxicity in vitro indicated that AmB-NLC presents lower toxicity to macrophages than free AmB (raw material) or AmB-D (Unianf®). Tadini et al (2017) observed that AmB-NLC also had reduced cytotoxic effects on L929 mouse fibroblasts after 24 h or 48 h treatment, in comparison to AmB and AmB-D. On the other hand, we found that L. braziliensis-infected macrophages exposed to AmB-NLC reduced the rate of infection similarly to AmB-D, as demonstrated by the comparable IC50 values obtained for both compounds. This result seems promising, given that clinically approved AmB lipid formulations generally require higher concentrations provide efficacy comparable to AmB-D in vitro.35,36 AmB is known to reduce parasite viability at low concentrations and presents 10-fold greater affinity for ergosterol in parasite cell membranes than cholesterol in mammalian cells.37,38 It follows that the controlled release profile of AmB-NLC could reduce the levels of available free AmB over time, which could lessen toxic effects on host cells, while still effectively controlling infection. In addition, the intracellular delivery of fluorochromic-associated NLCs was found to be efficient even at the earliest treatment time (1h). Rapid AmB-NLC uptake by macrophages should lead to reduced drug accumulation in healthy tissues, thereby increasing therapeutic efficacy, reducing toxicity and preventing side effects.39
The main mechanisms of action suggested for AmB against parasites are its binding to ergosterol and the formation of pores in the cell membrane, which would allow the passage of electrolytes, such as K+, Ca2+ and Mg2+, leading to cell death.40,41 In addition, the induction of oxidative burst and the modulation of immune response in host cells have also been proposed as alternative mechanisms.42–44 Herein, our assessment of oxidative burst and immune response modulation through the evaluation of Nitric oxide (NO) and TNF-α production revealed that the IC50 concentration of AmB-NLC did not significantly modulate either mediator. Thus, we conclude that NO and TNF-α do not participate in the leishmanicidal mechanism by which AmB-NLC acts in vitro, and rather suggests a direct effect on intracellular parasites. Indeed, the rapid uptake of NLCs by L. braziliensis-infected macrophages observed herein, associated with the faster disruption of AmB-NLC at acidic pH levels, may directly affect intracellular parasites. On the other hand, in contrast to AmB-D and AmB-NLC, free AmB was significantly induced the production of both NO and TNF-α in our in vitro assessments. AmB also presented the highest toxicity to host cells observed in our study. Several studies have suggested the production of inflammatory mediators, such as TNF-α and NO, as responsible for the toxicity observed in some amphotericin B formulations, which is not the case in AmB-L.45–52 Thus, we believe that the free active compound of amphotericin B (AmB) likely presented greater bioavailability to host cells than either AmB-NCL or AmB-D), and thereby promoted not only the greatest leishmanicidal effect, but also the greatest toxicity to host cells.
To evaluate the potential leishmanicidal effect of AmB-NLC in vivo, we employed a standardized murine model capable of reproducing natural characteristics of this disease similar to that occurring in humans, ie, ulcerated skin lesions with regular, raised and well-delimited edges and granular bottom, as well as parasite dissemination to the lymphoid areas. To our knowledge, this is also the first time an experimental model, that closely mimics the natural infection aspects of cutaneous leishmaniasis, is used to evaluating NLC containing AmB. In this model, Leishmania braziliensis infection is self-healing in BALB/c mice. Lesions reach a peak size around the fifth or sixth weeks post-infection and gradually decrease in size until achieving complete cure, normally around the ninth week. Similarly, parasite load in the ear decreases from the sixth week onward.26 In this context, an effective treatment should be capable of shortening healing time, ie, able to preclude lesion development and/or induce smaller lesions compared to controls, as well as able to reduce tissue inflammation and parasite load at the peak of disease. Herein, all data obtained from the ear lesion size development and its dermal characterizes were in accordance with model described by Moura et al as well as to previous studies.26,27,53,54 Intraperitoneal treatment with AmB-NLC was found to be the most effective in decreasing mouse ear lesion size. Although both AmB-NLC and Sb5+ similarly reduced parasitic load and inflammatory cell infiltrate in infected ears, none of the treatments employed were able to reduce parasitic load in dLNs. Santos et al, in an evaluation of the leishmanicidal effect of 17-AAG, an inhibitor of the HPS90 protein, similarly observed diminished lesion size and parasitic load at the infection site of L. braziliensis-infected mice, without observing alterations in parasite burden in the dLNs.54 Likewise, Celes et al analyzed the efficacy of diethyl dithiocarbamate (DETC)-containing bacterial cellulose membranes and found no decreases in dLN parasitic load, despite demonstrating leishmanicidal action at the site of infection.27 Falcão et al suggested that parasite persistence within the dLNs of L. braziliensis-infected BALB/c mice may be due to presence of the IL-10-secreting CD4+CD25+Foxp3+ subset of T regulatory cells, which reduces the frequency of effector T cells.55 The role of CD4+CD25+ T regulatory cells in suppressing CD4+CD25− effector T cells was also shown to inhibit the elimination of Leishmania major in the skin of C57BL/6 mice, via mechanisms both dependent on and independent of IL-10.56 It has been suggested that the persistence of pathogens, such as Leishmania, in the host may provide beneficial immunity to the host, and could also be a reflection of both host and parasite co-evolutionary survival strategies.56
Considering that current treatment options for cutaneous leishmaniasis are limited by cost, host toxicity and an increasing number of refractory cases, it is our sincere hope that AmB-NLC may not only increase the therapeutic index of leishmaniasis treatment, but also encourage future scientific study towards the provision of low-cost stable drug delivery systems.
Conclusion
The results herein demonstrate for the first time the efficacy of AmB-NLC in promoting the in vitro and in vivo clearance of intracellular L. braziliensis parasites, the main etiological agent of cutaneous leishmaniasis in Brazil. The developed nanoformulation presented pharmacokinetic advantages, including a nanometric size, spherical shape and Zeta potential suggestive of high stability. Moreover, the unique pH-sensitive phospholipid composition indicates that AmB-NLC may be an innovative cost-effective drug delivery system. The observed clearance of intracellular L. braziliensis parasites in vitro by AmB-NLC, in the absence of either TNF-α or nitric oxide production at the IC50 concentration, may be related to more rapid disruption of AmB-NLC in acidic environments, including the parasitophorous vacuoles of Leishmania species. Moreover, the rapid AmB-NLC uptake by macrophages, as well as the controlled release profile at physiological pH levels, may have led to the lack of observed cytotoxicity in AmB-NLC-treated cells as compared to AmB and AmB-D. The in vivo evaluation, which employed a murine model capable of reproducing natural characteristics of cutaneous leishmaniasis in humans, demonstrated the efficacy of AmB-NLC in reducing both lesion size and parasite load in the ears of treated animals. Considering the high toxicity associated with conventional administration of amphotericin B (AmB-D), as well as the high cost of its less toxic liposomal formulation (AmB-L), our results represent a promising new formulation, with demonstrated safety and a cheaper cost than current commercially available formulations of amphotericin B. Our results suggest that the presently described novel AmB-NLC delivery system represents a promising nanoformulation that warrants further investigation in the treatment of cutaneous leishmaniasis.
Acknowledgments
The authors thank Andrezza Souza for technical and logistics support, Valdomiro Moitinho, MSc, for his assistance in vivo experimentation and Dr. Claudio Figueira for technical assistance with confocal microscopy. The authors would like to thank Andris K. Walter for English Language revision and manuscript copyediting assistance. The authors are grateful to Apis Flora Indl. Coml. Ltda. For the facilities. This study was supported in part by the following financial grants and the intramural research program from FIOCRUZ (VMB), FAPESP Grant fellowships (2015/15948-5, 2016/10145-4, 2016/10345-3, 2016/11116-8 and 2017/22888-4) (FMO) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. AAB, CIO and VMB are senior scientist fellows at the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). JRS is supported by a scholarship from CAPES. PSSM holds a fellowship from Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB). ALM has a fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). None of the funders played any role in study design, data collection or analysis, the decision to publish, or in the preparation of the manuscript.
Author Contributions
All authors made substantial contributions to the study’s conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. These authors equally contributed to the work: Jéssica Rebouças-Silva and Maraine Catarina Tadini. These senior authors equally contributed to the work: Franciane Marquele-Oliveira and Valéria M Borges.
Disclosure
The authors declare that they do not have any commercial associations that might pose a conflict of interest. The authors report no conflicts of interest for this work.
References
- 1.World Health Organization. Leishmaniasis. Leishmaniasis; Published 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed May13, 2020.
- 2.Ramírez JL, Guevara P. Persistent infections by Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 1997;92(3):333–338. doi: 10.1590/S0074-02761997000300006 [DOI] [PubMed] [Google Scholar]
- 3.Boaventura VS, Cafe V, Costa J, et al. Short report: concomitant early mucosal and cutaneous leishmaniasis in Brazil. Am J Trop Med Hyg. 2006;75(2):267–269. doi: 10.4269/ajtmh.2006.75.267 [DOI] [PubMed] [Google Scholar]
- 4.De LEB, Porto C, Da MJOC, Sampaio RNR. Tratamento da Leishmaniose Tegumentar Americana *. An Bras Dermatol. 2007;82(2):111–124. doi: 10.1590/S0365-05962007000200002 [DOI] [Google Scholar]
- 5.Romero GAS, De Farias Guerra MV, Paes MG, De Oliveira Macêdo V. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65(5):456–465. doi: 10.4269/ajtmh.2001.65.456 [DOI] [PubMed] [Google Scholar]
- 6.Almeida RP, Brito J, Machado PL, et al. Successful treatment of refractory cutaneous leishmaniasis with GM-CSF and antimonials. Am J Trop Med Hyg. 2005;73(1):79–81. doi: 10.4269/ajtmh.2005.73.79 [DOI] [PubMed] [Google Scholar]
- 7.Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in peru. Clin Infect Dis. 2008;46(2):223–231. doi: 10.1086/524042 [DOI] [PubMed] [Google Scholar]
- 8.Ponte-Sucre A, Gamarro F, Dujardin JC, et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):1–24. doi: 10.1371/journal.pntd.0006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva SC, Guimarães LH, Silva JA, et al. Molecular epidemiology and in vitro evidence suggest that Leishmania braziliensis strain helps determine antimony response among American tegumenary leishmaniasis patients. Acta Trop. 2018;178:34–39. doi: 10.1016/j.actatropica.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taslimi Y, Zahedifard F, Rafati S. Leishmaniasis and various immunotherapeutic approaches. Parasitology. 2016;145(4):497–507. doi:doi: 10.1017/S003118201600216X [DOI] [PubMed] [Google Scholar]
- 11.Cohen B. Amphotericin B toxicity and lethality: a tale of two channels. Int J Pharm. 1998;162(12):95–106. doi:doi: 10.1016/s0378-5173(97)00417-1 [DOI] [Google Scholar]
- 12.Bekersky I, Fielding RM, Buell D, Lawrence I. Lipid-based amphotericin B formulations: from animals to man. Pharm Sci Technol Today. 1999;2(6):230–236. doi: 10.1016/S1461-5347(99)00160-1 [DOI] [PubMed] [Google Scholar]
- 13.Ministério da Saúde B. Orientações para uso racional do medicamento anfotericina B lipossomal; Published 2014. Available from: https://www.saude.gov.br/artigos/955-saude-de-a-a-z/leishmaniose-visceral/14190-orientacoes-para-uso-racional-do-medicamento-anfotericina-b-lipossomal. Accessed September29, 2020.
- 14.Saupe A, et al. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) – structural investigations on two different carrier systems. Biomed Mater Eng. 2005;15(5):393–402. [PubMed] [Google Scholar]
- 15.Pardeshi C, Rajput P, Belgamwar V, et al. Solid lipid based nanocarriers: an overview. Acta Pharm. 2012;62(4):433–472. doi: 10.2478/v10007-012-0040-z [DOI] [PubMed] [Google Scholar]
- 16.Tadini MC, de Freitas Pinheiro AM, Carrão DB, et al. Assessments of nanostructured lipid carriers loaded with amphotericin b: a proposal for increased stability and safety for infectious diseases. J Pharm Biomed Anal. 2017;145:576–585. doi: 10.1016/j.jpba.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 17.de Carvalho RF, Ribeiro IF, Miranda-Vilela AL, et al. Leishmanicidal activity of amphotericin B encapsulated in PLGA-DMSA nanoparticles to treat cutaneous leishmaniasis in C57BL/6 mice. Exp Parasitol. 2013;135(2):217–222. doi: 10.1016/j.exppara.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 18.Palma E, Pasqua A, Gagliardi A, Britti D, Fresta M, Cosco D. Antileishmanial activity of amphotericin B-loaded-PLGA nanoparticles: an overview. Materials. 2018;11(7):1167. doi: 10.3390/ma11071167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanza JS, Pomel S, Loiseau PM, Frézard F. Recent advances in amphotericin B delivery strategies for the treatment of leishmaniases. Expert Opin Drug Deliv. 2019;16(10):1063–1079. doi: 10.1080/17425247.2019.1659243 [DOI] [PubMed] [Google Scholar]
- 20.Abu Ammar A, Nasereddin A, Ereqat S, et al. Amphotericin B-loaded nanoparticles for local treatment of cutaneous leishmaniasis. Drug Deliv Transl Res. 2019;9(1):76–84. doi: 10.1007/s13346-018-00603-0 [DOI] [PubMed] [Google Scholar]
- 21.Gasco MR. Method for producing solid lipid microspheres having a narrow size distribution. Inventor: Maria R. Gasco. US5250236. Filled: Aug. 2, 1991. Date of Patent: Oct. 5, 1993. [Google Scholar]
- 22.Petersen ALDOA, Campos TA, Santos Dantas DAD, et al. Encapsulation of the HSP-90 chaperone inhibitor 17-AAG in stable liposome allow increasing the therapeutic index as assessed, in vitro, on Leishmania (L) amazonensis amastigotes-hosted in mouse CBA macrophages. Front Cell Infect Microbiol. 2018;8(AUG):1–14. doi: 10.3389/fcimb.2018.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines AE. Guideline on Bioanalytical Method Validation Guideline on Bioanalytical Method Validation Table of Contents. 44 United Kingdom, 2011;1–23. [Google Scholar]
- 24.Brasil MDS. Manual De VigilâNcia Da Leishmaniose Tegumentar. 2017. [Google Scholar]
- 25.Sharma R, Silveira-Mattos PS, Ferreira VC, et al. Generation and characterization of a dual-reporter transgenic leishmania braziliensis line expressing eGFP and luciferase. Front Cell Infect Microbiol. 2020;9(January):1–10. doi: 10.3389/fcimb.2019.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Moura TR, Novais FO, Oliveira F, et al. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by leishmania braziliensis. Infect Immun. 2005;73(9):5827–5834. doi: 10.1128/IAI.73.9.5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celes FS, Trovatti E, Khouri R, et al. DETC-based bacterial cellulose bio- curatives for topical treatment of cutaneous leishmaniasis. Sci Rep 2016;6:38330. doi: 10.1038/srep38330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titus RG, Marchand M, Boon TLJ. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x [DOI] [PubMed] [Google Scholar]
- 29.Ambisome. [homepage on the Internet]. Paraná: consulta Remédios; Published 2020. Available from: https://consultaremedios.com.br/ambisome/p. Accessed July3, 2020.
- 30.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech.2011;12(1):13–15. doi: 10.1208/s12249-010-9563-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khosa A, Reddi S, Saha RN. Biomedicine & pharmacotherapy nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103(April):598–613. doi: 10.1016/j.biopha.2018.04.055 [DOI] [PubMed] [Google Scholar]
- 32.Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomed. 2013;8:2733–2744. doi: 10.2147/IJN.S41521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–4300. doi: 10.1016/S0142-9612(03)00331-4 [DOI] [PubMed] [Google Scholar]
- 34.Antoine JC, Prima E, Jouanne C, Bongrand P. Parasitophorous vacuoles of Leishmania amzonensis infected macrophages maintain an acidic pH. Infect Immun. 1990;58((3)(3)):779–787. doi: 10.1128/IAI.58.3.779-787.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson LH, Stevens DA. Comparison of antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin B cholesteryl sulfate colloidal dispersion. Antimicrob Agents Chemother. 1992;36(2):486–488. doi: 10.1128/AAC.36.2.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson EM, Ojwang JO, Szekely A, Wallace TL, Warnock DW, Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob Agents Chemother. 1998;42(6):1412–1416. doi: 10.1128/AAC.42.6.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odds FC, Brown AJP, Gow NAR. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11(6):272–279. doi: 10.1016/S0966-842X(03)00117-3 [DOI] [PubMed] [Google Scholar]
- 38.Matsumori N, Tahara K, Yamamoto H, et al. Direct interaction between amphotericin B and ergosterol in lipid bilayers as revealed by 2H NMR spectroscopy. J Am Chem Soc. 2009;131(33):11855–11860. doi: 10.1021/ja9033473 [DOI] [PubMed] [Google Scholar]
- 39.Bruni N, Stella B, Giraudo L, Della Pepa C, Gastaldi D, Dosio F. Nanostructured delivery systems with improved leishmanicidal activity: A critical review. Int J Nanomed. 2017;12:5289–5311. doi: 10.2147/IJN.S140363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gale EF. The release of potassium ions from Candida albicans in the presence of polyene antibiotics. J Gen Microbiol. 1974;80(2):451–465. doi: 10.1099/00221287-80-2-451 [DOI] [PubMed] [Google Scholar]
- 41.Sen YT, Ou KL, Peng PW, et al. Quantifying membrane permeability of amphotericin B ion channels in single living cells. Biochim Biophys Acta - Biomembr. 2013;1828(8):1794–1801. doi: 10.1016/j.bbamem.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 42.Sokol-Anderson ML, Brajtburg J, Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis. 1986;154(1):76–83. doi: 10.1093/infdis/154.1.76 [DOI] [PubMed] [Google Scholar]
- 43.Mesa-arango AC, Scorzoni L, Zaragoza O. It only takes one to do many jobs: amphotericin B as antifungal and immunomodulatory drug. Front Immunol. 2012;3:1–10. doi: 10.3389/fmicb.2012.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesa-Arango AC, Trevijano-Contador N, Román E, et al. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother. 2014;58(11):6627–6638. doi: 10.1128/AAC.03570-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gigliotti F, Shenep JL, Lott LTD. Induction of prostaglandin synthesis as the mechanism responsible for the chills and fever produced by infusing amphotericin B. Send to J Infect Dis. 1987;156(5):784–789. doi: 10.1093/infdis/156.5.784 [DOI] [PubMed] [Google Scholar]
- 46.John KS, Chia Edward J, Manus M. In vitro tumor necrosis factor induction assay for analysis of febrile toxicity associated with amphotericin b preparations. Antimicrob Agents Chemother. 1990;34(5):906–908. doi: 10.1128/AAC.34.5.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arning M, Kliche K, Wehmeier A. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses. 1995;465:459–465. doi: 10.1111/j.1439-0507.1995.tb00020.x [DOI] [PubMed] [Google Scholar]
- 48.Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. The antifungal drug amphotericin b promotes inflammatory cytokine release by a toll-like receptor- and CD14-dependent mechanism *. J Biol Chem. 2003;278(39):37561–37568. doi: 10.1074/jbc.M306137200 [DOI] [PubMed] [Google Scholar]
- 49.Majumder N, Ganguly S, Ghosh AK, Kundu S, Banerjee A, Saha S. Chlorogenic acid acts upon Leishmania donovani arresting cell cycle and modulating cytokines and nitric oxide in vitro. Parasite Immunol. 2020;42(6):1–12. doi: 10.1111/pim.12719 [DOI] [PubMed] [Google Scholar]
- 50.Ries F, Alflen A, Lopez PA, et al. Antifungal drugs influence neutrophil effector functions. Antimicrob Agents Chemother. 2019;63(6):1–17. doi: 10.1128/AAC.02409-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawabe K, Takano K, Moriyama M, Nakamura Y. Amphotericin B increases transglutaminase 2 expression associated with upregulation of endocytotic activity in mouse microglial cell line BV-2. Neurochem Res. 2017;42(5):1488–1495. doi: 10.1007/s11064-017-2205-0 [DOI] [PubMed] [Google Scholar]
- 52.Rogers PD, Kramer RE, Chapman SW, Cleary JD. Amphotericin B – induced interleukin-1 b expression in human monocytic cells is calcium and calmodulin dependent. J Infect Dis. 1999;180(May):1259–1266. doi: 10.1086/315004 [DOI] [PubMed] [Google Scholar]
- 53.Khouri R, Novais F, Santana G, et al. DETC induces Leishmania parasite killing in human invitro and murine in vivo models: A promising therapeutic alternative in leishmaniasis. PLoS One. 2010;5(12):8–9. doi: 10.1371/journal.pone.0014394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos DM, Petersen ALOA, Celes FS, Borges VM, Veras PST, de Oliveira CI. Chemotherapeutic potential of 17-AAG against cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. PLoS Negl Trop Dis. 2014;8(10):e3275. doi: 10.1371/journal.pntd.0003275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falcão SC, De MTR, Clarêncio J, Brodskyn C, Barral A, De OCI. The presence of Tregs does not preclude immunity to reinfection with Leishmania braziliensis. Int J Parasitol 2012;42:771–780. doi: 10.1016/j.ijpara.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 56.Belkaid Y, Piccirillo CA, Mendez S. CD4 1 CD25 1 regulatory T cells control Leishmania major persistence and immunity. Nature 2002;420(6915):520–527. doi: 10.1038/nature01199.1. [DOI] [PubMed] [Google Scholar]