Abstract
Introduction:
Cardiovascular complications are the leading cause of death after noncardiac surgery. Major abdominal operations represent the largest category of procedures considered to have an increased risk of cardiovascular complications. The current aim was to examine trends in the incidence of mortality, perioperative myocardial infarction, and cardiac arrest to determine the presence of potential volume-outcome relationships.
Methods:
We performed a retrospective analysis of the Nationwide Inpatient Sample for patients undergoing elective, open abdominal esophagectomy, gastrectomy, pancreatectomy, nephrectomy, hepatectomy, splenectomy, and colectomy (major abdominal surgery) during 2008–2014. Univariate and multivariate analyses were performed to determine the impact of operative volume on rates of myocardial infarction, cardiac arrest, and mortality.
Results:
Of the 962,754 elective admissions for major abdominal surgery, 1.4% experienced in-hospital mortality, 0.7% myocardial infarction, and 0.35% cardiac arrest. Myocardial infarction and cardiac arrest were associated with a 24-fold increase in risk of perioperative mortality. Compared with institutions that have a very low volume of operations, those hospitals with larger volumes of operations had a decreased risk of cardiac arrest and incident mortality after cardiovascular complications, but the odds of myocardial infarction were greatest at higher operative-volume hospitals. The annual all-cause mortality and myocardial infarction rates decreased over time, but the incidence of cardiac arrest increased.
Conclusion:
Myocardial infarction or cardiac arrest after major abdominal surgery increased the odds of mortality with superior rescue after cardiovascular complications at higher volume institutions. Across all US hospitals performing major abdominal surgery, the rate of cardiac arrest increased without a concomitant increase in myocardial infarction or mortality. Novel targets for risk modification of myocardial infarction and cardiac arrest as well as investigation of processes that facilitate rescue after these complications at higher operative-volume hospitals are needed to delineate quality improvement opportunities.
Introduction
Substantial improvements in perioperative care throughout the past two decades have resulted in decreased mortality and enhanced safety after major operations. In particular, improved techniques of hemodynamic monitoring and the ability to successfully resuscitate patients with cardiovascular complications have yielded superior outcomes despite increasing operative complexity and patient comorbidities. Although 1.5% of patients undergoing noncardiac surgery do not survive beyond 30 days, major adverse cardiac events remain the leading cause of mortality.1 Accurate prediction of the risk of perioperative myocardial infarction (MI) and cardiac arrest (CAR) is critical to the selection of potential interventions and the informed consent process. The real world impact of stratification schemes, such as the myocardial infarction cardiac arrest score, validated using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP), remains a subject of debate.2,3
Surgery elicits sympathetic activation, inflammation, loss of vascular tone, and hemodynamic instability because of hemorrhage and fluid shifts.4 Beginning in 1999, a series of publications including the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trial provided evidence for the protective impact of beta blockers against perioperative major adverse cardiovascular events and led to the widespread use of these agents.5 More recently, however, their utility has been questioned, and the use of beta blockers was associated with increased mortality and stroke in the Perioperative Ischemic Evaluation Study (POISE) trial.6 Given that a postoperative adverse cardiac event is a significant predictor of perioperative mortality, a continued focus on understanding the predictors of a perioperative myocardial event is crucial.
Apart from preoperative interventions, advances in operative techniques, such as the rapid adoption of laparoscopic surgery and enhanced recovery protocols, have further resulted in overall improvements in operative mortality and other complications.7–10 Additionally, as suggested by Dimick et al.11 more than a decade ago, hospitals with high operative volumes may lead to decreased postoperative complications after adjusting for case mix. Nevertheless, the impact of such improvements and hospital volume on adverse cardiac ischemic events after major abdominal operations remains ill-defined. The present study examined recent national trends in the development of MI and CAR after major abdominal operations. We hypothesized a concomitant decrease in mortality and rates of perioperative MI and CAR during the study period. We further hypothesized that hospitals with greater operative volumes would result in a lesser risk of such events.
Methods
We used the National Inpatient Sample (NIS) from January 2008 to December 2014 to identify all adult patients (≥18 years of age) undergoing major open abdominal surgery (MAS), including abdominal esophagectomy, gastrectomy, pancreatectomy, splenectomy, nephrectomy, hepatectomy, and colectomy (Table 5). The NIS is maintained by the Agency for Healthcare Research and Quality.12 NIS data are generated from hospital discharge abstracts via extracting diagnosis and procedure codes, as well as data on hospital bed size, metropolitan versus rural location, teaching status, and region. Starting in 2012, NIS methodology changed from 100% of discharges to sampling 20% of discharges from participating institutions. Sampling probabilities for each stratum were used to obtain survey estimates representative of nearly 97% of the US population.
Table 5.
Case mix and outcomes from 2008–2014 standardized for age.
| Case volume | Proportion MAS (%) | Mortality (%) | MI (%) | CAR (%) | MI or CAR (%) | |
|---|---|---|---|---|---|---|
| Esophagectomy | 17,425 | 1.81 | 3.76 | 1.07 | 1.07 | 2.11 |
| Gastrectomy | 138,427 | 14.38 | 1.39 | 0.52 | 0.30 | 0.78 |
| Pancreatectomy | 65,073 | 6.76 | 2.81 | 0.85 | 0.72 | 1.51 |
| Splenectomy | 39,699 | 4.12 | 2.39 | 0.81 | 0.6 | 1.39 |
| Nephrectomy | 397,578 | 41.3 | 0.49 | 0.54 | 0.2 | 0.71 |
| Hepatectomy | 74,547 | 7.74 | 1.86 | 0.64 | 0.39 | 0.97 |
| Colectomy | 230,006 | 23.89 | 1.87 | 1.00 | 0.41 | 1.34 |
| MAS | 962,754 | 100 | 1.35 | 0.70 | 0.35 | 1.00 |
MI, postoperative myocardial infarction; CAR, cardiac arrest; MAS, major abdominal surgery.
The operations included in the definition of MAS were categorized as high risk, based on the 2014 guidelines of the American College of Cardiology/American Heart Association.13,14 Patient identification and assessment of comorbidity was performed using all International Classification of Disease (ICD9) administrative diagnosis and procedure fields available (Table 6). Operations requiring resection of more than one organ were excluded from the primary analysis. Preliminary analyses included urgent admissions to assess the proportion of cases that occur on an urgent or emergent basis. In an attempt to decrease heterogeneity and minimize the impact of preoperative MI on the data, we only focused the analyses on patients with elective admission status. The Elixhauser index, a previously validated composite score of 30 common comorbidities, was utilized as an additional measure of patient comorbidity15,16.
Table 6.
International Classification of Disease procedural codes.
| Esophagectomy | 42.40, 42.41, 42.42 |
| Gastrectomy | 43.5, 43.6, 43.7, 43.8, 43.81, 43.89, 43.91, 43.99 |
| Pancreatectomy | 52.51, 52.52, 52.53, 52.59, 52.6, 52.7 |
| Nephrectomy | 55.4, 55.5, 55.51, 55.54 |
| Splenectomy | 41.42, 41.43, 41.5 |
| Hepatectomy | 50.9, 50.22, 50.3 |
| Colectomy | 45.7, 45.71, 45.72, 45.73, 45.74, 45.75, 45.76, 45.79, 45.8, 45.81, 45.83 |
The primary outcomes of interest were in-hospital mortality, perioperative MI, and CAR. Patient and hospital characteristics were compared between several cohorts, using univariate and multivariate models adjusting for patient characteristics. Hospital-level metrics available in the NIS, such as teaching status, bed size, and region, were incorporated.
Operative volume was calculated for each hospital by the overall caseload for MAS annually. We divided the hospitals into quintiles and designated them as very-low volume (1–39 cases/year), low-volume (40–90 cases/year), moderate-volume (91–193 cases/year), high-volume (194–467 cases/year), and very-high volume institutions (472–3,375 cases/year). Chi-square analysis of survey-weighted data and adjusted Wald two-tailed t test were utilized to compare patient and hospital characteristics among the very low- and very high-volume institutions.
Multivariate regression analyses were performed to identify factors that significantly impact mortality, MI, and CAR. The risk-adjusted model included patient demographics, comorbidities, history of MI, and several hospital characteristics gathered by the NIS. Model design was optimized for discriminatory power greater than 75% but reached up to 85% for adjusted analyses of failure to rescue. Having the greatest operative volume of all elective admissions during the study period, nephrectomy was selected as the reference operation for analyses. Additionally, separate multivariate regression models were performed with the composite variable, MI or CAR, as a covariate to assess the interaction between this adverse event and hospital operative volume on postoperative mortality, costs, and duration of stay. Rates of mortality, MI, and CAR were analyzed over time, using survey-weighted, age-standardized estimates, and the Cochran-Armitage tests for trends.17,18
Results
Study population
From the years 2008 to 2014, a total of 1,404,990 adult patients underwent MAS at 6,440 unique hospitals. Urgent admissions comprised 30% of the MAS operations and were associated with increased risk of all-cause postoperative mortality (OR 4.3, 95% CI 4.09–4.61, P < .001), MI (OR 2.32, 95% CI 2.14–2.52, P < .0001), and CAR (2.9, 95% CI 2.6–3.3, P < .0001) compared with elective admissions. After excluding urgent or emergent admissions, 962,754 MAS operations remained, with an overall mortality rate of 1.4%, ranging from 0.5% for nephrectomy to 3.8% for open splenectomy (Table 5). MI and CAR were low-frequency events, occurring in 0.7%–2.1% of subjects (Table 5). Patients experiencing mortality, MI, and CAR were more often male, older, with a higher Elixhauser Comorbidity Index, and a greater prevalence of heart failure, peripheral vascular disease, and renal failure (Table 1). History of hyper-tension, diabetes, and prior MI were not associated with increased rates of mortality or MI or CAR on unadjusted analyses. The presence of MI, CAR was also associated with increased duration of duration and costs on univariate comparison (Table 1).
Table 1.
Comorbidities and hospitalization characteristics by outcome.
| Mortality | Myocardial infarction | Cardiac arrest | ||||
|---|---|---|---|---|---|---|
| Survived N = 949,293 | Died N = 12,972 | No MI N = 956,055 | MI N = 6,699 | No CAR N = 959,386 | CAR N = 3,368 | |
| Age* (y) | 59.8 | 69.6 | 59.8 | 71.4 | 59.9 | 67.7 |
| Female* (%) | 50.4 | 39.0 | 50.4 | 35.1 | 50.3 | 35.8 |
| Elixhauser† | 2.7 | 3.9 | 2.7 | 4.1 | 2.7 | 3.9 |
| HF* (%) | 4.3 | 13.9 | 4.2 | 32.1 | 4.4 | 14.9 |
| CAD* (%) | 11.9 | 16.2 | 11.7 | 42.9 | 11.9 | 21.5 |
| Prior MI* (%) | 3.2 | 3.1 | 3.2 | 6.9 | 3.2† | 2.6† |
| HTN* (%) | 45.2 | 26.1 | 45.0 | 36.6 | 45.0 | 33.4 |
| COPD* (%) | 6.4 | 10.0 | 6.4 | 11.7 | 6.4 | 9.3 |
| PVD* (%) | 3.1 | 10.9 | 3.2 | 10.1 | 3.2 | 9.1 |
| Diabetes* (%) | 20.3 | 14.8 | 20.2 | 27.0 | 20.2‡ | 18.0‡ |
| Renal failure* (%) | 7.8 | 12.7 | 7.8 | 18.1 | 7.9 | 14.1 |
| Adjusted costs* (%) | 21,835.28 | 78,949.42 | 22,362.72 | 58,279.44 | 22,453.96 | 67,363.23 |
| LOS* (days) | 6.4 | 17.2 | 6.5 | 15.6 | 6.5 | 16.1 |
Elixhauser, Elixhauser Comorbidity Index; MI, myocardial infarction; CAR, cardiac arrest; HF, heart failure; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease.
All other univariate P values are less < .001.
P = .4
P = .16
MI or CAR by sex, comorbidities, operation type, operative volume
After adjusting for patient and hospital characteristics, personal history of heart failure and peripheral vascular disease increased the odds of MI or CAR while female sex decreased the risk of MI or CAR after elective major abdominal operations (Table 2). Diabetes was associated with decreased odds of cardiac arrest without a significant correlation with risk of MI. History of prior MI was also associated with decreased odds of MI, CAR, and all-cause mortality (Table 2). Nephrectomy served as the reference operation and carried the least risk of MI and CAR. Esophagectomy, pancreatectomy, and splenectomy consistently had the greatest odds of MI and CAR, as presented in Table 2. Patients treated at very high-volume hospitals experienced a greater likelihood of MI but not CAR (Fig. 1). In contrast, the odds of all-cause mortality were inversely related to institutional operative volume with the greatest risk reduction for very high-volume hospitals compared with very low-volume hospitals (Fig. 1).
Table 2.
Predictors of MI, CAR, and MI or CAR.
| MI | CAR | MI or CAR | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (y) | 1.05 | 1.04–1.05 | 1.04 | 1.03–1.04 | 1.04 | 1.04–1.05 |
| Female | 0.67 | 0.59–0.75 | 0.60 | 0.51–0.71 | 0.65 | 0.59–0.72 |
| HF | 4.93 | 4.27–5.69 | 2.32 | 1.82–2.96 | 4.19 | 3.70–4.74 |
| COPD | 0.88 | 0.74–1.05 | 0.97 | 0.74–1.27 | 0.51 | 0.46–0.57 |
| PVD | 1.51 | 1.24–1.84 | 1.92 | 1.45–2.54 | 0.90 | 0.77–1.05 |
| Renal failure | 0.96 | 0.81–1.15 | 1.18 | 0.91–1.51 | 1.66 | 1.41–1.96 |
| Diabetes | 1.02 | 0.90–1.16 | 0.74 | 0.60–0.91 | 1.03 | 0.89–1.19 |
| Prior MI | 0.55 | 0.44–0.70 | 0.41 | 0.25–0.68 | 0.53 | 0.43–0.66 |
| Operation type | ||||||
| Esophagectomy | 1.75 | 1.28–2.41 | 4.44 | 3.04–6.49 | 2.63 | 2.04–3.39 |
| Gastrectomy | 1.28 | 1.06–1.54 | 2.00 | 1.54–2.61 | 1.48 | 1.26–1.74 |
| Pancreatectomy | 1.55 | 1.25–1.91 | 3.51 | 2.69–4.60 | 2.13 | 1.79–2.52 |
| Splenectomy | 1.64 | 1.26–2.14 | 3.13 | 2.21–4.44 | 2.16 | 1.73–2.70 |
| Colectomy | 1.48 | 1.17–1.87 | 2.26 | 1.63–3.12 | 1.69 | 1.38–2.07 |
| Hepatectomy | 1.30 | 1.13–1.49 | 1.66 | 1.34–2.06 | 1.40 | 1.24–1.58 |
| Nephrectomy | Reference | |||||
| Model C-statistic | 0.84 | 0.75 | 0.80 | |||
MI, myocardial infarction; CAR, cardiac arrest.
Fig. 1.
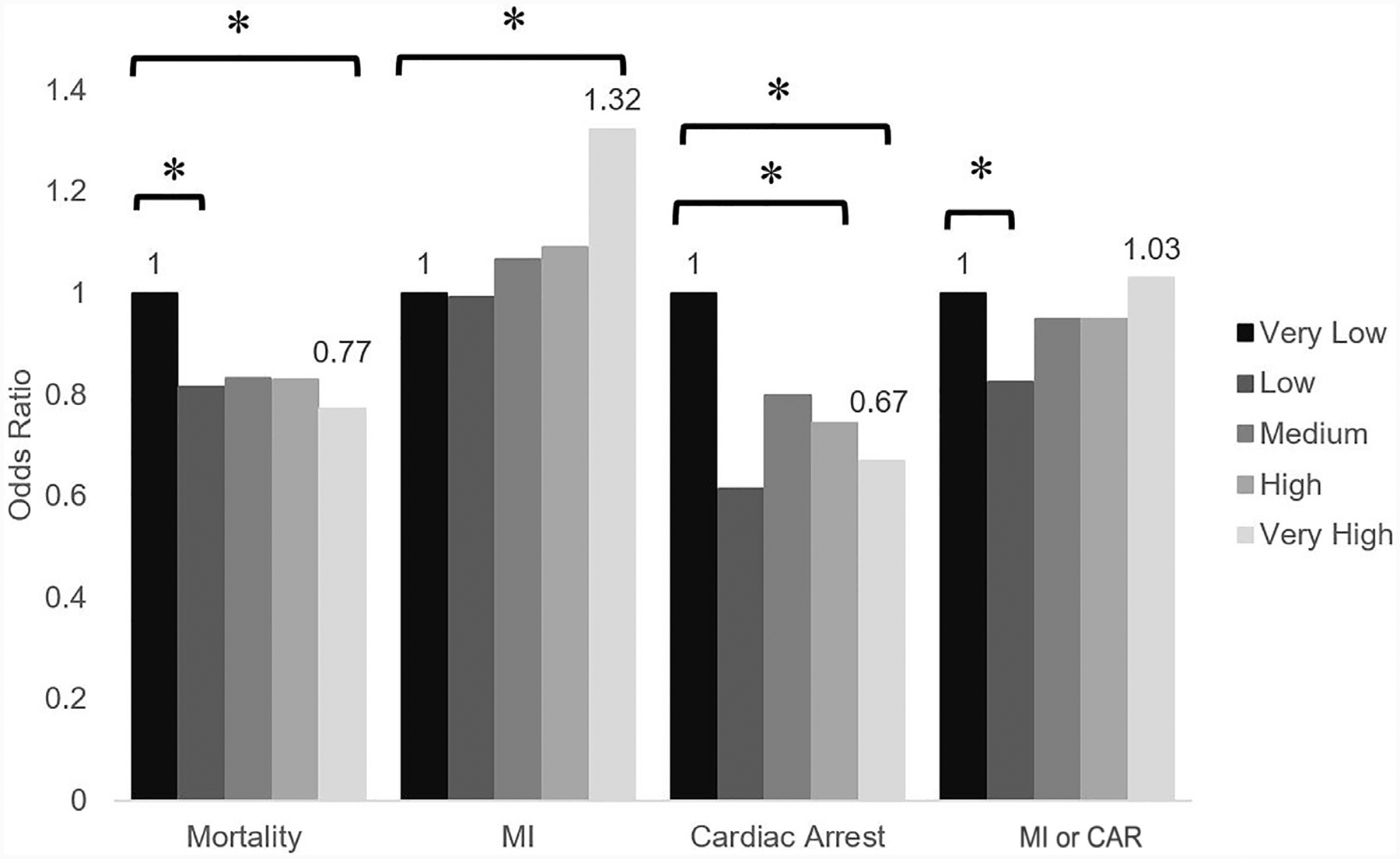
Impact of hospital operative volume on odds of all-cause mortality, MI, CAR, and MI or CAR. Reference condition is very low-volume hospitals, *P<.02.
Impact of MI or CAR and operative volume on mortality, duration of stay, and costs
Overall, MI or CAR (OR 23.9, 95% CI 18.2–31.3) contributed to significant mortality among patients undergoing MAS. The significantly greater odds of mortality associated with MI or CAR was because of the CAR component of this score (OR 56.8, 95% CI 37.3–86.5) rather than MI (OR 10.2, 95% CI 7.1–14.4). Compared with very low-volume institutions, mortality after MI (23.2% versus 13.3%, P = .007), and composite MI or CAR (34.1% versus 22.1%, P = .0007) were less at very high-volume hospitals without a significant difference for CAR (55.5% versus 44%, P = .059). Using a multivariate risk-adjusted model (Table 3), odds of mortality after MI or CA were highest at very low-volume hospitals (Fig. 2). As hospital operative volume increased, the odds of mortality after MI and CAR significantly decreased (Fig. 2). Similarly, composite analysis of MI or CAR correlated inversely with hospital operative volume (Fig 2).
Table 3.
Comparison odds of failure to rescue after MI, CA, or MI or CAR adjusted for patient and hospital Characteristics.
| Mortality after MI | Mortality after CAR | Mortality after MI or CA | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (y) | 1.05 | 1.04–1.05 | 1.05 | 1.05–1.06 | 1.05 | 1.05–1.05 |
| Female | 0.67 | 0.59–0.75 | 0.69 | 0.62–0.73 | 0.67 | 0.63–0.75 |
| HF | 4.93 | 4.27–5.69 | 1.47 | 1.77–2.36 | 2.05 | 1.26–1.72 |
| CAD | 0.84 | 0.74–0.96 | 0.76 | 0.76–0.99 | 0.87 | 0.67–0.88 |
| Prior MI | 0.77 | 0.60–1.00 | 0.85 | 0.63–1.05 | 0.81 | 0.64–1.11 |
| Hypertension | 0.36 | 0.32–0.39 | 0.34 | 0.34–0.41 | 0.37 | 0.31–0.38 |
| COPD | 0.88 | 0.74–1.05 | 0.97 | 0.74–1.27 | 0.51 | 0.46–0.57 |
| PVD | 1.51 | 1.24–1.84 | 1.92 | 1.45–2.54 | 0.90 | 0.77–1.05 |
| Diabetes | 0.63 | 0.56–0.71 | 0.64 | 0.56–0.72 | 0.64 | 0.57–0.72 |
| Renal failure | 1.02 | 0.90–1.16 | 0.74 | 0.60–0.91 | 1.03 | 0.89–1.19 |
| Hospital bed size | ||||||
| Small | Reference | |||||
| Medium | 1.23 | 1.04–1.46 | 1.21 | 0.99–1.40 | 1.18 | 1.02–1.45 |
| Large | 1.29 | 1.10–1.51 | 1.28 | 1.07–1.48 | 1.25 | 1.09–1.50 |
| Teaching status | ||||||
| Rural | Reference | |||||
| Urban nonteaching | 1.15 | 0.97–1.38 | 1.16 | 0.97–1.38 | 1.16 | 0.97–1.39 |
| Urban teaching | 1.23 | 1.03–1.47 | 1.22 | 0.99–1.40 | 1.18 | 1.02–1.47 |
| Hospital region | ||||||
| Northeast | Reference | |||||
| Midwest | 1.14 | 0.96–1.35 | 1.12 | 0.94–1.33 | 1.12 | 0.94–1.33 |
| South | 1.45 | 1.25–1.68 | 1.45 | 1.23–1.65 | 1.42 | 1.25–1.68 |
| West | 1.13 | 0.96–1.33 | 1.13 | 0.92–1.28 | 1.09 | 0.96–1.33 |
| Operation type | ||||||
| Esophagectomy | 6.91 | 5.60–8.53 | 6.47 | 5.09–8.10 | 6.42 | 5.18–8.07 |
| Gastrectomy | 3.96 | 3.42–4.58 | 3.88 | 3.38–4.57 | 3.93 | 3.35–4.51 |
| Pancreatectomy | 5.81 | 4.99–6.76 | 5.63 | 4.81–6.58 | 5.63 | 4.82–6.58 |
| Splenectomy | 5.01 | 4.12–6.10 | 4.79 | 4.00–6.01 | 4.90 | 3.91–5.88 |
| Colectomy | 4.62 | 3.91–5.46 | 4.59 | 3.91–5.54 | 4.65 | 3.87–5.45 |
| Hepatectomy | 2.89 | 2.53–3.30 | 2.88 | 2.55–3.34 | 2.92 | 2.52–3.29 |
| Nephrectomy | Reference | |||||
| Model C-statistic | 0.81 | 0.84 | 0.84 | |||
Fig. 2.
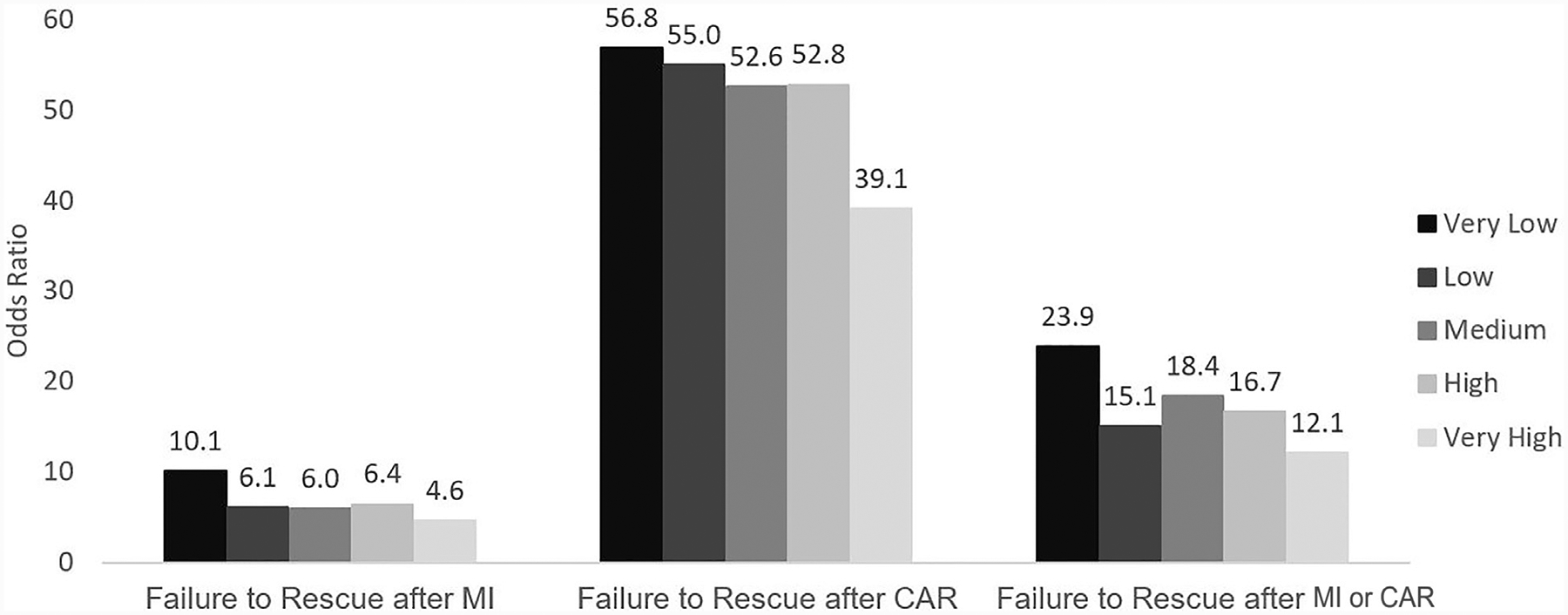
Impact of hospital operative volume on failure to rescue after MI, CAR, and or CAR. Reference condition is very low-volume hospitals, all comparisons P<.0001.
MI or CAR events contributed significantly to resource utilization. Occurrence of MI increased adjusted hospital costs by $20,193 (95% CI $12,428–$27,958). Compared with very low-volume institutions, very high-volume institutions had an additional $14,441 in inpatient expenditures after MI. Similarly, CAR was associated with a significantly greater incremental increase in hospitalization costs ($46,354, 95% CI $27,903–$64,805), with even greater incremental costs associated with this event at very high operative-volume hospitals ($41,325, 95% CI $5,976–$76,674). Although both MI (4.6 days, 95% CI 3.2–6.0 days) and CAR (13.8 days, 95% 6.2–21.4 days) resulted in greater durations of hospitalization, only MI demonstrated a weak correlation with operative volume and incremental increase in duration of stay (Table 4).
Table 4.
Incremental duration of stay after MI and CAR adjusted for patient comorbidities and hospital operative volume characteristics*.
| Adjusted incremental increase in duration of stay after MI or CR | ||||
|---|---|---|---|---|
| MI (days) | 95% CI | CA (days) | 95% CI | |
| Very low | 4.62 | 3.2–6.0 | 13.80 | −6.0–12.9 |
| Low | 4.13 | 1.1–7.2 | 3.44 | −6.1–12.1 |
| Moderate | 2.23 | 0.1–4.3 | 2.95 | −7.6–10.3 |
| High | 3.06 | 0.8–5.3 | 1.36 | −3.6–15.5 |
| Very high | 4.28 | 1.4–7.1 | 5.97 | −6.0–12.9 |
Model included age, sex, and patient comorbidities, including heart failure, coronary artery disease, prior MI, chronic pulmonary disease, peripheral vascular disease, diabetes, renal failure, hospital bed size, hospital teaching status, hospital region, and operation type only for patients who survived to discharge.
Trends in MI or CAR for elective admissions
Annual rates of mortality, MI, and CAR were analyzed in all elective admissions for MAS patients during the study period. A decrease in mortality and MI was accompanied by an overall increase in the occurrence of CAR, as shown in Fig. 3. Rescue after MI worsened during the study period, while mortality after CAR remained stable (Fig. 4). Subgroup analysis of trends by operative volume quintiles demonstrated a decrease in mortality in moderate (0.8%–0.6%, P = .001) and high volume (0.8%–0.4%, P < .0001) tiers, but an increase in mortality at very low-volume institutions (0.6%–0.9%, P < .0001). Throughout the study period, the incidence of MI decreased minimally in low-volume (0.3%–0.2%, P = .0003) and high-volume institutions (0.4%–0.2%, P = .03) but remained unchanged at others. The incidence of CAR increased at very low-volume hospitals (0.1%–0.3%, P < .0001) and moderate-volume hospitals (0.09%–0.2%, P < .0001), while this event decreased modestly in frequency at high operative-volume institutions (0.3%–0.2%, P < .0001).
Fig. 3.
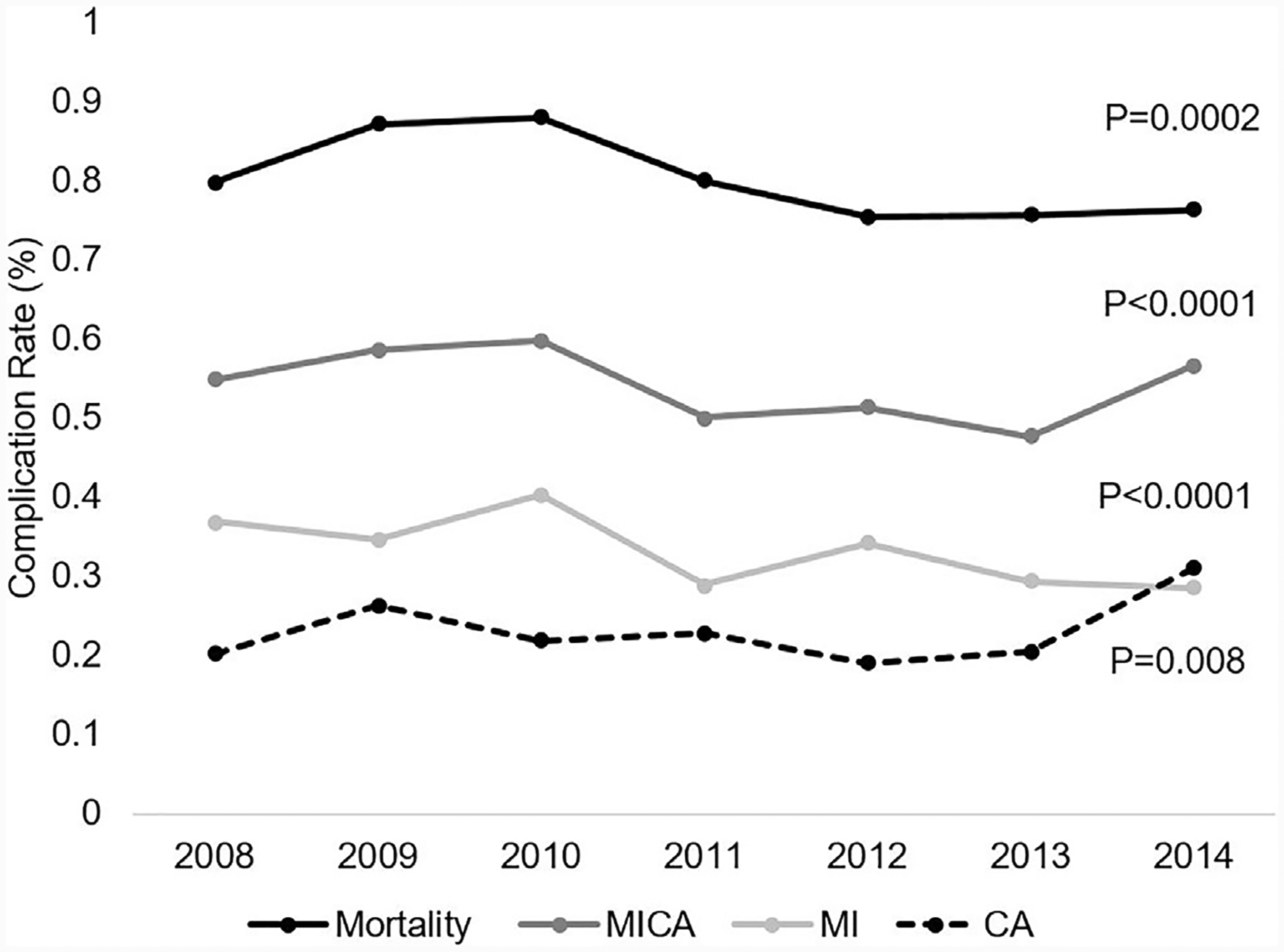
Rates of MI or CAR for Elective Major Abdominal Operations over 2008–2014 MI, myocardial infarction; CAR, cardiac arrest; MI or CAR, MI or cardiac arrest.
Fig. 4.
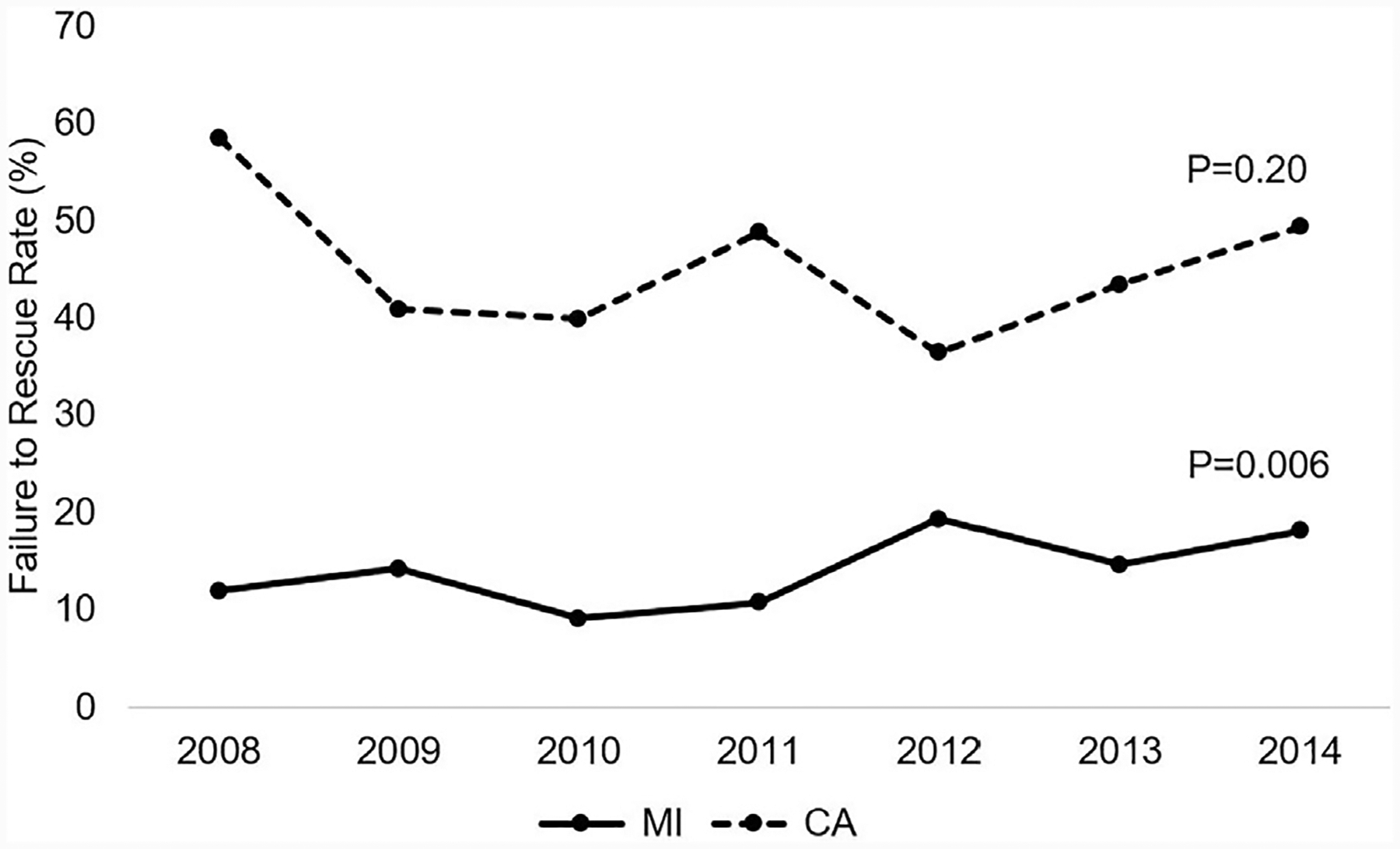
Failure to rescue after MI or CAR for elective major abdominal operations throughout 2008–2014. MI, myocardial infarction; CAR, cardiac arrest; MI or CAR, = MI or cardiac arrest.
Discussion
Despite improvements in perioperative management of major abdominal operations, MI or CAR remains a dreaded complication. The risk of MI or CAR with increasing complexity of operations performed and patient age has been mitigated by increased utilization of minimally invasive techniques and improved medical management.19 As one of the largest contemporary analyses of MI or CAR in high-risk abdominal operations, this study demonstrated that high operative volume was associated with an increased risk of MI and a decreased risk of CAR. Examining MI or CAR trends over time, the rate of MI decreased and the incidence of all cause CAR significantly increased at very low- and moderate-volume institutions. After the occurrence of MI or CAR, treatment at the greatest operative-volume institutions was associated with significantly decreased mortality.
Factors that determine rescue performance within different hospital systems may be attributable to inconsistent resources for recognition and intervention after a complication occurs. Nearly 20 years ago, groups examining outcomes after admission for MI found a dose-response effect of greater volume of admissions and survival.20 Ghaferi et al21 in 2011 concluded that at least for high-risk cancer operations, differences in mortality between high- and low-volume hospitals were not related to the incidence of complications but rather to the ability “to rescue” once these events occurred, which is concordant with the findings of the current study.22 Examining the volume-outcome relationship for procedures necessary for management of MI or CAR, Fanaroff et al23 demonstrated that an inverse relationship exists between the volume of percutaneous coronary interventions and in-hospital mortality. Although optimization of preoperative cardiac risk factors may have decreased rates of MI at low- and moderate-volume hospitals, capabilities to decrease rates of CAR, such as decreased use of opioids and respiratory monitoring at these centers warrants further investigation.
Possibly related to the ability to rescue after a complication, hospitals with greater operative volume had greater incremental costs after an MI or a CAR. As efforts for centralization of abdominal operations to high operative-volume institutions continues, policymakers must remain cognizant of the potential for increased disparities in the delivery of care.24 The current study provides further confirmation that for major abdominal operations, institutions with a greater operative volume have superior performance in rescuing patients who develop complications. This is associated with increased resource utilization and warrants the development of innovative systemwide interventions that address these patient outcome discrepancies and provide cost-effective solutions in resource-limited settings.
Beyond the volume-outcome debate, further understanding of the etiology of increasingly frequent CAR will allow targeted interventions to address this trend. As a limitation of this analysis, we are unable to provide a more granular explanation of the various etiologies that potentially contribute to CAR in this patient population. Although we expect that patient and hospital-system factors that potentially impact outcomes after an MI and CAR are interrelated, we acknowledge that the increasing trend in CAR may be unrelated to a primary cardiac ischemic etiology. Causes of CAR with greatest clinical visibility include fatal arrhythmia with or without ischemia, unrecognized hemorrhage, and acute respiratory failure.25 Although our analysis cannot further delineate the etiology of greater incidence of CAR throughout the study period, a contemporaneous evaluation of the role of opioid-related toxicity in perioperative CAR may also be warranted. [26]
Of note, patients in the present study with diabetes and prior MI were not at a greater adjusted risk for developing MI. Furthermore, administrative history of prior MI, conferred decreased risk of MI or CAR. This is in contrast with many reports that have suggested a previous MI to be a major risk factor for developing a perioperative ischemic cardiac event.2 Both the Revised Cardiac Risk and the Vascular Study Group of New England indices assign predictive weight to a history MI.2, 27–29 Given that hospital encounters are not linked over time and accuracy of billing data is occasionally biased by reimbursement-driven coding, the presence of these conditions in administrative databases may represent an artifact. Indeed, the NSQIP Gupta calculator does not utilize previous MI as one of its variables in agreement with our study. Among components of the Revised Cardiac Risk Index, only congestive heart failure was independently predictive of MI or CAR in our study.28
Additionally, little consensus exists regarding the elements of successful perioperative resuscitation and management. In a recent randomized trial of high-risk patients undergoing major gastrointestinal surgery, monitoring of cardiac output did not decrease the occurrence of complications or 30-day mortality30. Addition of the trial patients to a large group meta-analysis, however, revealed that cardiac-output–guided hemodynamic therapy for intravenous fluids and inotropes resulted in decreased complications.30 In regard to intraoperative fluid management, the debate regarding crystalloid and colloid resuscitation has been renewed with a recent, double-blinded, randomized controlled trial with evidence that postoperative morbidity and complications are less frequent with a closed-loop, goal-directed approach to use of colloids31. Although several additional targets for the prevention of MI, such as modulation of the sympathetic surge with beta blockers have been proposed, robust evidence for consistent and effective interventions are lacking at the present time.4,32 With continually changing guidelines on management of chronic conditions, such as atrial fibrillation and anti-platelet therapy for stable ischemic heart disease, the incidence of perioperative MI or CAR is likely to remain in flux.33 Finally, the definition of MI continues to evolve, and many investigators have advocated lower thresholds of troponin and early intervention upon detection of postoperative MIs.25
To our knowledge, this study is the first to report the influence of hospital volume on cost and duration of stay after MI or CAR for major abdominal operations. Of interest, cardiac complications were more costly, with a greater incremental increase in duration of stay at hospitals with a greater operative volume. Factors, such as monitoring capabilities and availability of interventions at high-volume hospitals, may be partially responsible for the increased costs of this complication. Moreover, such institutions are also more likely to be training facilities and possess the infrastructure for better detection of MIs. Given the overt clinical manifestations of CAR, this complication was not affected by hospital volume. Whether, the observed differences of rates of MI within different volume institutions represents a true increase in risk requires further investigation.
We acknowledge several noteworthy limitations of this study. Use of an administrative database limits clinical granularity of comparison among the patient cohort. Furthermore, the definition of MI is adapted from previous studies, using available ICD9 coding and does not reflect the changes in sensitivity and threshold of troponin I enzyme for clinical detection of MI.34 The temporal relationship between the occurrence of MI or CAR and the index operation is unknown. To decrease the impact of preoperative events, we only included patients with elective admissions for analysis. Absence of data on preoperative revascularization and control of heart rate and blood pressure diminished our ability to assess and adjust for preoperative fitness for major abdominal surgery. The deficiency of data on the extent of preoperative optimization is further confounded by the exclusion of patients who were admitted under emergent conditions and would not have been able to undergo thorough cardiac evaluation. Presumably, patients admitted for elective admissions may have already been stratified, skewing adverse event estimates.
As expected, MI or CAR increased the odds of in-hospital mortality significantly. The inverse relationship between operative volume and odds of mortality after MI or CAR supports the theory that failure to rescue patients after a complication may be the pernicious culprit that leads to discrepant outcomes. With the dawn of value-based health care delivery, a shift from aggressive preoperative revascularization to improved perioperative hemodynamic treatment strategies and appropriate management of complications is warranted.
Footnotes
Presented at the 13th Annual Academic Surgical Congress.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.surg.2018.04.030.
References
- 1.Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373:2258–2269. [DOI] [PubMed] [Google Scholar]
- 2.Gupta PK, Ramanan B, MacTaggart JN, Sundaram A, Fang X, Gupta H, et al. Risk index for predicting perioperative stroke, myocardial infarction, or death risk in asymptomatic patients undergoing carotid endarterectomy. J Vasc Surg. 2013;57:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juo Y-Y, Mantha A, Ebrahimi R, Ziaeian B, Benharash P. Incidence of myocardial infarction after high-risk vascular operations in adults. JAMA Surg. 2017;152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Periopera tive cardiac events in patients undergoing noncardiac surgery: A review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunkelgrun M, Boersma E, Schouten O, Koopman-van Gemert AW, van Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery. Ann Surg. 2009;249:921–926. [DOI] [PubMed] [Google Scholar]
- 6.POISE Study Group, Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management. Eur Heart J. 2014;35:2383–2431. [DOI] [PubMed] [Google Scholar]
- 8.Spanjersberg WR, van Sambeeck JDP, Bremers A, Rosman C, van Laarhoven CJHM. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc. 2015;29:3443–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan H-J, Wolf JS, Ye Z, Hafez KS, Miller DC. Population level assessment of hospital based outcomes following laparoscopic versus open partial nephrectomy during the adoption of minimally invasive surgery. J Urol. 2014;191:1231–1237. [DOI] [PubMed] [Google Scholar]
- 10.Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet. 2002;359:2224–2229. [DOI] [PubMed] [Google Scholar]
- 11.Dimick JB, Stanley JC, Axelrod DA, Kazmers A, Henke PK, Jacobs LA, et al. Variation in death rate after abdominal aortic aneurysmectomy in the United States: Impact of hospital volume, gender, and age. Ann Surg. 2002;235:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HCUP Databases. Healthcare Cost and Utilization Project (HCUP). 2006–2009 Agency for Healthcare Research and Quality, Website. www.hcup-us.ahrq.gov/databases.jsp. [PubMed] [Google Scholar]
- 13.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215–45. [DOI] [PubMed] [Google Scholar]
- 14.Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res. 2015;198:441–449. [DOI] [PubMed] [Google Scholar]
- 15.Van Walraven C, Oake N, Jennings A, Forster AJ. The association between continuity of care and outcomes: A systematic and critical review. J Eval Clin Pract. 2010;16:947–956. [DOI] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 17.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2000 Stat Notes. 2001;20:1–9. [PubMed] [Google Scholar]
- 18.Royston P. PTREND: Stata module for trend analysis for proportions. Stat Softw Components October 2014. https://ideas.repec.org/c/boc/bocode/s426101.html. Accessed January 15, 2018.
- 19.Smilowitz NR, Gupta N, Guo Y, Berger JS, Bangalore S. Perioperative acute myocardial infarction associated with non-cardiac surgery. Eur Heart J. 2017;38:2409–2417. [DOI] [PubMed] [Google Scholar]
- 20.Thiemann DR, Coresh J, Oetgen WJ, Powe NR. The association between hospital volume and survival after acute myocardial infarction in elderly patients. N Engl J Med. 1999;340:1640–1648. [DOI] [PubMed] [Google Scholar]
- 21.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. [DOI] [PubMed] [Google Scholar]
- 22.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanaroff AC, Zakroysky P, Dai D, Wojdyla D, Sherwood MW, Roe MT, et al. Outcomes of PCI in relation to procedural characteristics and operator volumes in the United States. J Am Coll Cardiol. 2017;69:2913–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco BA, Kothari AN, Blackwell RH, Brownlee SA, Yau RM, Attisha JP, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837–845. [DOI] [PubMed] [Google Scholar]
- 25.Botto F, Alonso-Coello P, Chan MT, illar JC, Xavier D, Srinathan S, et al. Myocardial injury after noncardiac surgery A large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. [DOI] [PubMed] [Google Scholar]
- 26.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. [DOI] [PubMed] [Google Scholar]
- 27.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complicatons more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg. 2010;52(674):e1–83 e3. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. [DOI] [PubMed] [Google Scholar]
- 29.Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850. [DOI] [PubMed] [Google Scholar]
- 30.Pearse RM, Harrison DA, MacDonald N, et al. Effect of a prioperative, cardiac output–Guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery. JAMA. 2014;311:2181. [DOI] [PubMed] [Google Scholar]
- 31.Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system. Anesthesiology. 2018;128:55–66. [DOI] [PubMed] [Google Scholar]
- 32.Brown DL, Redberg RF. Continuing use of prophylactic percutaneous coronary intervention in patients with stable coronary artery disease despite evidence of no benefit. JAMA Intern Med. 2016;176:597. [DOI] [PubMed] [Google Scholar]
- 33.Regan DW, Kashiwagi D, Dougan B, Sundsted K, Mauck K. Update in perioperative medicine: Practice changing evidence published in 2016. Hosp Pract. 2017;45 158–4. [DOI] [PubMed] [Google Scholar]
- 34.Kisten T, Biccard B. Incidence and hospital mortality of vascular surgery patients with perioperative myocardial infarction (PMI) or myocardial injury after non-cardiac surgery (MINS). South African J Anaesth Analg. 2017;23:64–68. [Google Scholar]


