Abstract
Purpose of Review
To describe the epidemiology, pathophysiology, management, and prognosis of patients with heart failure with mid-range ejection fraction (HFmrEF).
Recent Findings
In 2013, The American Heart Association (AHA)/American College of Cardiology (ACC) assigned an ejection fraction (EF) range to heart failure with reduced ejection fraction (HFrEF, EF ≤ 40%) and heart failure with preserved ejection fraction (HFpEF, EF ≥50%). This classification created a “gray zone” of patients with EFs between 41% and 49% that ultimately came to be known as heart failure with borderline or mid-range ejection fraction. HFmrEF patients represent a group with heterogeneous clinical characteristics that at times resembles HFrEF, at others HFpEF, and at others still a unique phenotype altogether. No randomized controlled trials exist in those with HFmrEF, though HFrEF and HFpEF studies that include overlap suggest some potential benefit of beta blockers, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitors. Mortality rates among the HFmrEF population are significant, and are similar to those in patients with HFrEF and HFpEF.
Summary
HFmrEF is a complex disorder that remains poorly understood. Future research is needed to better elucidate the pathophysiology, management, and prognosis of this condition.
Keywords: Heart failure, Mid-range ejection fraction
Introduction
Though the term ejection fraction (EF) was first coined in 1965, it did not come to dominate the classification of heart failure (HF) until the 2005 American Heart Association (AHA)/American College of Cardiology (ACC) Guideline for the Management of Heart Failure [1]. Here, HF characterization transitioned from a framework of systolic versus diastolic dysfunction to one of HF with reduced (HFrEF) versus preserved ejection fraction (HFpEF) [1, 2]. In 2013, the ACC/AHA Guideline for the Management of Heart Failure formally assigned EF ranges to HFrEF (EF ≤ 40%) and HFpEF (EF ≥ 50%) and, in doing so, created a “gray zone” of patients with EFs between 41 and 49% [3•]. The Guideline called this zone heart failure with borderline ejection fraction, and in 2014, this phenotype was labeled heart failure with mid-range ejection fraction (HFmrEF)—a term that was formally adopted in the 2016 European Society of Cardiology (ESC) HF Guidelines [3•, 4•, 5].
Patients with HFmrEF have traditionally been excluded from large HF trials, which have hindered study on HFmrEF prevalence, clinical characteristics, and response to traditional HF therapy. Since the ACC/AHA and ESC guideline classifications, there has been a flurry of research on the clinical entity of HFmrEF. In this review, we provide an up-to-date overview of the epidemiology, clinical characteristics, morbidity and mortality, and treatment for HFmrEF.
Epidemiology and Clinical Characteristics
Heart failure affects 6.2 million Americans and costs $30.7 billion dollars a year [6]. Of those with HF, an estimated 7–25% have HFmrEF [7•, 8, 9, 10•, 11•, 12•, 13•, 14•, 15•]. Early population level studies demonstrated that patients with HFmrEF have clinical characteristics and outcomes similar to those of patients with HFpEF [8, 9]. A recent analysis of 39,982 hospitalized heart failure patients from the Get With The Guidelines cohort affirmed these earlier findings [7•]. More recent studies, however, appear to paint a slightly different picture. In the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) Program, HFmrEF patients more closely resembled HFrEF patients in terms of age, sex, ischemic heart disease, and history of myocardial infarction [10•]. In 42,061 patients from the Swedish Heart Failure registry, HFmrEF patients resembled HFrEF patients in terms of the prevalence of diabetes mellitus (DM), coronary artery disease (CAD), valve disease, statin use, and platelet inhibitor use. In the same cohort, HFmrEF patients resembled HFpEF patients in terms of problematic alcohol use, potassium levels, and N-terminal pro B-type natriuretic peptide levels (NT-proBNP). For several other characteristics, including New York Heart Association class, pulmonary edema, and diuretic use, there was no pattern observed [11•]. In the TIME-CHF (Trial of Intensified versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure) cohort, the HFmrEF group was found to be intermediate to HFrEF and HFpEF in terms of clinical characteristics, though was more like the HFrEF population in terms of CAD and response to NT-proBNP-guided therapy [12•]. In the ESC Long Term and PINNACLE (Practice Innovation and Clinical Excellence Registry) groups, HFmrEF patients were similarly found to resemble HFrEF patients in terms of CAD, while the CHART-2 (Chronic Heart Failure Analysis and Registry in the Tohoku District-2) cohort had clinical characteristics intermediate between HFrEF and HFpEF (Table 1) [13•, 14•, 15•]. Streng et al. analyzed the prevalence of noncardiac comorbidities in 3499 patients from the BIOSTAT-CHF (Biological Study to Tailored Treatment in Chronic Heart Failure) study and found that prevalence of DM, thyroid dysfunction, stroke, chronic obstructive pulmonary disease, chronic kidney disease, anemia, obesity, and peripheral arterial disease in HFmrEF patients was intermediate to that observed in those with HFrEF and HFpEF [16].
Table 1.
Clinical characteristics of patients with heart failure stratified by ejection fraction
| Swedish Heart Failure Registry [11] | Get With The Guidelines-HF [7] | CHARM Program [10] | PINNACLE Registry [14] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | |
| Characteristic | n = 23,402 | n = 9019 | n = 9640 | n = 18,398 | n = 3285 | n = 18,299 | n = 4323 | n = 1322 | n = 1953 | n = 316,628 | n = 56,527 | n = 324,387 |
| Demographics | ||||||||||||
| Age (years) | 72 | 74 | 77 | 79 | 81 | 82 | 65 | 65 | 67 | 68 | 70 | 70 |
| Female, % | 29 | 39 | 55 | 41 | 52 | 68 | 26 | 30 | 46 | 33 | 33 | 51 |
| Body mass index, kg/m2 | 26 | 27 | 28 | 26 | 27 | 27 | 27 | 28 | 29 | 29 | 30 | 30 |
| Comorbidities, % | ||||||||||||
| Atrial fibrillation | 51 | 58 | 63 | 35 | 37 | 39 | 26 | 26 | 31 | 33* | 40* | 34* |
| Coronary artery disease | 54 | 53 | 42 | 57 | 55 | 44 | – | – | – | 64 | 70 | 56 |
| Diabetes mellitus | 27 | 27 | 28 | 38 | 42 | 39 | 29 | 29 | 28 | 26 | 30 | 26 |
| Hypertension | 56 | 64 | 72 | 70 | 75 | 78 | 49 | 56 | 69 | 69 | 79 | 79 |
| Peripheral vascular disease | 10 | 10 | 10 | 14 | 15 | 12 | – | – | – | 12 | 15 | 13 |
| Smoking | 60 | 55 | 50* | 11 | 8 | 7 | 16 | 16 | 12 | 61 | 61 | 54 |
| Valvular disease | 21 | 21 | 28 | 10 | 11 | 13 | – | – | – | – | – | – |
| Medical therapy | ||||||||||||
| ACEi/ARB | 90 | 84 | 72 | 55 | 50 | 49 | 57† | 27† | 16† | 66 | 61 | 51 |
| Beta blockers | 90 | 86 | 78 | 38 | 37 | 36 | 55 | 58 | 54 | 78 | 75 | 62 |
| Aldosterone antagonist | 33 | 24 | 26 | 10 | 7 | 5 | 21 | 11 | 12 | – | – | – |
| Diuretics | 80 | 74 | 85 | 65 | 60 | 62 | 89 | 74 | 75 | 56 | 52 | 43 |
| Digoxin | 18 | 16 | 18 | 21 | 15 | 14 | 53 | 35 | 25 | 5 | 4 | 3 |
| Statins | 48 | 48 | 39 | 45 | 43 | 39 | 41‡ | 45‡ | 40‡ | – | – | – |
| Hydralazine | – | – | – | 5 | 5 | 5 | – | – | – | – | – | – |
| Nitrates | 16 | 17 | 18 | 19 | 19 | 16 | – | – | – | – | – | – |
| CRT/CRT-D | 3.5 | 0.9 | 0.4 | 2 | 1 | 0.5 | – | – | – | – | – | – |
| ICD without CRT | 2.6 | 1.3 | 0.6 | 15 | 4 | 1 | 4§ | 2§ | 0.5§ | – | – | – |
Abbreviations: ACEi, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker; CHARM, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity; CRTD, Cardiac Resynchronization Therapy-Defibrillator; ESC, European Society of Cardiology; HF, Heart Failure; HFpEF, Heart Failure with Preserved Ejection Fraction; HFmrEF, Heart Failure with Mid-Range Ejection Fraction; HFrEF, Heart Failure with Reduced Ejection Fraction; PINNACLE, Practice Innovation and Clinical Excellence
Includes atrial flutter
ACEi only
Any lipid lowering therapy
Study does not specify if ICD with or without CRT
Pathophysiology
HFrEF and HFpEF have traditionally been thought to be a result of systolic and diastolic dysfunction, respectively. As research progresses, it is increasingly recognized that there may be significant overlap between the two conditions. While many pharmacologic interventions have proven effective in HFrEF, no drug to date has demonstrated improved mortality outcomes in the HFpEF population, a finding that underscores our incomplete understanding of this disease state [17–24]. The pathophysiology among patients with HFmrEF is similarly not well characterized. To investigate further, Rickenbacher et al. used echocardiographic data from TIME-CHF [12•]. In this cohort, left ventricular cavity dimension progressively increased, and parameters of systolic function gradually decreased from HFpEF to HFmrEF to HFrEF. Elevated left ventricular filling pressures were present in all groups. All three groups also demonstrated evidence of left ventricular hypertrophy. Concentric remodeling was seen in HFpEF and to a lesser degree in HFmrEF compared to eccentric hypertrophy in the HFrEF group. Diastolic dysfunction was not different between the groups [12•].
From a biomarker perspective, NT-proBNP levels are elevated in HFrEF and HFmrEF to a similar extent, with levels in these groups being much higher than in those with HFpEF [12•]. Patients with HFmrEF and HFrEF are similar with regard to higher serum creatinine and troponin T levels when compared to those with HFpEF [12•]. In contrast, HFmrEF patients resemble HFpEF patients with respect to higher cystatin C and lower hemoglobin levels [12•]. Tromp et al. evaluated a panel of 37 biomarkers from different pathophysiologic domains across a wide range of ejection fractions. HFrEF patients were found to have a profile predominantly associated with cardiac stretch, HFpEF patients with cardiac inflammation, and HFmrEF patients with both cardiac stretch and inflammation [25]. In the SHOP (Singapore Heart Failure Outcomes and Phenotypes) cohort, cardiac troponin values among HFmrEF patients were intermediate to those with HFrEF and HFpEF [26].
On a signaling level, Vergaro et al. investigated the neuroendocrine profiles of patients with HF, ultimately demonstrating similar profiles between HFpEF and HFmrEF patients with comparatively higher levels of neurohormones (NT-proBNP, renin to aldosterone ratio, aldosterone, and norepinephrine) in the HFrEF group [27]. Pugliese et al. evaluated responses to exercise in 169 patients and demonstrated that exercise intolerance among those with HFpEF and HFmrEF was predominantly attributable to peripheral factors (arterial-venous oxygen content difference) whereas intolerance in those with HFrEF was due to low increases in stroke volume [28].
Management
There have been no randomized controlled trials (RCT) designed specifically to evaluate pharmacologic therapy in those with HFmrEF. HFrEF and HFpEF trials that include overlap into the 40–50% range may provide insights into this population’s pharmacologic management. The CHARM Program consisted of three separate RCTs evaluating the impact of candesartan (versus placebo) on the primary outcome of cardiovascular death or HF admission among those with reduced (≤ 40%) and preserved (> 40%) EF [21, 29, 30]. A post hoc analysis of the CHARM Program evaluated the impact of candesartan on patients with HFmrEF for the primary outcome of cardiovascular death or HF hospitalization [10•]. Over a mean follow up of 2.9 years, candesartan lowered incidence of the primary outcome among patients with HFmrEF (HR 0.76, 95% CI 0.61–0.96) [10•]. Similarly, a meta-analysis of 11 trials demonstrated that beta blockers reduced the incidence of cardiovascular mortality among patients with HFmrEF in sinus rhythm (HR 0.48, 95% CI 0.24–0.97) [31•]. This analysis did not reach significance for all-cause mortality (HR 0.59, 95% CI 0.34–1.03) [31•]. The TOPCAT (Treatment of Cardiac Function with an Aldosterone Antagonist) trial evaluated the efficacy of spironolactone versus placebo on the primary outcome of cardiovascular mortality, aborted cardiac arrest, or HF hospitalization among patients with an EF ≥ 45% [32]. Though the overall trial was neutral, a subgroup analysis of patients from the Americas with EF of 45–50% suggested a benefit of spironolactone versus placebo for the primary outcome (HR 0.55, 95% CI 0.33–0.91) as well as for the outcomes of cardiovascular (HR 0.46, 95% CI 0.23–0.94) and all-cause mortality (HR 0.58, 95% CI 0.34–0.99) [33•]. Last, PARAGON-HF (Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor [ARNI] with Angiotensin Receptor Blocker [ARB] Global Outcomes in Heart Failure with Preserved Ejection Fraction) found no significant effect of ARNI therapy (compared to ARB) on a composite outcome of cardiovascular mortality or HF hospitalization among those with an EF ≥ 45% [34•]. However, in a pre-specified subgroup of patients with EF ≤ 57%, there was potential benefit for the composite outcome (HR 0.78, 95% CI 0.64–0.95) (Table 2) [34•].
Table 2.
Impact of guideline-directed medical therapy on cardiovascular outcomes in heart failure with mid-range ejection fraction
| Study | Type of analysis | Cohort | Therapy tested | Outcome | Hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Lund et al. [10] | Post hoc analysis of CHARM Program | EF 40–49% | Candesartan | CV death or HF hospitalization | 0.76 (0.61–0.96) |
| Cleland et al. [31] | Meta-analysis of 11 RCTs | EF 40–49% | Beta blockers | CV death | 0.48 (0.24–0.97) |
| Solomon et al. [33] | Post hoc analysis of TOPCAT | EF 40%-< 50%, Americas | Spironolactone | CV death, HF hospitalization, aborted cardiac arrest | 0.55 (0.33–0.91) |
| Solomon et al. [34] | Post hoc analysis of PARAGON-HF | EF ≤ 57% | Sacubitril-valsartan | CV death, HF hospitalization | 0.78 (0.64–0.95) |
CAD, Coronary Artery Disease; CHARM, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity; CI, Confidence Interval; CV, Cardiovascular; EF, Ejection Fraction; HF, Heart Failure; PARAGON, Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor with Angiotensin Receptor Blocker Global Outcomes in Heart Failure with Preserved Ejection Fraction; RCT, Randomized Control Trial; TOPCAT, Treatment of Cardiac Function with an Aldosterone Antagonist
Observational studies have further evaluated the association of conventional HF therapeutics with cardiovascular outcomes in the HFmrEF population. In the CHART-2 cohort, beta blocker use was associated with improved mortality among those with HFmrEF [13•]. A similar relationship was seen in the Swedish Heart Failure Registry, though only among patients with both CAD and HFmrEF (HR 0.74, 95% CI 0.59–0.92) [11•]. In a study of the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) cohort linked to long-term Medicare date, beta blockers were not associated with improvements in all-cause mortality in those with EF ≥ 40% [35, 36].
Given the lack of formal studies evaluating traditional HF therapies in those with HFmrEF, guidelines currently suggest the use of diuretics for volume management and the treatment of comorbidities [5]. The management of CAD and noncardiac comorbidities such as CKD and hypertension is likely to provide significant benefit [13•, 37–39]. Given the findings above, it may be reasonable to consider the use of ARBs, beta blockers, mineralocorticoid receptor antagonists, and ARNIs in the management of those with HFmrEF while recognizing that the available data suggest a stronger signal for reduction in HF hospitalization than for improvement in cardiovascular and all-cause mortality (Fig. 1).
Fig. 1.
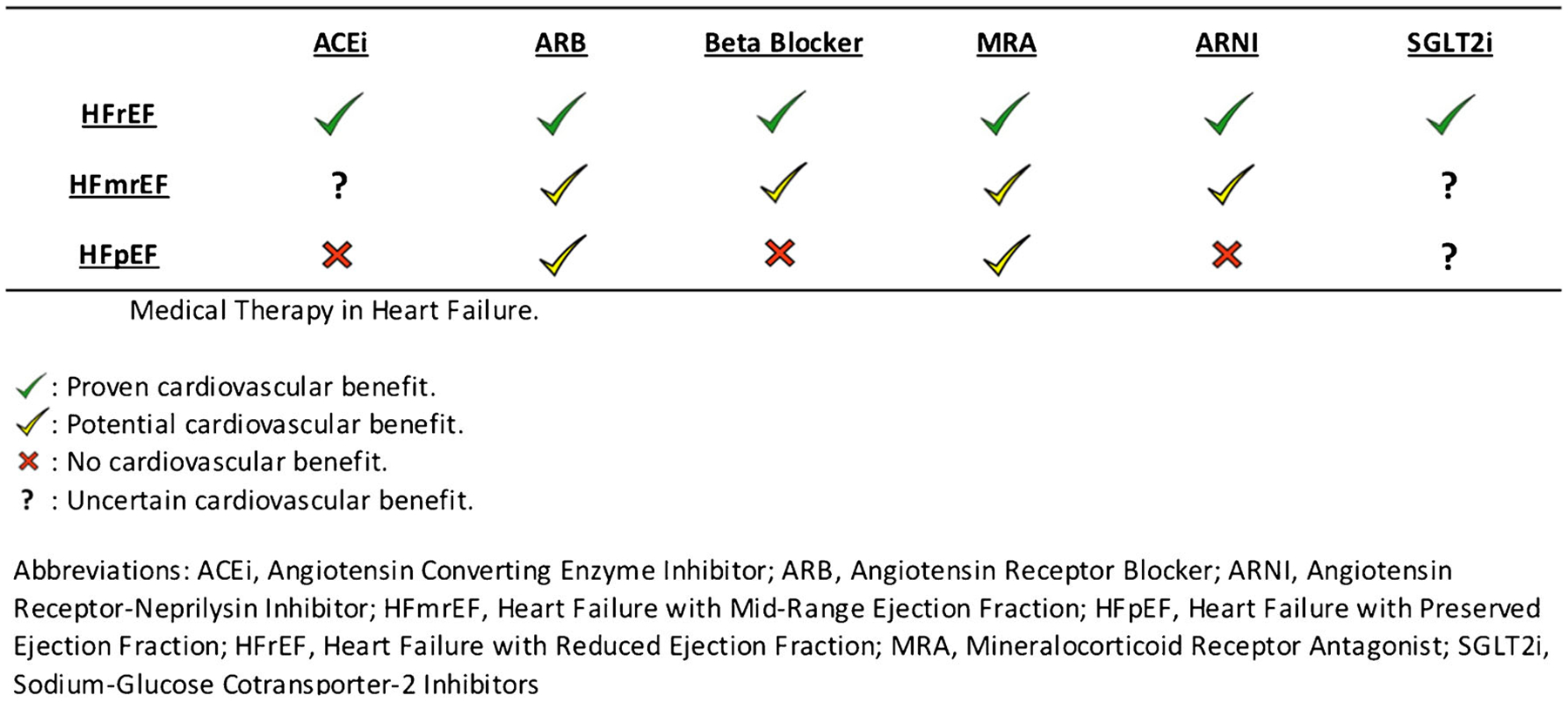
Medical therapy in heart failure. Proven cardiovascular benefit (green check). Potential cardiovascular benefit (yellow check). Uncertain cardiovascular benefit (question mark). No cardiovascular benefit (red x). Abbreviations: ACEi, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker; ARNI, Angiotensin Receptor-Neprilysin Inhibitor; HFmrEF, Heart Failure with Mid-Range Ejection Fraction; HFpEF, Heart Failure with Preserved Ejection Fraction; HFrEF, Heart Failure with Reduced Ejection Fraction; MRA, Mineralocorticoid Receptor Antagonist; SGLT2i, Sodium-Glucose Cotransporter-2 Inhibitors
Prognosis
Several large databases have published morbidity and mortality data for patients with HFmrEF. In the ESC Heart Failure Long-Term Registry, mortality at 1 year for HFmrEF (7.6%) patients was intermediate to that for HFrEF (8.8%) and HFpEF (6.3%) patients, though this difference was not statistically significant. Heart failure hospitalization was 14.6%, 8.7%, and 9.7% in the HFrEF, HFmrEF, and HFpEF groups, respectively [15•]. The Get With The Guidelines Registry linked to Medicare claims reported a 5-year mortality of 75.4% with no significant differences seen when stratified by EF among patients 65 years or older hospitalized with HF. In this group, patients with HFrEF and HFmrEF had higher readmission rates than patients with HFpEF [7•]. Similar to the Get With The Guidelines registry, the TIME-CHF cohort demonstrated no difference in mortality between those with HFrEF, HFmrEF, and HFpEF [12•]. Data from the MAGGIC (Meta-analysis Global Group in Chronic Heart Failure) group revealed similar hazard of mortality in those with HFpEF and HFmrEF and an increased hazard of mortality in the HFrEF group compared to the HFpEF group [40]. In a propensity matched cohort, HFmrEF patients were at higher risk of sudden cardiac death (HR 2.73, 95% CI 1.07–6.98) and cardiovascular death (HR 1.71, 95% CI 1.13–2.57) than those with HFpEF [41]. Finally, in CHARM, HFrEF patients were found to have a higher hazard of cardiac and all-cause mortality than HFmrEF and HFpEF patients [10•].
Future Considerations
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel drug class that have recently been shown to significantly influence cardiovascular outcomes. In CANVAS (Canagliflozin Cardiovascular Assessment Study), canagliflozin reduced major adverse cardiovascular events as well as renal progression and HF hospitalization in diabetic patients at high risk for cardiovascular disease [42]. In EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients), empagliflozin improved all-cause mortality, cardiovascular mortality, and HF hospitalization in a similar population [43]. DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) studied the impact of dapagliflozin in those with EF ≤ 40% and found that this drug significantly reduced a composite outcome of worsening HF or cardiovascular death (HR 0.74, 95% CI 0.65–0.85) [44]. RCTs are currently underway to evaluate the impact of SGLT2 inhibitors on HF outcomes among those with EF ≥ 40%.
Large RCTs in the HFmrEF population are needed, though their design and implementation pose challenges. First, it is unclear whether HFmrEF represents a distinct clinical phenotype or if it is simply a transition zone between HFrEF and HFpEF [45]. Indeed, large studies have demonstrated the dynamic nature of EF. In the Swedish Heart Failure registry, more than 1/3 of HFmrEF patients had worsening EF during follow up, while 1/4 experienced improved EF [46]. In the Washington University Heart Failure registry, the majority of those with HFmrEF had prior reduced EF, while 17% had deteriorated from a previously preserved EF [47]. In the Olmsted County Minnesota cohort, EF increased by around 7% in HFrEF and decreased by around 6% in HFpEF over 5 years, crossing 50% in almost 40% of patients [38]. The second issue lies in the accuracy of echocardiographic EF evaluation. A study evaluating the accuracy of the Simpson’s rule demonstrated high interobserver (8%–21%) and intraobserver (6%–13%) variability, suggesting risk for misclassification [48]. Potential solutions to these issues have been proposed by Lam et al. and include (1) expanding the EF range of HFpEF or HFrEF trials to include HFmrEF or to (2) study the entire range of EF in each study [45]. The former approach has delivered some data on ARB, MRA, and ARNI therapy in the HFmrEF population. While Lam et al. point out that large goal-directed medical therapy trials are unlikely to be repeated with expanded EF ranges, this strategy may play an important role in the design of future studies. The latter approach is limited by the risk of being underpowered and relies on grouping together populations of patients known to have pathologically different diseases [45].
Conclusion
There have been expanding insights into HFmrEF since its first introduction. Overall, this disease entity appears to represent a group of patients with clinical characteristics, pathophysiology, therapeutic responses, and prognosis that is at times similar to HFrEF, at others to HFpEF, and at others still to a completely unique phenotype. The management of this group of patients is challenging. While there are no RCTs in this population, post hoc analyses and observational studies suggest potential efficacy of traditional HFrEF guideline-directed medical therapy, especially for the outcome of reducing HF hospitalization. Though significant progress has been made in this area, future research is still needed to better elucidate optimal classification of HFmrEF and to identify strategies that will best achieve improvements in patient-centered outcomes.
Acknowledgments
BZ: Supported by the American Heart Association SDG 17SDG33630113 and the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number KL2TR001882. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
GCF: Consulting for Abbott, Amgen, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
Footnotes
Conflict of Interest PKS and JJH have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with humans or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. [DOI] [PubMed] [Google Scholar]
- 2.Bartle SH, Sanmarco ME, Dammann JF. Ejected Fraction: An Index of Myocardial Function (abstr.). Am J Cardiol. 1965;15(1):1.14248275 [Google Scholar]
- 3.•.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):e240–327 [DOI] [PubMed] [Google Scholar]; This reference first defined heart failure with borderline ejection fraction.
- 4.•.Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%). Eur J Heart Fail. 2014;16(10):1049–55 [DOI] [PubMed] [Google Scholar]; This reference first coined the term, “Heart Failure with Mid-Range Ejection Fraction”.
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke Statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 7.•.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86 [DOI] [PubMed] [Google Scholar]; This reference provides descriptions of the epidemiology, clinical characteristics and long-term outcomes for those with Heart Failure with Mid-Range Ejection Fraction in large population-based cohorts.
- 8.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol. 2007;50(8):768–77. [DOI] [PubMed] [Google Scholar]
- 9.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101(8): 1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•.Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20(8):1230–9 [DOI] [PubMed] [Google Scholar]; This reference provides data to support the use of traditional HFrEF therapies in those with HFmrEF.
- 11.•.Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, et al. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail. 2017;19(12):1624–34 [DOI] [PubMed] [Google Scholar]; This reference provides descriptions of the epidemiology, clinical characteristics and long-term outcomes for those with Heart Failure with Mid-Range Ejection Fraction in large population-based cohorts.
- 12.•.Rickenbacher P, Kaufmann BA, Maeder MT, Bernheim A, Goetschalckx K, Pfister O, et al. Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF). Eur J Heart Fail. 2017;19(12):1586–96 [DOI] [PubMed] [Google Scholar]; This reference provides descriptions of the epidemiology, clinical characteristics and long-term outcomes for those with Heart Failure with Mid-Range Ejection Fraction in large population-based cohorts.
- 13.•.Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 study. Eur J Heart Fail. 2017;19(10):1258–69 [DOI] [PubMed] [Google Scholar]; This reference provides descriptions of the epidemiology, clinical characteristics and long-term outcomes for those with Heart Failure with Mid-Range Ejection Fraction in large population-based cohorts.
- 14.•.Ibrahim NE, Song Y, Cannon CP, Doros G, Russo P, Ponirakis A, et al. Heart failure with mid-range ejection fraction: characterization of patients from the PINNACLE registry(R). ESC Heart Fail. 2019;6(4):784–92 [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference provides descriptions of the epidemiology, clinical characteristics and long-term outcomes for those with Heart Failure with Mid-Range Ejection Fraction in large population-based cohorts.
- 15.•.Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC heart failure long-term registry. Eur J Heart Fail. 2017;19(12):1574–85 [DOI] [PubMed] [Google Scholar]; This reference provides descriptions of the epidemiology, clinical characteristics and long-term outcomes for those with Heart Failure with Mid-Range Ejection Fraction in large population-based cohorts.
- 16.Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. 2018;271:132–9. [DOI] [PubMed] [Google Scholar]
- 17.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353(9169): 2001–7. [PubMed] [Google Scholar]
- 18.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. [DOI] [PubMed] [Google Scholar]
- 19.Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. [DOI] [PubMed] [Google Scholar]
- 20.Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429–35. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. 2003;362(9386):772–6. [DOI] [PubMed] [Google Scholar]
- 22.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 24.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875–85. [DOI] [PubMed] [Google Scholar]
- 25.Tromp J, Khan MAF, Mentz RJ, O’Connor CM, Metra M, Dittrich HC, et al. Biomarker profiles of acute heart failure patients with a mid-range ejection fraction. JACC Heart Fail. 2017;5(7):507–17. [DOI] [PubMed] [Google Scholar]
- 26.Gohar A, Chong JPC, Liew OW, den Ruijter H, de Kleijn DPV, Sim D, et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1638–47. [DOI] [PubMed] [Google Scholar]
- 27.Vergaro G, Aimo A, Prontera C, Ghionzoli N, Arzilli C, Zyw L, et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int J Cardiol. 2019;296:91–7. [DOI] [PubMed] [Google Scholar]
- 28.Pugliese NR, Fabiani I, Santini C, Rovai I, Pedrinelli R, Natali A, et al. Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid-range ejection fraction. Eur Heart J Cardiovasc Imaging. 2019;20(7):828–36. [DOI] [PubMed] [Google Scholar]
- 29.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-added trial. Lancet. 2003;362(9386):767–71. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362(9386):777–81. [DOI] [PubMed] [Google Scholar]
- 31.•.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference provides data to support the use of traditional HFrEF therapies in those with HFmrEF.
- 32.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92. [DOI] [PubMed] [Google Scholar]
- 33.•.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37(5):455–62 [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference provides data to support the use of traditional HFrEF therapies in those with HFmrEF.
- 34.•.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–20 [DOI] [PubMed] [Google Scholar]; This reference provides data to support the use of traditional HFrEF therapies in those with HFmrEF.
- 35.Patel K, Fonarow GC, Ekundayo OJ, Aban IB, Kilgore ML, Love TE, et al. Beta-blockers in older patients with heart failure and preserved ejection fraction: class, dosage, and outcomes. Int J Cardiol. 2014;173(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) registry. J Am Coll Cardiol. 2009;53(2):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. 2016;4(6):464–72. [DOI] [PubMed] [Google Scholar]
- 38.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5(6):720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meta-analysis Global Group in Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33(14):1750–7. [DOI] [PubMed] [Google Scholar]
- 41.Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I, Vazquez R, Delgado-Jimenez J, Alvarez-Garcia J, et al. Mid-range left ventricular ejection fraction: clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol. 2017;240: 265–70. [DOI] [PubMed] [Google Scholar]
- 42.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. [DOI] [PubMed] [Google Scholar]
- 43.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. [DOI] [PubMed] [Google Scholar]
- 44.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019. [DOI] [PubMed] [Google Scholar]
- 45.Lam CS, Solomon SD. Fussing over the middle child: heart failure with mid-range ejection fraction. Circulation. 2017;135(14):1279–80. [DOI] [PubMed] [Google Scholar]
- 46.Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, et al. Significance of Ischemic Heart Disease in Patients With Heart Failure and Preserved, Midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ Heart Fail. 2017;10(6). [DOI] [PubMed] [Google Scholar]
- 47.Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid-range ejection fraction. Eur J Heart Fail. 2017;19(12):1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGowan JH, Cleland JG. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods. Am Heart J. 2003;146(3):388–97. [DOI] [PubMed] [Google Scholar]


