The balance between HIPK2-mediated phosphorylation and neddylation-dependent degradation controls spastin protein levels, revealing novel therapeutic targets for hereditary spastic paraplegia.
Abstract
Hereditary Spastic Paraplegia (HSP) is a neurodegenerative disease most commonly caused by autosomal dominant mutations in the SPG4 gene encoding the microtubule-severing protein spastin. We hypothesise that SPG4-HSP is attributable to reduced spastin function because of haploinsufficiency; thus, therapeutic approaches which elevate levels of the wild-type spastin allele may be an effective therapy. However, until now, how spastin levels are regulated is largely unknown. Here, we show that the kinase HIPK2 regulates spastin protein levels in proliferating cells, in differentiated neurons and in vivo. Our work reveals that HIPK2-mediated phosphorylation of spastin at S268 inhibits spastin K48-poly-ubiquitination at K554 and prevents its neddylation-dependent proteasomal degradation. In a spastin RNAi neuronal cell model, overexpression of HIPK2, or inhibition of neddylation, restores spastin levels and rescues neurite defects. Notably, we demonstrate that spastin levels can be restored pharmacologically by inhibiting its neddylation-mediated degradation in neurons derived from a spastin mouse model of HSP and in patient-derived cells, thus revealing novel therapeutic targets for the treatment of SPG4-HSP.
Introduction
Spastin is an AAA ATPase microtubule (MT)-severing enzyme that regulates cytoskeleton rearrangement associated with membrane remodeling. It is a critical regulator in cytokinesis and nuclear envelope resealing during cell division and it is also involved in intracellular traffic (1, 2, 3). In neurons, spastin has key roles in axonal transport and regeneration (4, 5, 6). This enzyme functions as hexamers that drive the remodeling and severing of MT by tugging the C-terminal tail of tubulin through the central pore of the hexamer (7, 8, 9). Thus far, four spastin isoforms have been identified, M1 (68 kD), a shorter isoform lacking the first 86 aa (M87, 60 kD), and splice variants of both of these, excluding exon 4 (M1∆4, 64 kD, and M87∆4, 55 kD). M1 and M87 are synthesized simultaneously but at largely different levels because the first AUG is surrounded by a weak Kozak sequence resulting in a preferred translation from the second AUG (10, 11). Splice variants are the least abundant isoforms, for which no specific functions have been reported thus far.
It has been suggested that precise regulation of spastin is critical to prevent its dysfunction (5, 6). SPG4 mutations are the most common cause of autosomal dominant Hereditary Spastic Paraplegia (HSP), a neurodegenerative disease characterized by progressive spasticity of the lower limbs (12, 13, 14, 15, 16). Because most of the SPG4 pathogenic mutations are nonsense, frameshift or large insertion/deletion mutations that are generally associated with nonsense mediated degradation of mRNA, haploinsufficiency is considered one of the main molecular mechanisms of the disease (17, 18, 19). In a subset of patients carrying missense mutations dominant-negative effect, threshold-effect-model and gain-of-function appear to be relevant (16, 20, 21). Axonal swellings, characterized by aberrant accumulation of neurofilaments and mitochondria, and abnormal organelle distribution/trafficking are the hallmarks of axonal defects in mouse and human SPG4-HSP models (22, 23, 24, 25). Loss-of-function mouse SPG4-HSP models show motor behavior deficits and axon trafficking impairment; heterozygous mice show very mild phenotypes compared with the homozygous ones, further supporting a dosage effect (22, 23). Studies on neurons generated from SPG4-HSP patient-derived induced Pluripotent Stem cells, reported spastin reduction associated with alterations in neurite morphology, swellings and transport deficits (24, 25). Currently, there is no cure for HSP. Importantly, in patients’ neurons a spastin gene dosage-dependent rescue of the defects has been recently reported by lentiviral spastin expression, providing proof of principle that spastin elevating approaches could be a therapeutic strategy (25). However, at present little is known about the molecular mechanisms regulating endogenous spastin protein levels.
Recently, we identified spastin as novel target of the multifunctional kinase HIPK2 (26), that is highly expressed in the nervous system (27, 28). We demonstrated that HIPK2-mediated phosphorylation of spastin at serine 268 is required for its midbody localisation and successful abscission. Here, we show that spastin levels are regulated by HIPK2-mediated phosphorylation and neddylation-dependent degradation, providing evidence that it is possible to restore spastin levels and reduce axonal swelling pathology by targeting these pathways in SPG4-HSP models.
Results
Spastin protein levels are regulated by HIPK2 at post-transcriptional level
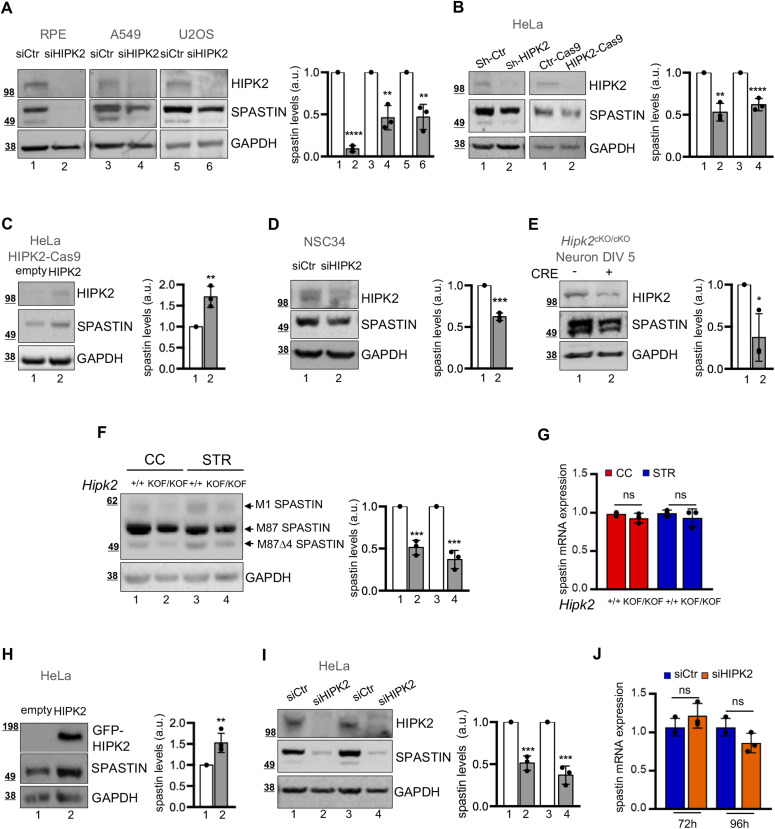
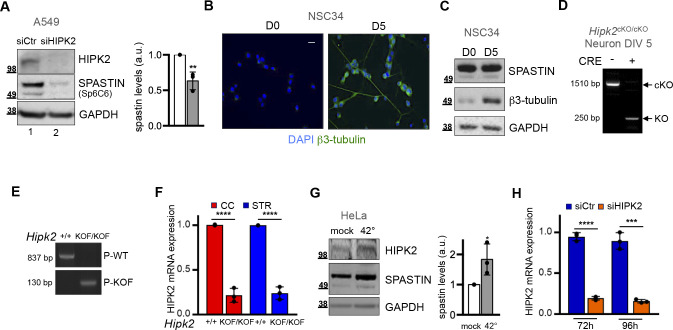
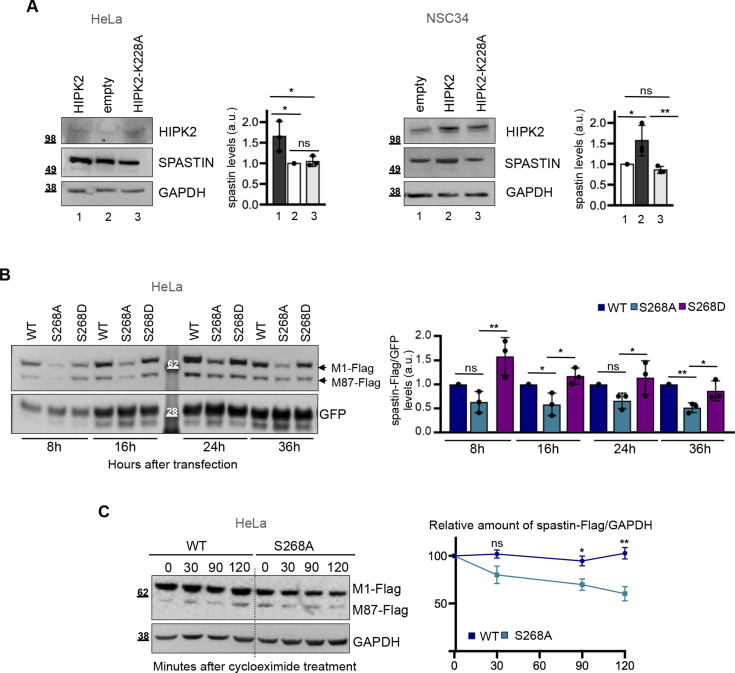
During previous studies we noticed a reduction of spastin protein levels upon HIPK2 depletion in HeLa cells, suggesting that HIPK2 might control spastin protein levels (26). To verify this hypothesis, we performed HIPK2-depletion by RNAi, CRE-Lox or CRISPR/Cas9 recombination in cells of different origin and analysed spastin protein levels with different anti-spastin Abs (Figs 1A and B and S1A). Compared with control cells, reduction of spastin levels was observed in all the HIPK2-deficient cells obtained (Figs 1A and B and S1A) and was rescued after HIPK2 re-expression in HeLa HIPK2-Cas9 cells (Fig 1C). Notably, spastin reduction was observed upon HIPK2 depletion in murine neuron-like NSC34 differentiated cells (Figs 1D and S1B and C), in human SH-SY-5Y neuron-like differentiated cells (data not shown) and in primary cortical neurons explanted from CRE-inducible Hipk2 KO mice upon infection with an adenovirus expressing the recombinase CRE (Figs 1E and S1D), showing that spastin levels are regulated by HIPK2 in postmitotic differentiated primary neurons.
Figure 1. HIPK2 regulates spastin at posttrascriptional levels.
(A) Indicated cells were transfected with a mix of three human HIPK2-specific (siHIPK2) or negative control (siCtr) stealth siRNAs and analysed by Western blot (WB) 96 h posttransfection. Unless otherwise indicated, here and in the following figures representative WB of three independent experiments was shown and the intensity of the spastin bands quantified, normalized with GAPDH, and reported in a.u. relative to control, as mean ± SD of three independent experiments. Molecular weight markers are reported in kilodalton. Here, spG311/1 Ab was used for spastin detection and statistical differences are relative to corresponding siCtr, unpaired t test. (B) HeLa cells were transfected with vectors expressing control LacZ sh-RNA (sh-Ctr) or a mix of two HIPK2-specific shRNAs (sh-HIPK2) and analysed by WB 72 h posttransfection (left panels). HeLa HIPK2-Cas9 cells and their parental control cells (Ctr-Cas9) were analysed by WB 24 h after plating (right panels). Statistical differences are relative to corresponding control, unpaired t test. (C) HeLa HIPK2-Cas9 cells were transfected with low dose of HIPK2-HA–expressing vector to avoid apotosis induction and analysed by WB 24 h posttransfection with anti-HIPK2 and anti-spastin Abs. Statistical difference is relative to empty vector transfected cells used as control, unpaired t test. (D) NSC34 cells were transfected with a mix of three mouse HIPK2-specific (siHIPK2) or negative control (siCtr) stealth siRNAs and incubated in differentiation medium; 5 d post siRNA transfection cells were analysed by WB. Statistical difference, unpaired t test. (E) Cortical neurons at 5 days in vitro (DIV5) derived from indicated mouse infected with adenovirus expressing the recombinase CRE were analysed by WB 96 h postinfection. Statistical difference, unpaired t test. (F, G) Indicated neural tissues were explanted from Hipk2+/+ and Hipk2KOF/KOF adult mice (5 mo) and spastin levels were analysed by WB and real time RT-PCR. (F) Representative WB showing the reduction of spastin isoforms is reported; statistical differences are relative to corresponding control tissue, unpaired t test. (G) Relative fold-change of spastin mRNA levels, using actin mRNA as normalizer, are represented as mean ± SD of three independent experiments in (G); ns, no statistically significance, unpaired t test. (H) Representative WB of HeLa cells transfected with GFP (empty) or GFP-HIPK2–expressing vectors and lysed 24 h posttransfection. Statistical difference, unpaired t test. (I, J) HeLa cells transfected as in Fig 1A were analysed by WB and real-time RT-PCR at the indicated time post transfection. In (I), representative WB, statistical differences are relative to corresponding siCtr, unpaired t test; in (J), relative fold-change of spastin mRNA levels, using GAPDH mRNA as normalizer, as mean ± SD of three independent experiments. ns, unpaired t test.
Source data are available for this figure.
Figure S1. Spastin expression is regulated by HIPK2.
(A) Total cell extracts of A549 cells shown in Fig 1A were run twice and Western blot (WB) analysis performed by using the sp6C6 monoclonal spastin Ab for spastin detection. Representative WB and its quantification are reported. Statistical difference, unpaired t test. (B, C) NSC34 cells were incubated in differentiation medium. Differentiation was evaluated 5 d after treatment by IF and WB using β3-tubulin neuronal marker. In (B), representative images of β3-tubulin staining at indicated days after differentiation are reported. Scale bar, 10 μM. In (C), representative WB with indicated Abs is shown. (D) Indicated cortical neurons were analysed by PCR using primers amplifying cKO and KO Hipk2 alleles. The effect of CRE recombinase in Hipk2cKO/cKO is showed by the amplification of the KO allele. (E) PCR genotyping analysis of the genomic DNA extracted from tails derived from indicated mice by using primers specifically amplifying WT (P-WT) or KOF Hipk2 alleles (P-KOF). (F) HIPK2 mRNA residual levels were analysed by real time RT-PCR in tissues analysed in Fig 1F and G. Relative fold-change of HIPK2 mRNA levels, using actin mRNA as normalizer, are represented as mean ± SD of three independents. Statistical differences, relative to +/+ counterparts, unpaired t test. (G) HeLa cells were incubated in standard culture condition at 37°C (mock) or shifted to 42°C for 1 h to induce HIPK2 stabilization and analysed by WB with indicated Abs. Statistical difference, unpaired t test. (H) HeLa cells transfected as in Fig 1A were analysed by real-time RT-PCR at the indicated time post transfection. Relative fold-change of HIPK2 mRNA levels, using GAPDH mRNA as normalizer, are represented as mean ± SD of three independent experiments. Statistical differences, unpaired t test.
Consistent with these observations, analysis of spastin expression in cerebral cortex (CC) and striatum (STR) explanted from Hipk2KOF/KOF mice, showing strongly reduced HIPK2 levels compared with wild-type (WT) siblings (Fig S1E and F), revealed a significant reduction of the protein levels of all detectable spastin isoforms, whereas spastin mRNA levels were unchanged (Fig 1F and G). This suggests that HIPK2 might also control spastin protein levels in the central nervous system (CNS) in vivo.
As reciprocal approach, we showed that HIPK2 up-regulation, obtained by either overexpression of the exogenous protein or heat shock–mediated stabilization (29) of the endogenous one, was associated with increased spastin expression (Figs 1H and S1G).
Altogether these findings demonstrate that spastin is regulated by HIPK2 in proliferating cells, in differentiated neurons and in vivo.
To investigate HIPK2–mediated regulation of spastin, we analysed both spastin mRNA and protein levels in HIPK2-depleted (siHIPK2) and control cells (siCtr). The decrease in spastin protein expression occurs without significant change at the mRNA level upon HIPK2 depletion (Figs 1I and J and S1H), indicating post-transcriptional regulation of protein levels.
Spastin is polyubiquitinated at K554R and degraded via proteasome in HIPK2-defective cells
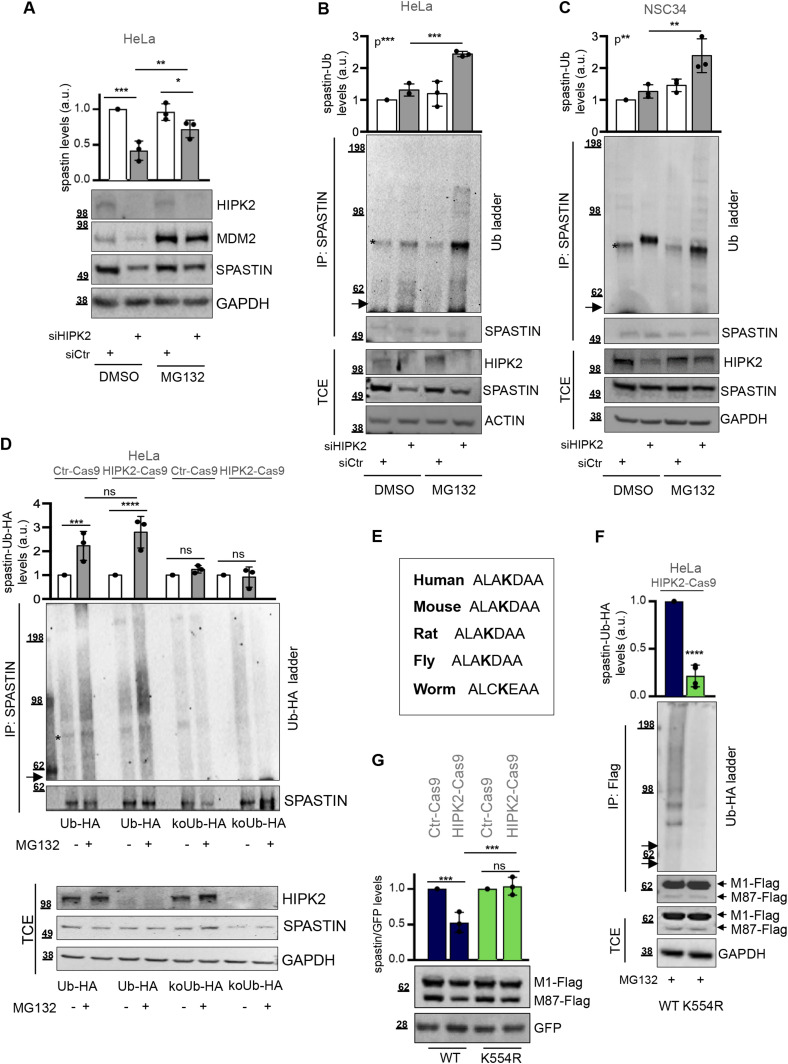
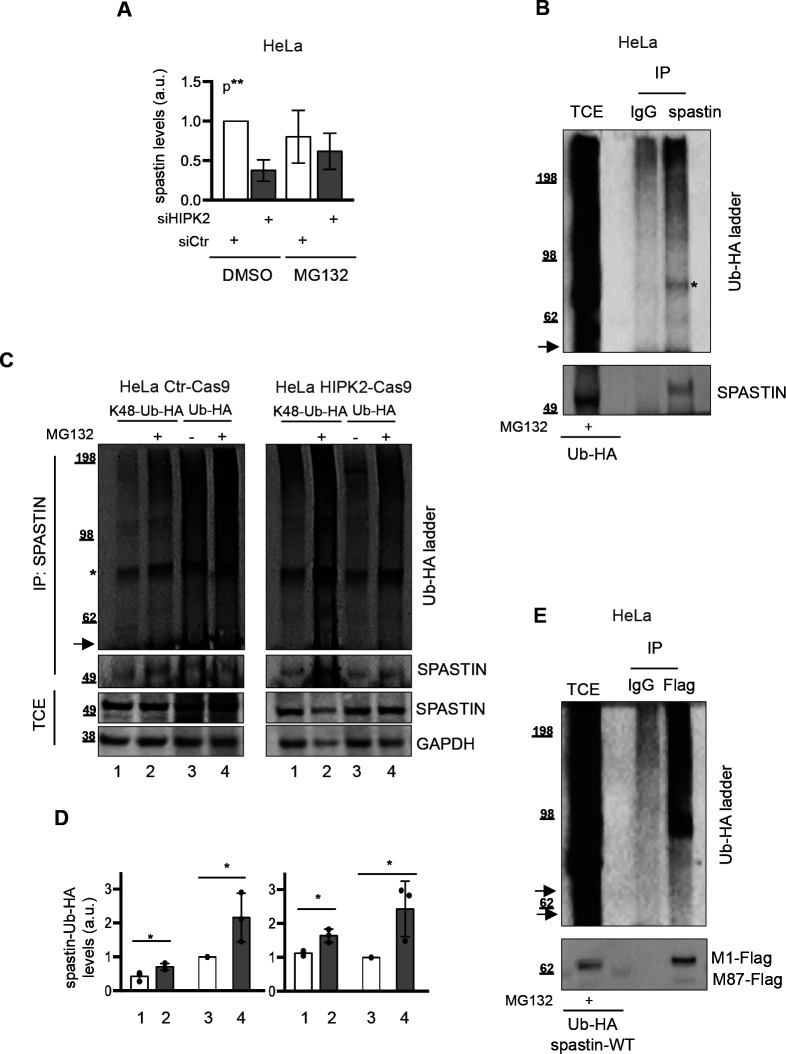
Next, we performed protein degradation tests and observed that spastin protein levels could be rescued by treatment with the proteasome inhibitor MG132 in HIPK2-depleted cells (Fig 2A), suggesting that degradation of spastin is mediated by the ubiquitin proteasome pathway (UPP). Thus, we investigated whether spastin is polyubiquitinated in HIPK2-defective cells. By using an anti-Ub Ab recognising mono- and poly-Ub conjugates, a pattern of slowly migrating spastin-Ub conjugates was detectable after spastin IP in HIPK2-depleted cells post MG132 treatment (Figs 2B and C and S2A). To further validate spastin polyubiquitination, we performed ubiquitination assays in HeLa HIPK2-Cas9 and in Ctr-Cas9 cells, using HA-tagged Ub-WT (Ub-HA) or lysine-free Ub mutant (koUb-HA), which allows only monoubiquitination. IP with normal mouse IgG immunoglobulins was used as negative control (Fig S2B). No ubiquitination signals were observed in cells expressing koUb-HA mutant, whereas a smear of slowly migrating spastin-Ub-HA conjugates accumulated in Ub-WT-expressing cells after MG132 treatment in HIPK2-Cas9 cells (Fig 2D), indicating spastin polyubiquitination. Similar results were obtained also in parental Ctr-Cas9 cells (Fig 2D). Next, we performed a ubiquitination assay using a K48-only Ub mutants (K48-Ub-HA), which allows only K48-linked polyubiquitinated chains, the canonical proteasomal degradation signal. Spastin K48-polyubiquitination was detectable after MG132 treatment in HeLa HIPK2-Cas9 and in Ctr-Cas9 cells (Fig S2C and D). Altogether, these findings indicate the involvement of the UPP pathway in the regulation of spastin, even if we cannot rule out the involvement of other non-proteolytic processes.
Figure 2. HIPK2 regulates spastin via proteasomal degradation through K554 polyubiquitination.
(A) Representative Western blot (WB) of HeLa cells transfected as in Fig 1A and lysed 96 h posttransfection and 8 h after treatment with 20 μM MG132 or its solvent DMSO. MDM2 stabilization has shown as MG132 positive control. Statistical differences, ANOVA test. (B) HeLa cells were transfected as in Fig 1A and harvested 96 h posttransfection and 8 h after treatment with 20 μM MG132 or DMSO. Total cell extract (TCE) was analysed by WB for the indicated proteins and immunoprecipitated with anti-spastin Ab. IPs were analysed by WB with anti-Ub and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a nonspecific band. The intensity of spastin-Ub ladder was normalized by the intensity of spastin band in IP and reported relative to siCtr DMSO–treated cells as mean ± SD (n = 3). Statistical differences, ANOVA test. (C) NSC34 cells were transfected as in Fig 1B and harvested 5 d posttransfection and 8 h after treatment with 20 μM MG132. TCE was analysed by WB for the indicated proteins and immunoprecipitated with anti-spastin Ab. IPs were analysed by WB with anti-Ub and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a specific band. The intensity of spastin-Ub ladder was calculated and reported as in Fig 2B. Statistical differences, ANOVA test. (D) HeLa Ctr-Cas9 and HIPK2-Cas9 cells were transfected with vectors expressing HA-tagged Ub-WT (Ub-HA) or its derivative KoUb-HA (i.e., Ub with all lysines mutated in arginines) and treated 24 h posttransfection with 20 μM MG132 or DMSO for 8 h. TCEs were analysed as in Fig 2B. IPs were analysed by WB with anti-HA and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a nonspecific band. The intensity of spastin-Ub-HA ladder was normalized by the intensity of spastin band in IP and reported relative to the correspondent DMSO-treated cells as mean ± SD (n = 3). Statistical differences, ANOVA test. (E) Spastin amino acid sequence encompassing the K554 is reported for indicated organisms. Fly = Drosophila melanogaster; worm = Caenorhabditis elegans. (F) HIPK2-Cas9 HeLa cells were transfected with vectors expressing flag-myc–tagged spastin-WT or spastin-K554R in combination with the vector expressing HA-tagged Ub-WT and treated 24 h posttransfection with 20 μM MG132 for 8 h. TCE were analysed by WB and immunoprecipitated with anti-Flag Ab (mouse Ab by Origene Technologies). IPs were analysed by WB with anti-HA and anti-Flag Ab (rabbit Ab by Sigma-Aldrich). The arrows indicate the position of the unmodified spastin isoforms. The intensity of spastin-Ub-HA ladder was normalized by the intensity of spastin bands in IP and reported as mean ± SD (n = 4). Statistical difference, unpaired t test. (G) HIPK2-Cas9 and Ctr-Cas9 HeLa cells were transfected with vectors expressing spastin-WT or spastin-K554R in combination with peGFP vector at 10:1 molar ratio and analysed by WB 24 h posttransfection. GFP expression was used as internal control for transfection efficiency. Representative WB is shown. The intensity of spastin-Flag bands was normalized by the intensity of GFP and reported relative to correspondent Ctr-Cas9 control cells. Statistical differences, ANOVA test.
Source data are available for this figure.
Figure S2. Spastin is K48-polyubiquitinated.
(A) Data quantification of spastin/loading control levels by Western blot (WB) on total cell extract (TCE), relative to data shown in Fig 2B. Quantification was performed as in Fig 2A. Bars are mean ± SD of three independent experiments; Statistical differences, ANOVA test. (B) As in Fig 2D, HeLa cells were transfected with vector expressing Ub-HA and treated 24 h posttransfection with 20 μM MG132 for 8 h. TCE was immunoprecipitated with anti-spastin mouse monocolonal Ab, sp3G11/1. Mouse IgG were used as negative IP control. TCE and IPs were analysed by WB with anti-HA and anti-spastin Abs. The arrow indicates the position of the unmodified spastin and the asterisk indicates a non-specific band. (C, D) HeLa HIPK2-Cas9 and Ctr-Cas9 cells were transfected with vectors expressing HA-Ub or its derivative K48-Ub-HA (i.e., Ub with only K48, the other lysines are mutated in arginines) and treated 24 h post transfection with 20 μM MG132 or DMSO for 8 h. TCE were analysed by WB and IP as in Fig 2D. In (C), representative WB of three independent experiment is shown. The arrow indicates the position of the unmodified spastin and the asterisk indicates a non-specific band. In (D), relative spastin-Ub-HA levels were quantified and reported as in Fig 2C. Statistical differences, unpaired t test. (E) As in Fig 2F, HeLa cells were transfected with vectors expressing flag-myc tagged spastin in combination with Ub-HA and 24 h post transfection cells were treated with 20 μM MG132 for 8 h. TCE was immunoprecipitated with anti-Flag mouse monoclonal Ab. Mouse IgG were used as negative IP control. TCE and IPs were analysed by WB with anti-HA and anti-Flag Abs. The arrow indicates the position of the unmodified spastin.
Among all the lysines of spastin that are possible sites for Ub conjugation, K554 was reported as a putative site in a large-scale mass spectrometry (MS) screen (https://www.phosphosite.org; (30)). Thus, we focused our attention on this lysine residue which is evolutionary conserved (Fig 2E) and located in the ATPase domain common to all spastin isoforms. We generated a spastin K554 mutant (spastin-K554R), and tested it in ubiquitination assays. Flag-myc tagged spastin-K554R and spastin-WT were expressed in combination with Ub-HA in HIPK2-Cas9 cells. As shown in Figs 2F and S2E, spastin-WT displayed a smear of slowly migrating bands comparable to that of the endogenous protein, whereas spastin-K554R showed a very strong reduction of this pattern, indicating that K554 is a key spastin polyubiquitination site (Fig 2F). Accordingly, when transfected at comparable efficiency, spastin-WT levels were reduced in HIPK2-Cas9 cells compared with HIPK2 proficient cells, whereas spastin-K554R was expressed at similar levels in both cell types, suggesting that spastin-K554R is more resistant to protein degradation than spastin-WT (Fig 2G). These results show that K554 polyubiquitination is required for spastin degradation in HIPK2-defective cells.
HIPK2 regulates spastin protein levels through its kinase activity
We have demonstrated that HIPK2 binds and phosphorylates spastin at S286 (26), thus we analysed whether HIPK2 regulates spastin protein levels through its kinase activity. Compared with HIPK2-WT–overexpressing cells, no increase in spastin protein levels was observed upon transfection of the kinase-defective HIPK2-K228A mutant (Fig 3A), consistent with a role for HIPK2 kinase activity in coordinating the ubiquitination of spastin. By using vectors encoding non-phosphorylatable or phosphomimetic spastin mutants, S268A and S268D, respectively, we noticed that spastin-S268A was reproducibly and consistently expressed at lower levels than spastin-WT or spastin-S268D when transiently transfected at comparable efficiency (Fig 3B). Accordingly, spastin-S268A displayed a shorter half-life than spastin-WT in the presence of cycloheximide (Fig 3C), indicating that HIPK2-mediated S268 phosphorylation contributes to spastin stability.
Figure 3. Kinase activity of HIPK2 regulates spastin protein levels.
(A) Representative Western blot (WB) of HeLa (right panels) and NSC34 cells (left panels) transfected with vectors expressing HA-tagged HIPK2-WT, its derivative -K228A mutant or HA-alone (empty). Statistical differences, Anova test. (B) Representative WB of HeLa cells transfected with vectors expressing flag-myc spastin-WT or indicated spastin mutants in combination with peGFP vector at 10:1 molar ratio and lysed at indicated time post transfection. GFP expression was used as internal control for transfection efficiency. The intensity of spastin-Flag bands was normalized by the intensity of GFP and reported relative to spastin-WT for each time point. Statistical differences, ANOVA test. (C) HeLa cells were transfected with vectors expressing flag-myc–tagged spastin-S268A or spastin-WT, treated with 25 μg/ml cycloeximide 36 h posttransfection and analysed by WB at indicated times after treatment. Note that to minimize differences in spastin levels at the time 0, cells were transfected with different doses of the expressing vectors, that is, 1 μg of spastin-S268A–expressing vector and 0.5 μg of spastin-WT–expressing vectors. Representative WB is shown. The levels of spastin-Flag bands relative to those of GAPDH were measured at each time point and reported as mean ± SEM of four different independent experiments in the right panel. Statistical differences were calculated and reported for each time point, unpaired t test.
Source data are available for this figure.
HIPK2-dependent S268 phosphorylation prevents spastin polyubiquitination and degradation
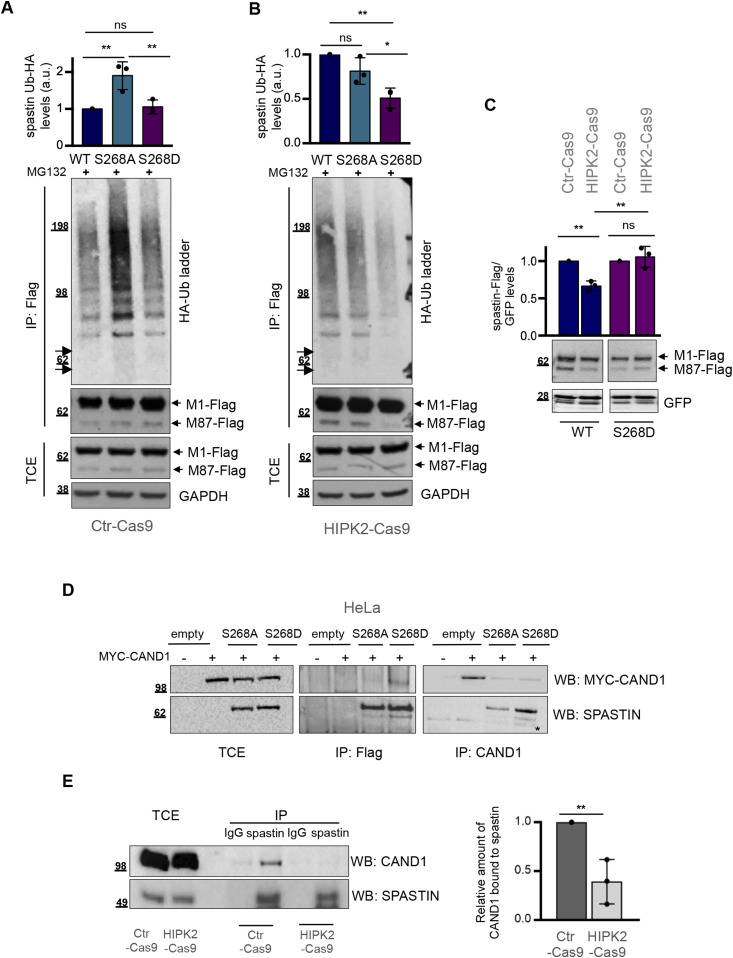
Next, we asked whether S268 phosphorylation is required to prevent spastin polyubiquitination. Thus, we compared on the same blot the polyubiquitination levels among WT, non-phosphorylatable, and phosphomimetic spastin forms in the presence or absence of HIPK2. For clarity, we have presented these data in two separate panels (Fig 4A and B). The non-phosphorylatable spastin-S268A mutant is the most polyubiquitinated in HIPK2 proficient Ctr-Cas9 cells (Fig 4A), whereas the phosphomimetic spastin-S268D mutant is the least polyubiquitinated in the HIPK2-Cas9 cells (Fig 4B), indicating that HIPK2-mediated S268-phosphorylation prevents spastin polyubiquitination. Accordingly, when transfected at comparable efficiency in HIPK2 proficient and in HIPK2-KO cells, the phosphomimetic spastin-S268D mutant, but not the WT form, is resistant to degradation (Figs 4C and S3). Overall, these findings indicate that HIPK2-mediated S268 phosphorylation protects spastin from polyubiquitination at K554 and degradation.
Figure 4. Phosphorylation in S268 prevents spastin polyubiquitination and degradation.
(A, B) HeLa Ctr-Cas9 (A) and HIPK2-Cas9 (B) cells were transfected with vectors expressing indicated flag-myc–tagged spastin-WT or its derivative mutants in combination with the vector expressing HA-tagged Ub-WT and treated 24 h posttransfection with 20 μM MG132 for 8 h. Total cell extracts (TCEs) were analysed by Western blot (WB) and IP as in Fig 2F. Samples were processed in parallel and analysed on the same blot to make comparison between HIPK2 proficient and null cells. The arrows indicate the position of unmodified spastin isoforms. The intensity of spastin-Ub-HA ladder was normalized by the intensity of spastin-Flag bands in IP and reported as mean ± SD (n = 3). Statistical differences, ANOVA test. (C) HeLa Crt-Cas9 and HIPK2-Cas9 cells were transfected with vectors expressing indicated flag-myc–tagged spastin WT or its derivative S268D in combination with peGFP vector as in Fig 2G and analysed by WB 24 h post transfection. GFP expression was used as internal control for transfection efficiency. Representative WB is shown. The intensity of spastin-Flag bands was normalized by the intensity of GFP and reported relative to Ctr-Cas9 control cells. Statistical differences, Anova test. (D) Representative Co-IP showing that spastin-S268D preferentially binds CAND1. HeLa cells were co-transfected with the plasmid expressing MYC-CAND1 in combination with empty vector or vectors expressing spastin-S268A or spastin-S268D. Cells were collected 24 h posttransfection. TCE were analysed by WB or immunoprecipitated with anti-Flag or anti-CAND1 Abs and analysed as indicated. The asterisk indicates an aspecific band. TCE and IP samples were loaded on the same gel and processed on the same filter. Blots were vertically cropped to show appropriate expositions. Full blots are shown in the source data F4 file. (E) Co-IP showing that spastin interaction with CAND1 is stronger in HeLa Ctr-Cas9 cells compared with HIPK2-Cas9 cells. TCE from Ctr-Cas9 and HIPK2-Cas9 cells were analysed by WB or immunoprecipitated with anti-spastin Ab and analysed with indicated Abs. IgG immunoglobulins were used as IP negative control. Band intensities of co-immunoprecipitated CAND1 were normalized by band intensities of spastin immunoprecipitated and their relative values are reported as mean ± SD (n = 3). Statistical difference, unpaired t test.
Source data are available for this figure.
Figure S3. Comparison of spastin-Flag WT and S268D levels in HeLa Ctr-Cas9 cells.
Data quantification relative to Fig 4C. Spastin-Flag intensity relative to our internal efficiency transfection control (GFP) was reported relative to M87-spastin-Flag, M1-spastin-Flag and to spastin-Flag (i.e., M1+ M87). Statistical differences, unpaired t test.
We next addressed the mechanism by which S268 phosphorylation regulates ubiquitination. We hypothesised that S268 phosphorylation can protect spastin from polyubiquitination by impairing the recruitment of proteins belonging to the ubiquitination pathway or by promoting interactions with factors that mask the domain of spastin necessary for efficient ubiquitination/degradation. To investigate this, we used MS to analyse the interactome of exogenous spastin-S268A and spastin-S268D in HeLa cells (Supplemental Data 1 (27.8KB, xlsx) ). Among the known interacting proteins, we found atlastin (31), and spastin itself (7, 8, 32). Interestingly, we found Cullin-Associated NEDD8-Dissociated Protein 1 (CAND1) as a unique spastin-S268D interactor and this preferential interaction was confirmed by co-IP experiments (Fig 4D). Next, we verified spastin/CAND1 endogenous interaction (Fig 4E). According to exogenous co-IP results, we observed that the interaction is stronger in HIPK2 proficient cells than in HIPK2-Cas9 cells, further supporting a preferential interaction between CAND1 and phosphorylated spastin (Fig 4E). CAND1 is a known inhibitor of Cullin-RING ubiquitin E3-Ligase (CRL) complexes (33), its interaction with spastin suggests involvement of the neddylation pathway in spastin degradation via UPP.
LSA-2020-00799_Supplemental_Data_1.xlsx (27.8KB, xlsx) Identification of proteins contained in spastin-S268A and spastin-S268D immunoprecipitates from HeLa cells. The original list of proteins was filtered to include only proteins that are absent from the negative control experiments.
Spastin levels and neurite defects can be restored by preventing neddylation-dependent degradation in different SPG4-HSP models
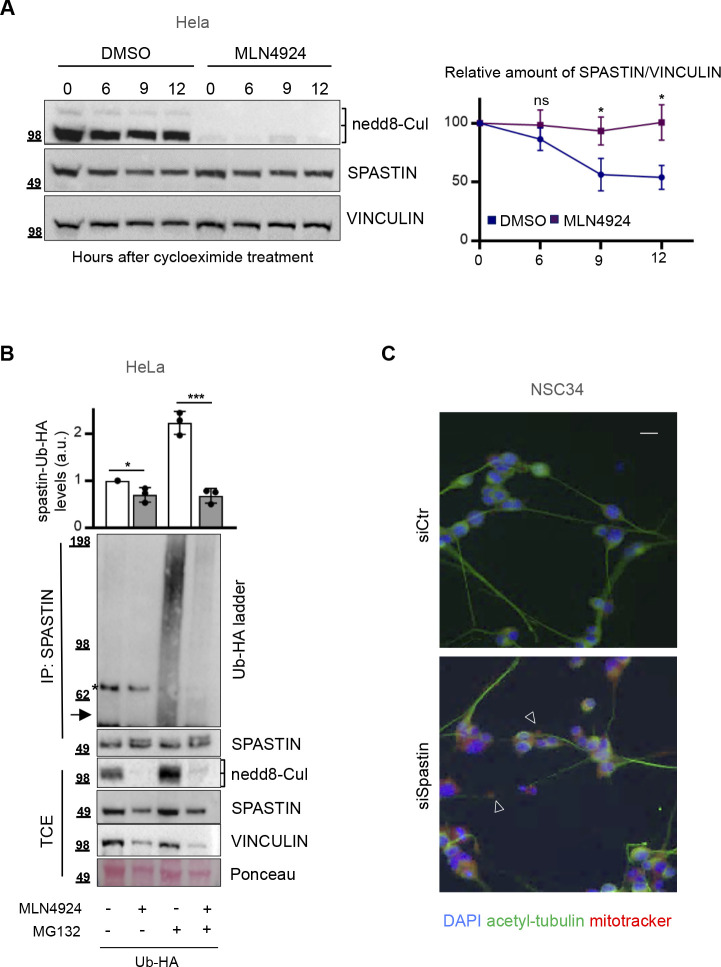
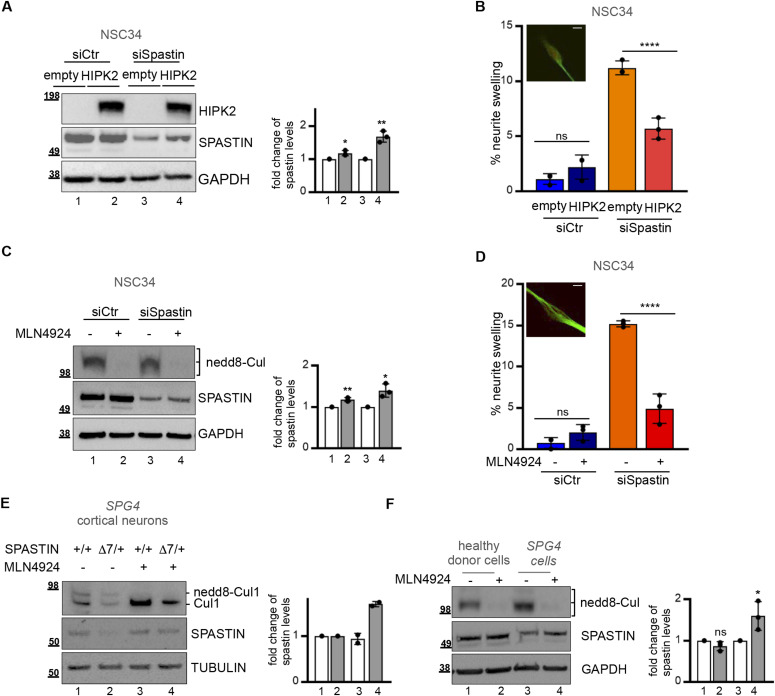
To investigate the biological relevance of HIPK2-mediated spastin protein regulation, we analysed the effects of neddylation-mediated UPP inhibition and HIPK2 overexpression on spastin protein levels in pathological conditions due to spastin reduction. We hypothesised that as HIPK2 expression, the neddylation inhibitors, such as MLN4924/pevonedistat (33, 34), would stabilize spastin preventing its degradation and subsequently rescue the pathological phenotypes associated to spastin reduction. To test this hypothesis, first we verified whether MNL4924 treatment stabilizes spastin by preventing its polyubiquitination (Fig S4A and B). Next, we mimicked typical pathological SPG4-HSP phenotypes depleting spastin via RNAi in NSC34 differentiated cells. As expected, spastin reduction caused neurite swelling defects (5, 23; Fig S4C) and we observed and quantify these defects (Fig 5B and D, see the light orange columns). In this spastin RNAi neuronal cell model, we observed that HIPK2 overexpression (Fig 5A) or treatment with a low non-toxic dose of MLN4924 (Fig 5C) led to an increase in spastin protein levels and a rescue of neurite swelling (Fig 5A–D), confirming our hypotheses. These results provide evidence that targeting the HIPK2/spastin axis might be a strategy to develop spastin-elevating therapeutic approaches.
Figure S4. MLN4924 prevents spastin polyubiquitination stabilizing its protein levels.
(A) HeLa cells were treated with 25 μg/ml cycloeximide and 0.1 μM MLN4924 or its solvent DMSO and analysed by Western blot (WB) at indicated times after treatment. Representative WB of four different experiments is reported. Cullin deneddylation is shown as MNL4924 positive control and vinculin is shown as loading control. The levels of spastin bands relative to those of vinculin were measured at each time point and reported as mean ± SEM of four different independent experiments in the right panel. Statistical differences were calculated and reported for each time point, unpaired t test. (B) HeLa cells were transfected with vectors expressing Ub-HA and pretreated 6 h posttransfection with 0.1 μM MLN4924 or its solvent, DMSO, for 16 h and treated 24 h posttransfection with 20 μM MG132 or its solvent DMSO for additional 8 h. As in Fig 2C, total cell extracts were immunoprecipitated with anti-spastin mouse monocolonal Ab, sp3G11/1. Total cell extracts and IPs were analysed by WB with anti-HA and anti-spastin Abs. Representative WB of three independent experiment is shown. The arrow indicates the position of unmodified spastin protein and the asterisk indicates a non-specific band. Cullin deneddylation is shown as MNL4924 positive control; Ponceau staining is shown as further loading control beside vinculin immunostaining. The intensity of spastin-Ub-HA ladder was normalized by the intensity of spastin bands in IP and reported relative to the DMSO-treated sample as mean ± SD (n = 3). Statistical differences, unpaired t test. (C) NSC34 cells were transfected as in Fig 5A, incubated in differentiation medium and analysed by IF with anti-acetylated-tubulin Ab (green) and mitotracker (red) 5 d after siRNA transfection and differentiation for neurite swelling evaluation. Representative fields of indicated cells are reported. The arrowheads indicate neurite swelling. Scale bar, 10 μM.
Figure 5. Restoration of spastin protein levels and neurite swelling in SPG4-(HSP) Hereditary Spastic Paraplegia models.
(A, B) NSC34 cells were transfected with 40 nM spastin-specific (siSpastin) or negative control stealth siRNAs and incubated in differentiation medium. 48 h post siRNA transfection cells were transfected with empty or HIPK2 expressing vectors and analysed by Western blot (WB) and by IF using anti-acetylated-tubulin Ab (green) and mitotracker (red) 5 d after siRNA transfection and differentiation. (A) Representative WB is shown in (A); the intensity of the spastin bands were quantified, normalized and reported relative to empty vector in siCtr and in siSpastin cells as mean ± SD of three independent experiments. Statistical differences, unpaired t test. (B) The percentage of swelling is reported in (B) as mean ± SD of three different experiments in which >100 cells were analysed. Statistical differences, ANOVA test. Representative image of neurite swelling is shown. Scale bar, 10 μM. (A, C, D) NSC34 cells were transfected as in (A) and incubated in differentiation medium. 72 h post siRNA transfection cells were treated with MLN4924 0.1 μm or its solvent DMSO and analysed 16 h after treatment. (C) Representative WB is shown in (C). Cullin deneddylation is shown as MNL4924 positive control. The intensity of the spastin bands were quantified, normalized and reported relative to solvent-treated cells in siCtr and in siSpastin as mean ± SD of three independent experiments. Statistical differences, unpaired t test. (D) The percentage of swelling is reported in (D) as mean ± SD of three different experiments in which >100 cells were analysed. Statistical differences, Anova test. Representative image of neurite swelling is shown. Scale bar, 10 μM. No dead cells were observed after treatment. (E) Representative WB showing the increase of endogenous spastin protein levels 72 h after 0.1 μM MLN4924 treatment in indicated primary cortical neurons derived from SPG4 WT (+/+) and heterozygous (+/Δ7) E5 mice. The intensity of the spastin bands were quantified, normalized and reported relative to solvent-treated cells in neurons derived from SPG4 WT (+/+) and heterozygous (+/Δ7) as mean ± SD of two independent experiments. (F) Representative WB showing the increase of endogenous spastin protein levels 16 h after 0.1 μM MLN4924 treatment in SPG4-HSP lymphoblastoid cells. The intensity of the spastin bands were quantified, normalized and reported relative to solvent-treatment in healthy donors lymphoblastoid cells and in patient’s derived SPG4-HSP lymphoblastoid cells. Statistical differences, unpaired t test.
Source data are available for this figure.
Relevant from a clinical point of view, MLN4924 is a CNS-penetrant drug that is currently in several phase 1–3 clinical trials for different malignancies (https://clinicaltrials.gov/ct2/results?term=pevonedistat). Thus, we evaluated whether MLN4924 treatment is able to increase the levels of normal spastin allele in the context of pathological heterozygous SPG4-HSP mutations. To address this issue, we used two different SPG4-HSP models. First, cortical neurons explanted from heterozygous ΔE7 SPG4 mice and second patient’s derived human lymphoblastoid cells carrying heterozygous c751insA mutation. Both models express approximately 50% levels of spastin WT and undetectable levels of truncated proteins (23 and data not shown). MLN4924 treatment increased spastin protein levels in both models (Fig 5E and F). Importantly, the increase in spastin levels produced by MNL4924 treatment restored levels close to those of control cells (Fig 5E and F), demonstrating that MLN4924 is able to restore para-physiological spastin levels in SPG4-HSP haploinsufficient context.
Discussion
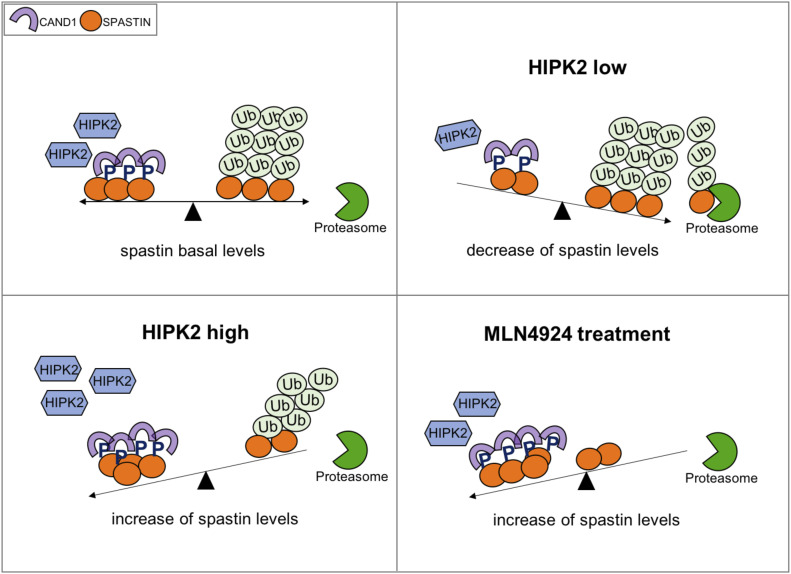
We have identified and characterized a pathway responsible for the stability of the spastin protein, which is mutated in the most common form of autosomal dominant HSP, and exploited this knowledge to propose a novel approach for corrective elevation of spastin levels in disease by preventing its neddylation-dependent degradation. In particular, the present study shows that HIPK2-mediated phosphorylation of spastin at S268 inhibits spastin K48-polyubiquitination at K554 and prevents its neddylation-dependent proteasomal degradation, correlating HIPK2-mediated phosphorylation and spastin stability. Our findings support the hypothesis that spastin protein levels are controlled by a balance between the S268 phosphorylation and polyubiquitination/degradation processes (Fig 6).
Figure 6. Schematic model depicting the regulation of spastin protein levels.
Because S268-phosphorylation (indicated with a blue P) prevents polyubiquitination of spastin, its protein levels are the consequence of a balance between phosphorylation and degradation events depending on HIPK2 kinase activity and the function of a still unknown CRL complex. Based on this model, low HIPK2 activity reduces the spastin phosphorylated forms leading to an increase of spastin polyubiquitination with a consequent decrease of spastin protein levels due to proteasomal degradation. In contrast, high HIPK2 activity protects spastin from polyubiquitination, increasing its protein levels. When polyubiquitination is prevented using the neddylation inhibitor, MLN4924, spastin protein levels increase independently of its phosphorylation status.
The precise mechanism by which S268 phosphorylation protects from polyubiquitination remains to be further characterized. The preferential interaction of phosphorylated spastin with CAND1, which functions as substrate receptor exchange factor for CRL1 and 4 complexes (34, 35, 36), supports a model in which the phosphorylation of spastin might alter the dynamic equilibrium with its specific receptor. Therefore, we hypothesized that the enhanced CAND1/spastin interaction might induce a decrease of the exchange possibility necessary for the active CRL complex formation. Nevertheless, we cannot exclude that binders of the p-S268 spastin might act sterically inducing conformational changes that hide site/s for successful ubiquitination. Interestingly, katanin, a MT-severing enzyme belonging to the same ATPase family as spastin, is regulated via phosphorylation/polyubiquitination crosstalk involving similar regions of the protein as we describe for spastin (37, 38, 39), opening the possibility of a more general mechanism for spatio-temporal regulation of ATPases presenting the same domain architecture as spastin and katanin.
Furthermore, because both spastin and HIPK2 have been shown to play roles in different biological processes, our data open the possibility that HIPK2-mediated spastin regulation can function as a fine-tuning regulator in multiple spastin-dependent processes, such as nuclear envelope resealing, cytokinesis and trafficking (1, 3). Recently, spastin severing activity was shown to elicit two different outcomes: the extraction of tubulin subunit from the MT might be counteract by spontaneous incorporation of soluble GTP-tubulin or provoke the severing when extraction outpaces repair (7). We might hypothesize cellular modulation of these two outcomes by locally controlling spastin protein levels acting on the balance between its phosphorylation and ubiquitination/degradation (Fig 6). Further layers of complexity are conceivable considering that spastin levels appear strictly regulated to avoid its accumulation, likely via the action of specific phosphatase/s, deubiquitinase/s, and other factors regulating spastin expression.
Curative therapies and approaches to manage HSP diseases are completely lacking and therapy is only symptomatic. Based on literature evidence (17) and our findings, we hypothesized that elevating spastin by inhibiting its neddylation-dependent degradation might be a novel possible therapeutic approach in SPG4-HSP. The existence of a CNS-penetrant drug, such as MLN4924, that (i) blocks NEDD8-activating enzyme, (ii) is currently in several clinical trials, (iii) has been shown to increase the survival of Spinal Muscolar Atrophy- and Amyothrophic Lateral Sclerosis-derived motorneurons and ameliorate the in vivo phenotype of Spinal Muscolar Atrophy mouse model (40), prompted us to directly assess whether this drug increases the levels of functional spastin in SPG4-HSP haploinsufficient contexts. Our findings indicate that MNL4924 might be a novel spastin-elevating compound, without adverse effects on cell viability.
There are several limitations in this study. We have to consider that neddylation-dependent degradation controls a broad set of proteins and preclinical studies in opportune animal models have to be carried out to assess whether MNL4924 is effective in relieving HSP pathological features. SPG4-HSP shows mild phenotype in homozygote mouse models compared with the severity of the symptoms in HSP patients. The mouse model that we used in this study was an essential mammalian model to be tested for a rescue of spastin levels in neurons. However, its “very weak” swelling phenotype in heterozygous mutant neurons prevents us from assessing the rescue of axon swelling phenotype. Therefore, studies on animal models with a stronger phenotype at the heterozygous status (such as, Drosophila melanogaster or Caenorhabditis elegans) would be required to fully validate the therapeutic impact of this compound.
Spastin-elevating approaches are not the best therapeutic strategy for the less common missense variants of spastin, acting with pathogenic mechanism other than haploinsufficiency (e.g., dominant-negative or gain-of-function mechanisms). However, it will be interesting to assess the response to UPP of each of these variants. We might speculate to extend this approach to those that show a partial loss of activity triggering degradation. Spastin WT stabilization could alleviate also the pathogenicity of mutants resulting in loss of ATPase activity, such as the I344K variant (41). Preclinical studies with MT-targeting drugs have shown preliminary promising effects for HSP treatment (42, 43), the effects of very low dose of these drugs combined with spastin inhibition of degradation might be also evaluated.
Finally, the possibility to pharmacologically modulate spastin levels opens the way to potential clinical implications also in promoting axon regeneration after nerve injury (6, 44) and in neurodegenerative diseases associated to spastin dysfunctions, such as Alzheimer’s disease (45, 46).
In conclusion, we have identified and characterized a novel pathway regulating spastin protein levels via HIPK2-mediated phosphorylation that prevents spastin neddylation-dependent degradation. Intriguingly, we show that inhibition of neddylation by using the CNS penetrant drug MLN4924 is able to restore spastin levels and rescue neurite defects. Even though many factors have to be considered before initiating preclinical studies for MLN4924 repurposing in SPG4-HSP, our novel findings provide proof of principle that elevating spastin by inhibiting its UPP-mediated degradation has therapeutic potential for SPG4-HSP patients.
Materials and Methods
Cells, culture conditions, and treatments
HeLa (a gift of N Corbi), A549 (a gift of R Falcioni), U2OS (a gift of F Moretti), and retinal pigment epithelial (a gift of G Guarguaglini) were cultured at 37°C and 5% CO2 in DMEM GlutaMAX supplemented with 10% heat-inactivated FBS (Life Technologies); mouse motoneuronal NSC34 (a gift of M Cozzolino) was cultured in DMEM-F12 1:1, supplemented with 10% FBS. Patient-derived lymphoblastoid cells, carrying the pathogenetic heterozygous c751insA SPG4 mutation, were cultured in RPMI supplemented with 10% FBS. To obtain homogeneous neuronal cell cultures, SNSC34 were differentiated by incubation in serum-free medium supplemented daily with 10 μM retinoic acid (Sigma-Aldrich) and 10 ng/ml GDNF (Peprotech). HeLa HIPK2 null (HIPK2-Cas9) and their parental control (Ctr-Cas9) cells (47) were kindly provided by ML Schmitz. Cells were routinely tested for mycoplasma contamination.
Mice carrying Hipk2 KO first conditional-ready alleles (Hipk2KOF/KOF; https://www.mousephenotype.org/data/genes/MGI:1314872) were generated by the International KO mouse consortium by inserting into the HIPK2 loci a lacZ cassette, which traps and truncates the nascent transcript, leading to 80% of Hipk2 mRNA reduction. Primary cortical neurons from CRE-inducible Hipk2 KO alleles (Hipk2cKO/cKO) were obtained by inbreeding Hipk2KOF/KOF mice with mice carrying FLP recombinase (https://www.jax.org/strain/005703), and neurons from SPG4 ΔE7/+ and +/+ mice (23) were prepared and cultured as previously described in reference 23. Adenovirus expressing CRE is a kind gift of D Pajalunga.
The following inhibitors were used: MG132 and cycloeximide (Sigma-Aldrich) and MLN4924 (Cayman Chemical). For the evaluation of cell viability, trypan blue dye exclusion test was used to determine the number of viable cells.
RNAi and real-time RT-PCR
HIPK2 RNAi was obtained in human cells by using specific stealth siRNAs (a mix of three different validated siRNAs by Life Technologies, as in reference 48) or by transfecting HIPK2-specific sh-RNA–expressing vectors (a mix of pRetroSuper-1376 and -789, (49)). HIPK2 RNAi in murine cells and spastin RNAi in human cells were obtained by using a mix of specific validated stealth siRNAs by Life Technologies; for spastin, RNAi-specific siRNAs were selected among which targeting sequences common to all spastin isoforms as in reference 26. siRNAs were transfected using Lipofectamine RNAi MAX (Life Technologies). RNA extraction and real-time RT-PCR were performed as in reference 48, and relative fold-change were determined by the 2−ΔΔCt method using GAPDH or actin mRNA as normalizer. All reactions were performed in triplicate, in three independent experiments. Primers for HIPK2 and spastin amplification are as references 48 and 50, respectively. The spastin primers amplify a region common to all spastin isoforms. The sequences of the siRNAs used are the following:
murine-specific siHIPK2 #1: CCACCAACUUGACCAUGACCUUUAA; #2: GAGCCAAGUUCCAACUGGGACAUGA; #3: GCCCAUGUCAAAUCUUGUUUCCAAA.
human-specific siHIPK2 #1: CCCGAGUCAGUAUCCAGCCCAAUUU; #2: CCACCAACCUGACCAUGACCUUUAA; #3: CAGGGUUUGCCUGCUGAAUAUUUAU.
human/murine-specific siSpastin #1: CCAGUGAGAUGAGAAAUAUUCGAUU, #2 CGGACGUCUAUAACGAGAGUACUAA.
Expression vectors
The following plasmids were used: peGFP-c2 (Clontech); peGFP-human-HIPK2, flag empty and flag-HIPK2, pET-HA, pET-human-HIPK2-HA, and pET-human-HIPK2-K228A-HA (Kind gifts of ML Schmitz); pSUPER-LacZ and pSUPER-HIPK2-1376 and pSUPER-HIPK2-789 (49), pEF-HA-Ub-WT, and pEF-HA-KoUb (kind gift of A Pollice); pRK5-HA-Ub-K48, pRK5-HA-Ub-WT, and pcDNA3-Myc3-CAND1 (Addgene); Flag-myc empty vector (pCMV6-Entry) and flag-myc–tagged spastin expressing vector (OriGene Technologies). The latter vector expresses higher levels of the M1 isoform than the corresponding M87, in agreement with data showing that M1 is the most abundant form when both isoforms have equally good Kozak’s sequences (51). Spastin-S268A and Spastin-S268D mutants were described in reference 26, and spastin-K554R was obtained by site-directed mutagenesis in the spastin-flag-myc–tagged vector using the QuikChange Lightning Kit (Stratagene) and analysed by sequencing. Vectors were transfected by using Lipofectamine LTX and Plus reagent (Life Technologies).
Western blot (WB) and immunopecipitation (IP)
Total cell extracts were prepared in RIPA buffer (50 mM Tris–HCl, pH 8, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP40, and 1 mM EDTA) supplemented with protease and phosphatase inhibitors (Roche). For ubiquitination assays, we first performed WB analysis with anti-spastin or anti-Flag antibodies (Abs) on 1/15 of our IP reactions to determine the efficiency of IP in each sample. After this quantification, we loaded similar levels of immunoprecipitated spastin and performed WB with anti-ubiquitin (Ub) or anti-HA Abs, depending on the experimental conditions. Proteins were resolved by SDS–PAGE using Bolt Novex Bis-Tris Gels 4–12% gradient or 8% (Life Technologies). Immunoreactivity was determined using ECL-Prime (Amersham), and image acquisition and densitometric analysis were performed with Image Lab software (Bio-Rad). The following antibodies were used: anti-HIPK2 (rat monoclonal Ab C5C6 kindly provided by ML Schmitz); anti-GAPDH #sc-32233 (1:1,000), anti-α-tubulin #sc-5286 (1:1,000), anti-vinculin #73614 (1:1,000), anti-spastin mouse monoclonal Abs (1:100; sp3G11/1 #sc-53443 or sp6c6 #sc-81624), and anti-spastin goat polyclonal Ab #sc-49528 (1:1,000) by Santa Cruz Biotechnology (anti-spastin monoclonal Abs were indifferently used because they produce comparable results, whereas anti-spastin polyclonal Ab was used by WB to detect immunoprecipitated spastin in murine cells); anti β3-tubulin #5568 (1:1,000) and anti-MYC #2276 (1:500) mouse monoclonal Abs (Cell Signaling Technology); anti-CAND1 #NBP1-49918 (1:1,000) rabbit polyclonal Ab (Novus Biological); anti-Ub #BML-PW8810-0100 (1:1,000, clone FK2; Enzo Life Sciences); anti-HA #11583816001 (1:1,000) mouse monoclonal Ab by Roche; anti-GFP (1:800, mouse monoclonal Ab by Roche or 1:200 #sc-390394 rabbit polyclonal Ab by Santa Cruz Biotechnology); anti-Flag (1:1,000; rabbit polyclonal Ab, #F7425, by Sigma-Aldrich or mouse monoclonal Ab, #TA50011; OriGene Technologies); anti-Nedd8 #ab81264 (1:500) rabbit monoclonal Ab by Abcam, anti-Cul1 #612040 (1:500) mouse monoclonal Ab by BD bioscience; and anti–HRP-conjugated goat anti-mouse #7076, anti-rat #7077, and anti-rabbit #7074 (Cell Signaling Technology).
Normal mouse IgG #sc-2025 by Santa Cruz Biotechnology was used as isotype control immunoglobulins in IP experiments.
MS analysis
24 h after transfection with flag-myc–tagged spastin-S268A or -S268D expressing vectors in HeLa cells extracts were obtained by lysing the cells in RIPA buffer supplemented with protease and phosphatase inhibitors (Roche). IP was performed by incubation of 2 mg of total cell extract with anti-flag Ab (mouse Ab by Origene Technologies) covalent coupled to Dynabeads M-280 Tosylactivated according to the according to manufacturer’s protocol (Life Technologies). Beads coupled to an unrelated Ab (i.e., anti-E1A mouse monoclonal Ab; BD Bioscience) were used as negative control. Each sample was separated on a 1D-gel NuPAGE 4–12% (Invitrogen), run in MOPS buffer, and stained with the Colloidal Blue Staining kit (Invitrogen). Gel lanes were entirely subdivided into slices and subjected to reduction, alkylation, and trypsin digestion (52). The resulting peptide mixtures were desalted in a trap column (Acclaim PepMap 100 C18, LC Packings; DIONEX), and then separated in a 10-cm reverse-phase column (Silica Tips FS 360-75-8; New Objective) and slurry-packed in house with 5 μm, 200. Å pore size C18 resin (Michrom BioResources). Separation was performed with an Ultimate 3000 nanoflow HPLC (DIONEX) connected with an LTQ-XL mass spectrometer (ThermoElectron), equipped with a nano-electrospray ion source (ESI).
The list of identified interactors by one experimental replicate has been deposited to the ProteomeXchange Consortium (see the Data Availability section).
Immunofluorescence (IF) and swelling analysis
Cells were seeded onto poly-L-lysine–coated coverslips, fixed in 2% formaldehyde, permeabilized in 0.25% Triton X-100 in PBS for 10 min, and then blocked in 5% bovine serum albumin in PBS before the primary Ab was applied. The following Abs were used: anti β3-tubulin #5568 (1:500 mouse monoclonal Abs by Cell Signaling Technology) and anti-acetyl-α-tubulin #T7451 (1:500 mouse monoclonal Ab by Sigma-Aldrich). Secondary mouse FITC Ab #A32723 (Alexa-fluor; Life Technologies) was used. DNA was marked with DAPI (Sigma-Aldrich) and mitochondria with mitotracker Red #M22425 (Life Technologies). Cells were examined under inverted microscope (Eclipse Ti; Nikon) using a Clara camera (ANDOR Technology). Images for each sample were taken in parallel using identical microscope by Nis-Elements H.C. 5.11 using the JOBS module for automated acquisitions. Swelling quantitative analysis was performed as in reference 23. Briefly, swelling was defined along the neurite revealed with acetyl-tubulin and inside mitochondrial staining as larger than 2 μm in diameter; random fields were analysed after staining with mitotracker and the number of swelling scored per at least 100 DAPI stained nuclei.
Genotyping PCR
Genomic DNA was extracted using DirectPCR lysis reagent (Viagen), and PCR analysis was carried out using GoTaq DNA polymerase (Promega) following the supplier’s conditions. Alleles were identified using the following primers: for Hipk2 wild type (Forward 5′-CGATCGAGTAAGGGTCGGTG-3′, Reverse 5′-GATGTGTGCTTGAGGCTTGC-3′); for Hipk2 KOF (Forward 5′-TTATGGTCTGAGCTCGCCATCAGT-3′, Reverse 5′-TGTTTCTCCATGGACAGTGGGTT-3′); for Hipk2 cKO and KO alleles (distinguishable by different amplicon size; Forward 5′-AAGGCGCATAACGATACCACG-3′, Reverse 5′-CTGTTTCTCCATGGACAGTGGGTT-3′).
Statistical analysis
Data analyses were performed using the GraphPad Prism software. Each experiment has been repeated from three to four times and the results obtained presented as mean ± SD, unless otherwise indicated. The unpaired t test or ANOVA test were applied to determine the significance of quantitative experiments. Statistical significance was set at P < 0.05 and was reported by asterisks according to the following scheme ****P < 0.00001, ***P < 0.0001, **P < 0.001, and *P < 0.05.
Ethics statement
All mouse experimental procedures were conformed to protocols approved by the Regina Elena National Cancer Institute Animal Care and Use Committee and were performed in accordance with the Guide for the care and use of laboratory animals and the guidelines of the National Institutes of Health, according to the current National Legislation (Art. 31 D.lgs 26/2014, 4 March, 2014) and were approved by National Institutes of Health (1056/2015). The ethics committee of Asl Roma 2, Rome, Italy, approved this study on patient derived cells (0074975/2020). Written informed consent was obtained and data analysis was performed anonymously.
Data Availability
MS data have been deposited to the ProteomeXchange Consortium via the partner repository PRIDE (53) with the data set identifier PXD021945.
Supplementary Material
Acknowledgements
We thank Embo for short-term mobility fellowship to F Sardina. We are grateful to all people cited in the text for their gifts of cells and reagents. We thank Dr F Magi, Dr. B Ciani, and Dr. G Della Zanna for their help and advice. Funding sources: This work was supported by grants from Fondazione Telethon (GGP16072), AFM-Telethon (#22157), Latium Region (85-2017-15348), UK-HSP group, and Italian Association for Cancer Research to C Rinaldo (IG #17739).
Author Contributions
F Sardina: conceptualization, data curation, formal analysis, funding acquisition, investigation, and writing—review and editing.
A Pisciottani: investigation and methodology.
M Ferrara: investigation.
D Valente: investigation and methodology.
M Casella: investigation and methodology.
M Crescenzi: supervision.
A Peschiaroli: supervision and methodology.
C Casali: resources and methodology.
S Soddu: data curation, supervision, and writing—review and editing.
AJ Grierson: resources, supervision, and writing—review and editing.
C Rinaldo: conceptualization, data curation, formal analysis, supervision, funding acquisition, and writing—original draft.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- 1.Connell JW, Lindon C, Luzio JP, Reid E (2009) Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic 10: 42–56. 10.1111/j.1600-0854.2008.00847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison R, Lumb JH, Fassier C, Connell JW, Martin DT, Seaman MN, Hazan J, Reid E (2013) An ESCRT-spastin interaction promotes fission of recycling tubules from the endosome. J Cell Biol 202: 527–543. 10.1083/jcb.201211045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H (2015) Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 522: 231–235. 10.1038/nature14408 [DOI] [PubMed] [Google Scholar]
- 4.Trotta N, Orso G, Rossetto MG, Daga A, Broadie K (2004) The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol 14: 1135–1147. 10.1016/j.cub.2004.06.058 [DOI] [PubMed] [Google Scholar]
- 5.Riano E, Martignoni M, Mancuso G, Cartelli D, Crippa F, Toldo I, Siciliano G, Di Bella D, Taroni F, Bassi M, et al. (2009) Pleiotropic effects of spastin on neurite growth depending on expression levels. J Neurochem 108: 1277–1288. 10.1111/j.1471-4159.2009.05875.x [DOI] [PubMed] [Google Scholar]
- 6.Stone MC, Rao K, Gheres KW, Kim S, Tao J, La Rochelle C, Folker CT, Sherwood NT, Rolls MM (2012) Normal spastin gene dosage is specifically required for axon regeneration. Cell Rep 2: 1340–1350. 10.1016/j.celrep.2012.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vemu A, Szczesna E, Zehr E, Spector J, Grigorieff N, Deaconescu A, Roll-Mecak A (2018) Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 361: 6404 10.1126/science.aau1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandate RC, Szyk A, Zehr AE, Lande CG, Roll-Mecak A (2019) An allosteric network in spastin couples multiple activities required for microtubule severing. Nat Struct Mol Biol 26: 671–678. 10.1038/s41594-019-0257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han H, Schubert HL, McCullough J, Monroe N, Purdy MD, Yeager M, Sundquist WI, Hill CPJ (2020) Structure of spastin bound to a glutamate-rich peptide implies a hand-over-hand mechanism of substrate translocation. J Biol Chem 295: 435–443. 10.1074/jbc.ac119.009890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claudiani P, Riano E, Errico A, Andolfi G, Rugarli E (2005) Spastin subcellular localization is regulated through usage of different translation start site and active export from the nucleus. Exp Cell Res 309: 358–369. 10.1016/j.yexcr.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 11.Mancuso G, Rugarli E (2008) A cryptic promoter in the first exon of the SPG4 gene directs the synthesis of the 60-kDa spastin isoform. BMC Biol 6: 31 10.1186/1741-7007-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solowska JM, Garbern JY, Baas PW (2010) Evaluation of loss of function as an explanation for SPG4-based hereditary spastic paraplegia. Hum Mol Genet 19: 2767–2779. 10.1093/hmg/ddq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazan J, Fonknechten N, Mavel D, Paternotte C, Samson D, Artiguenave F, Davoine CS, Cruaud C, Dürr A, Wincker P, et al. (1999) Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat Genet 23: 296–303. 10.1038/15472 [DOI] [PubMed] [Google Scholar]
- 14.Lindsey JC, Lusher ME, McDermott CJ, White KD, Reid E, Rubinsztein DC, Bashir R, Hazan J, Shaw PJ, Bushby KM (2000) Mutation analysis of the spastin gene (SPG4) in patients with hereditary spastic paraparesis. J Med Genet 37: 759–765. 10.1136/jmg.37.10.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soderblom C, Blackstone C (2006) Traffic accidents: Molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol Ther 109: 42–56. 10.1016/j.pharmthera.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 16.Blackstone C. (2012) Cellular pathways of hereditary spastic paraplegia. Ann Rev Neurosci 35: 25–47. 10.1146/annurev-neuro-062111-150400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solowska JM, Baas PW (2015) Hereditary spastic paraplegia SPG4: What is known and not known about the disease. Brain 138: 2471–2484. 10.1093/brain/awv178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bürger J, Fonknechten N, Hoeltzenbein M, Neumann L, Bratanoff E, Hazan J, Reis A (2000) Hereditary spastic paraplegia caused by mutations in the SPG4 gene. Eur J Hum Genet 8: 771–776. 10.1038/sj.ejhg.5200528 [DOI] [PubMed] [Google Scholar]
- 19.Shoukier M, Neesen J, Sauter SM, Argyriou L, Doerwald N, Pantakani DV, Mannan AU (2009) Expansion of mutation spectrum, determination of mutation cluster regions and predictive structural classification of SPAST mutations in ereditary spastic paraplegia. Eur J Hum Genet 17: 187–194. 10.1038/ejhg.2008.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinnery PF, Keers SM, Holden MJ, Ramesh V, Dalton A (2004) Infantile hereditary spastic paraparesis due to codominant mutations in tht spastin gene. Neurology 63: 710–712. 10.1212/01.wnl.0000135346.63675.3e [DOI] [PubMed] [Google Scholar]
- 21.Svenson IK, Kloos MT, Gaskell PC, Nance MA, Garbern JY, Hisanaga S, Pericak-Vance MA, Ashley-Koch AE, Marchuk DA (2004) Intragenic modifiers of hereditary spastic paraplegia due to spastin gene mutations. Neurogenetics 5: 157–164. 10.1007/s10048-004-0186-z [DOI] [PubMed] [Google Scholar]
- 22.Tarrade A, Fassier C, Courageot S, Mailly P, Dalard C, Delga S, Roblot N, Lefèvre J, Job D, Hazan J, et al. (2006) A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubulecomposition. Hum Mol Genet 24: 3544–3558. 10.1093/hmg/ddl431 [DOI] [PubMed] [Google Scholar]
- 23.Kasher PR, De Vos KJ, Wharton SB, Manser C, Bennett EJ, Bingley M, Wood JD, Milner R, McDermott CJ, Miller CC, et al. (2009) Direct evidence for axonal transport defects in a novel mouse model of mutant spastin-induced hereditary spastic paraplegia (HSP) and human HSP patients. J Neurochem 110: 34–44. 10.1111/j.1471-4159.2009.06104.x [DOI] [PubMed] [Google Scholar]
- 24.Denton KR, Lei L, Grenier J, Rodionov V, Blackstone C, Li XJ (2014) Loss of spastin function results in disease-specific axonal defects in human pluripotent stem cell-based models of hereditary spastic paraplegia. Stem Cells 32: 414–423. 10.1002/stem.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havlicek S, Kohl Z, Mishra HK, Prots I, Eberhardt E, Denguir N, Wend H, Plötz S, Boyer L, Marchetto MC, et al. (2014) Gene dosage-dependent rescue of HSP neurite defects in SPG4 patients’ neurons. Hum Mol Genet 23: 2527–2541. 10.1093/hmg/ddt644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisciottani A, Biancolillo L, Ferrara M, Valente D, Sardina F, Monteonofrio L, Camerini S, Crescenzi M, Soddu S, Rinaldo C (2019) HIPK2 phosphorylates the microtubule-severing enzyme spastin at S268 for abscission. Cells 8: 684 10.3390/cells8070684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Orazi G, Rinaldo C, Soddu S (2012) Updates on HIPK2: A resourceful oncosuppressor for clearing cancer. J Exp Clin Cancer Res 31: 63 10.1186/1756-9966-31-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz ML, Rodriguez-Gil A, Hornung J (2014) Integration of stress signals by homeodomain interacting protein kinases. Biol Chem 95: 375–386. 10.1515/hsz-2013-0264 [DOI] [PubMed] [Google Scholar]
- 29.Upadhyay M, Bhadauriya P, Ganesh S (2016) Heat shock modulates the subcellular localization, stability, and activity of HIPK2. Biochem Biophys Res Commun 472: 580–584. 10.1016/j.bbrc.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 30.Wagner SA, Beli P, Weinert BT, Schölz C, Kelstrup CD, Young C, Nielsen ML, Olsen JV, Brakebusch C, Choudhary C, et al. (2012) Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol Cell Proteomics 11: 1578–1585. 10.1074/mcp.m112.017905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans K, Keller C, Pavur K, Glasgow K, Conn B, Lauring B (2009) Interaction of two hereditary spastic paraplegia gene products, spastin and atlastin, suggests a common pathway for axonal maintenance. Proc Natl Acad Sci U S A 103: 10666–10671. 10.1073/pnas.0510863103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roll-Mecak A, Vale RD (2008) Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451: 363–367. 10.1038/nature06482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458: 732–736. 10.1038/nature07884 [DOI] [PubMed] [Google Scholar]
- 34.Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, Guo A, Chen YN, Rush J, Hsu PW, et al. (2011) Global identification of modular cullin-RING ligase substrates. Cell 147: 459–474. 10.1016/j.cell.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Reitsma JM, Mamrosh JL, Zhang Y, Straube R, Deshaies RJ (2018) Cand1-mediated adaptive exchange mechanism enables variation in F-box protein expression. Mol Cell 69: 773–786.e6. 10.1016/j.molcel.2018.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichermeier KM, Straube R, Reitsma JM, Sweredoski MJ, Rose CM, Moradian A, denBesten W, Hinkle T, Verschueren E, Petzold G, et al. (2020) PIKES analysis reveals response to degraders and key regulatory mechanisms of the CRL4 network. Mol Cell 77: 1092–1106.e9. 10.1016/j.molcel.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 37.Sharp DJ, Ross JL (2012) Microtubule-severing enzymes at the cutting edge. J Cell Sci 125: 2561–2569. 10.1242/jcs.101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead E, Heald R, Wilbur JD (2013) N-terminal phosphorylation of p60 katanin directly regulates microtubule severing. J Mol Biol 425: 214–221. 10.1016/j.jmb.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han H, Monroe N, Votteler J, Shakya B, Sundquist WI, Hill CP (2015) Binding of substrates to the central pore of the Vps4 ATPase is autoinhibited by the microtubule interacting and trafficking (MIT) domain and activated by MIT interacting motifs (MIMs). J Biol Chem 290: 13490–13499. 10.1074/jbc.m115.642355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Muela N, Litterman NK, Norabuena EM, Mull JL, Galazo MJ, Sun C, Ng SY, Makhortova NR, White A, Lynes MM, et al. (2017) Single-cell analysis of SMN reveals its broader role in neuromuscular disease. Cell Rep 18: 1484–1498. 10.1016/j.celrep.2017.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim JH, Kang HM, Jung HR, Kim DS, Noh KH, Chang TK, Kim BJ, Sung DH, Cho HS, Chung KS, et al. (2018) Missense mutation of SPAST protein (I344K) results in loss of ATPase activity and prolonged the half-life, implicated in autosomal dominant hereditary spastic paraplegia. Biochim Biophys Acta Mol Basis Dis 1864: 3221–3233. 10.1016/j.bbadis.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 42.Fassier C, Tarrade A, Peris L, Courageot S, Mailly P, Dalard C, Delga S, Roblot N, Lefèvre J, Job D, et al. (2013) Microtubule-targeting drugs rescue axonal swellings in cortical neurons from spastin knockout mice. Dis Model Mech 6: 72–83. 10.1242/dmm.008946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan Y, Wali G, Sutharsan R, Bellette B, Crane DI, Sue CM, Mackay-Sim A (2014) Low dose tubulin-binding drugs rescue peroxisome trafficking deficit in patient-derived stem cells in hereditary spastic paraplegia. Biol Open 3: 494–502. 10.1242/bio.20147641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin YF, Xie Z, Zhou J, Chen HH, Shao WW, Lin HD (2019) Effect of exogenous spastin combined with polyethylene glycol on sciatic nerve injury. Neural Regen Res 14: 1271–1279. 10.4103/1673-5374.251336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zempel H, Mandelkow EM (2015) Tau missorting and spastin-induced microtubule disruption in neurodegeneration: Alzheimer disease and hereditary spastic paraplegia. Mol Neurodegener 10: 68 10.1186/s13024-015-0064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM (2013) Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J 32: 2920–2937. 10.1038/emboj.2013.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Gil A, Ritter O, Hornung J, Stekman H, Krüger M, Braun T, Kremmer E, Kracht M, Schmitz ML (2016) HIPK family kinases bind and regulate the function of the CCR4-NOT complex. Mol Biol Cell 27: 1969–1980. 10.1091/mbc.e15-09-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinaldo C, Moncada A, Gradi A, Ciuffini L, D’Eliseo D, Siepi F, Prodosmo A, Giorgi A, Pierantoni GM, Trapasso F, et al. (2012) HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody. Mol Cell 47: 87–98. 10.1016/j.molcel.2012.04.029 [DOI] [PubMed] [Google Scholar]
- 49.Cecchinelli B, Porrello A, Lazzari C, Gradi A, Bossi G, D’Angelo M, Sacchi A, Soddu S (2006) Ser58 of mouse p53 is the homologue of human Ser46 and is phosphorylated by HIPK2 in apoptosis. Cell Death Differ 13: 1994–1997. 10.1038/sj.cdd.4401933 [DOI] [PubMed] [Google Scholar]
- 50.Dráberová E, Vinopal S, Morfini G, Liu PS, Sládková V, Sulimenko T, Burns MR, Solowska J, Kulandaivel K, de Chadarévian JP, et al. (2011) Microtubule-severing ATPase spastin in glioblastoma: Increased expression in human glioblastoma cell lines and inverse roles in cell motility and proliferation. J Neuropathol Exp Neurol 70: 811–826. 10.1097/nen.0b013e31822c256d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solowska JM, D’Rozario M, Jean DC, Davidson MW, Marenda DR, Baas PW (2014) Pathogenic mutation of spastin has gain-of-function effects on microtubule dynamics. J Neurosci 34: 1856–1867. 10.1523/jneurosci.3309-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858. 10.1021/ac950914h [DOI] [PubMed] [Google Scholar]
- 53.Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, et al. (2019) The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res 47: D442–D450. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source Data for Figure 1LSA-2020-00799_SdataF1.1.xlsx (24.7KB, xlsx) LSA-2020-00799_SdataF1.2.tif (8.8MB, tif)
Source Data for Figure 2LSA-2020-00799_SdataF2.1.xlsx (25.1KB, xlsx) LSA-2020-00799_SdataF2.2.tif (9.8MB, tif)
Source Data for Figure 3LSA-2020-00799_SdataF3.1.xlsx (19KB, xlsx) LSA-2020-00799_SdataF3.2.tif (7.3MB, tif)
Source Data for Figure 4LSA-2020-00799_SdataF4.1.xlsx (14.8KB, xlsx) LSA-2020-00799_SdataF4.2.tif (8.8MB, tif)
LSA-2020-00799_Supplemental_Data_1.xlsx (27.8KB, xlsx) Identification of proteins contained in spastin-S268A and spastin-S268D immunoprecipitates from HeLa cells. The original list of proteins was filtered to include only proteins that are absent from the negative control experiments.
Source Data for Figure 5LSA-2020-00799_SdataF5.1.xlsx (18.8KB, xlsx) LSA-2020-00799_SdataF5.2.tif (9.4MB, tif)
Data Availability Statement
MS data have been deposited to the ProteomeXchange Consortium via the partner repository PRIDE (53) with the data set identifier PXD021945.