Abstract
Background/Aim: This multi-institutional study aimed to investigate the efficacy and feasibility of proton beam therapy (PBT) for renal cell carcinoma (RCC) in Japan. Patients and Methods: The survival, local control, and toxicities in 22 RCC patients treated between 2001 and 2016 at 6 Japanese PBT institutes were analyzed. Results: The 22 patients comprised 20 men and had a median age of 67 (range=42-88) years. The total irradiation dose was 60-79.6 Gy (relative biological effectiveness). Over a median follow-up of 37 months, the 3-year overall and disease-specific survival rates were 95% and 100%, respectively, and no recurrence occurred. No patient experienced grade 3 or higher adverse events. The serum blood urea nitrogen (p=0.25) and creatinine levels (p=0.95) were not significantly affected, although the mean estimated glomerular filtration rate was reduced by 7.1±11.2 ml/min/1.73 m2. Conclusion: Despite the small number of patients, high-dose PBT can control RCC while maintaining their renal function with high probability, and could be and alternative curative therapy especially for inoperable patients.
Keywords: Renal cell carcinoma, proton beam therapy, local control, toxicity, radiotherapy
The incidence of renal cancer continues to increase. In Japan, there were approximately 25,000 estimated new cases of renal cancers, according to the Cancer Registry and Statistics, in 2015 (1), and renal cell carcinoma (RCC) accounts for the majority of these cancers. RCC predominantly affects the older population and males.
The standard therapy for patients with RCC is surgery (2,3). Surgical treatment usually involves removing either the tumor (partial nephrectomy) or the entire kidney and surrounding tissues (radical nephrectomy). Alternative treatments are used for patients deemed ineligible for surgery and for whom active surveillance is an inappropriate treatment choice. Radiofrequency ablation involves either percutaneous or intraoperative insertion of electrodes to induce thermal ablation in the tumor (4). Cryoablation delivered via a laparoscopic approach involves supercooled liquid introduced to create an ice ball, which causes ablation (5). Both these treatments are invasive, requiring access to the kidney via percutaneous incisions, and are problematic for patients who require continuous anticoagulative medications.
RCC has traditionally been regarded as radioresistant, yet a pathological complete response after radiotherapy (RT) has been reported (6,7). A few clinical studies have reported benefits of stereotactic body RT (SBRT) on RCC outcomes, such as high local tumor control and few adverse events (8-10). A systematic review showed that SBRT is comparable with radiofrequency ablation or cryotherapy in terms of local effectiveness, with a local progression rate of 6.1% (11). RT is occasionally used to treat RCC patients, especially those who prefer a non-invasive procedure or who have a tumor size beyond the range of alternative treatments.
Regarding particle beam therapy, carbon ion RT showed 5-year and 10-year overall survival (OS) rates of 89.2% and 58.7% in 19 patients, respectively (12). Proton beam therapy (PBT), which is another type of particle beam therapy, has since long been used to treat various cancers (13-16), but there are no clinical reports of its use for RCC. Because the numbers of RCC patients treated with PBT at a single institution are very small and thus the data from a single institution are insufficient for evaluation, we conducted a multi-institutional study in Japan to assess the treatment effects and toxicities of PBT in RCC patients.
Patients and Methods
A retrospective observational study of patients who underwent PBT for their primary RCCs at six Institutions was conducted. The participating Institutions at which PBT was used between 2001 and 2016 were Medipolis Proton Therapy and Research Center, University of Tsukuba Hospital, Southern TOHOKU Proton Therapy Center, Hyogo Ion Beam Medical Center, Nagoya City West Medical Center, and Fukui Prefecture Hospital. The present study was approved by the institutional review board of each institution (no. 31-3). The clinical information of each patient was anonymized and collected using an electronic data capture system.
A total of 22 patients who received PBT for histologically (including cytologically) diagnosed or clinically diagnosed RCC from January 2001 to December 2016 were enrolled. Exclusion criteria were the presence of any metastasis or having received palliative treatment. The clinical diagnosis of RCC was based on radiographic findings. The relative biological effectiveness (RBE) was calculated as 1.1 and the biological effective dose (BED) was calculated using the linear-quadratic model (17). The BED was defined as nd (1+d/α/β), where n is the fractionation number, d is the daily dose, and the α/β ratio is 10 Gy for RCC and 3 Gy for the normal kidney.
The follow-up period was defined from the first day of PBT to the date of death or the last follow-up visit. Principally, patients were followed up at 6-month intervals after PBT. The OS and disease-specific survival rates were calculated from the first day of PBT to the date of the event or the last follow-up visit using the Kaplan-Meier method. Local tumor control was classified as a complete response, partial response, stable disease, or progressive disease according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (18). Radiation-related toxicities were assessed based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0 (19). Renal function was estimated by measuring the serum blood urea nitrogen (BUN) level, creatinine (Cr) level, and estimated glomerular filtration rate (eGFR) before PBT and at the last follow-up.
Results
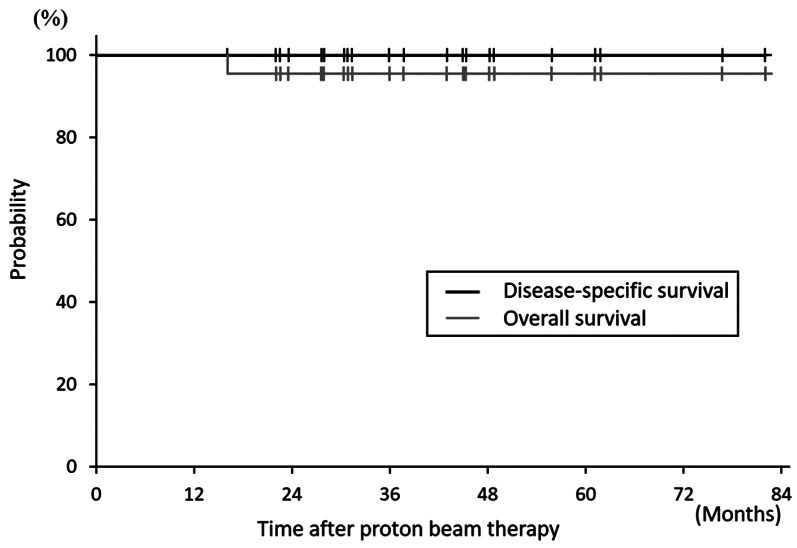
Of the 22 patients, 20 (91%) were men, and 2 (9%) were women, and the median age was 67 (range=42-88) years. Table I shows the patient characteristics. The total irradiation dose was 60-79.6 Gy (relative biological effectiveness [RBE]) delivered in 10-36 fractions and the BEDs of RCC ranged from 93.9 to 109.6 Gy (median: 104.9 Gy). The median follow-up period was 37 (range=22-82) months. A total of 21 patients survived until the last follow-up, whereas the remaining patient died of a thoracic aortic dissection at 16 months after PBT without any signs of RCC recurrence or PBT-related toxicities. The 3-year OS and disease-specific survival rates were 94.7±10.0% and 100% at 3 years (Figure 1).
Table I. Characteristics of patients with renal cell carcinoma.
Gy (RBE)/fr: Gray (relative biological effectiveness)/fraction.
Figure 1. Kaplan-Meier curves for overall and disease-specific survival.
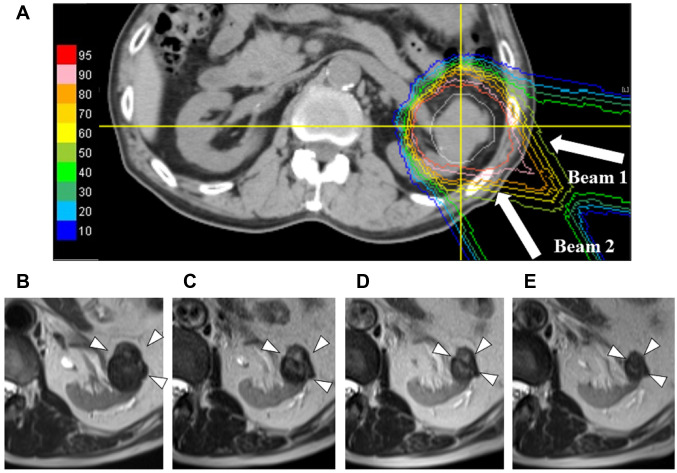
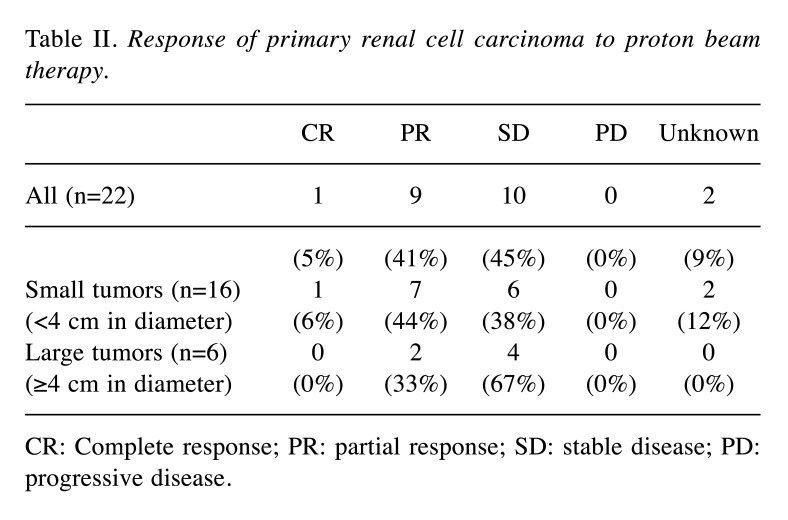
No recurrence was observed in the present study. Tumor shrinkage after PBT usually occurred gradually (Figure 2). The local response at the last follow-up was defined as a complete response, a partial response, stable disease, and progressive disease in 1, 9, 10, and 0 patients, respectively; the response was unknown in 2 patients (Table II). The respective numbers in the 16 tumors <4 cm in diameter were 1, 7, 6, and 0, and those in the remaining 6 tumors ≥4 cm in diameter were 0, 2, 4, and 0 (Table II).
Figure 2. A representative case of renal cell carcinoma after proton beam therapy. Dose distribution of proton beam therapy (a) and changes in a tumor observed on MRI at 18 (b), 24 (c), 30 (d), and 36 (e) months after treatment.
Table II. Response of primary renal cell carcinoma to proton beam therapy.
CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease.
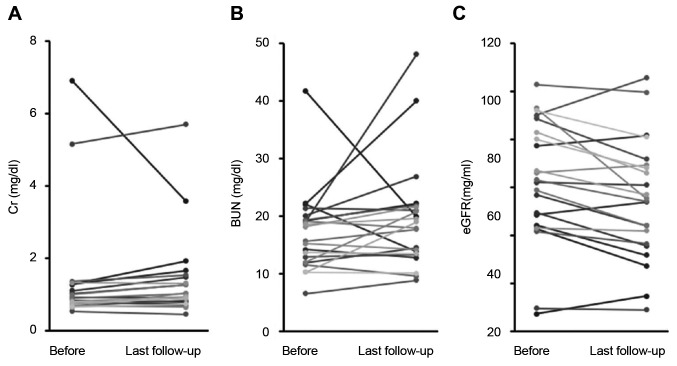
Grade 2 or higher adverse events were observed in one patient during the acute phase (dermatitis) and two patients during the late phase (renal dysfunction at 9 and 28 months after PBT, respectively). The serum blood urea nitrogen (BUN) level, creatinine (Cr) level, and estimated glemerular filtration rate (eGFR) were 17.1±7.0 mg/dl, 1.4±1.6 mg/dl, and 62.2±1.6 ml/min/1.73 m2, respectively, at the start of PBT and 19.4±9.3 mg/dl, 1.4±1.2 mg/dl, and 55.0±24.8 ml/min/1.73 m2, respectively, at the last follow-up examination (Figure 3). The serum BUN and Cr levels were not significantly increased compared to baseline (p=0.25 and p=0.95, respectively). The serum eGFR after PBT did not decrease at less than 50% of baseline levels in any patient, although the mean decrease after treatment was significant (p=0.007).
Figure 3. Changes in renal function before and after proton beam therapy. The serum blood urea nitrogen (BUN) levels (a), creatinine (Cr) levels (b), and estimated glomerular filtration rate (eGFR) (c) before proton beam therapy and at the last follow-up, are shown.
Discussion
There are very few scientific reports on RCC treated with RT and even fewer on RCC treated with PBT. The principal reason is probably the small number of such cases within a single facility. We conducted a nationwide study involving all PBT facilities in Japan. As a result, we collected data from 22 patients with RCC treated with PBT from multiple institutions, and these data were sufficient for analyzing the efficacy of PBT on primary RCC as a curative intent, although the sample size was still small. To the best of our knowledge, this is the first report to evaluate the effect of PBT in RCC patients.
Adverse events were found in one patient in the acute phase and two patients in the late phase. However, these events were grade 2, and no severe (grade 3 or higher) adverse events were observed in any patient. To assess renal function, changes in the serum BUN levels, Cr levels, and eGFR were measured in blood samples. The results indicated no significant increase of the BUN or Cr level after ipsilateral irradiation of the RCC (Figure 3). Regarding the eGFR, the greatest change observed was from 93 ml/min/1.73 m2 before PBT to 55.5 ml/min/1.73 m2 at the last follow-up. However, the change in the remaining patients was relatively small; the mean eGFR reduction was 7.1±11.2 ml/min/1.73 m2 over a median follow-up of >3 years and there were no grade 3 renal toxicities or need for hemodialysis. Siva et al. evaluated changes in renal function after photon SBRT for RCC in a prospective trial, which included a similar number of patients (n=21) to that in our study (9). They reported that eGFR gradually decreased after SBRT, with a reduction of 8.7±13.4 ml/min/1.73 m2 at 1 year. Similarly, the Cr levels significantly increased at 1 year post SBRT compared with the baseline level (p=0.02). In their study, there were exponential decreases in the GFR in the affected kidney of 39% at a dose of 26 Gy/fraction and of 25% at 42 Gy/3 fractions for every 10 Gy of physical dose delivered (9). Correa et al. reported the latest systematic review of RT for RCC and showed a rate of grade 3/4 adverse events of 1.5% and an eGFR reduction of 7.7 ml/min after using mainly photon SBRT (10). On the other hand, Kasuya et al. reported that renal function after carbon ion RT was maintained in all 14 patients evaluated, without pre-treatment definitive renal comorbidities, and the average reduction in the eGFR was 6.1 ml/min/1.73 m2 (12). Taken together, PBT can be used without inflicting any severe damage to the body, including renal function, and appears to be sufficiently safe.
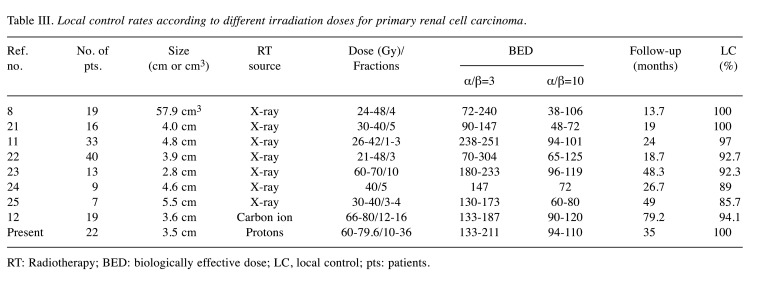
In the present study, a relatively high BED10 ranging from 93.9 to 109.6 Gy was delivered irrespective of the patient or tumor background characteristics, and neither local progression nor severe toxicity was observed. Kasuya et al. also suggested that a higher BED delivered to the tumor might yield a better local control rate and recommended a higher dose of 72 Gy (RBE)/16 fractions, corresponding to a BED10 of 104 Gy. Since RCC is generally considered a radioresistant tumor, dose escalation may be a reasonable approach to improve outcomes. However, an analysis of nine international multi-institutional studies of SBRT using a BED10 range of 80-87.5 Gy reported a 4-year local control rate of 97.8% (20). Table III summarizes the clinical outcomes of previous studies evaluating RT for primary RCC (8,11,12,22-25). Those studies used a wide range of dose fractionation schedules and BED10 values (range=38-125 Gy), but local tumor control was achieved in almost all patients irrespective of the BED10.
Table III. Local control rates according to different irradiation doses for primary renal cell carcinoma.
RT: Radiotherapy; BED: biologically effective dose; LC, local control; pts: patients.
On the other hand, a recent systematic review (10) showed a local control rate after SBRT of 97.2% (95% CI=93.9-99.5), and a higher rate of local failure in the low-dose arm compared with the high-dose group or after a dose reduction to mitigate toxicity (8,10,26). Furthermore, the studies using a BED10 range of 60-80 Gy with a median follow-up for ≥2 years reported a relatively low local control rates (range=85.7-89%) as shown in Table III (24,25). Conversely, local control rates in all the 3 studies and the present study using a BED10 ≥90 Gy with a median follow-up of ≥2 years were higher than 90% (11,12,23). Taking the previous reports of SBRT and particle beam therapy together, BED10 ≥90 Gy may be necessary and high-dose RT using advanced technology can accomplish good local tumor control. We consider that our PBT results in RCC patients are similar to those of previous studies in terms of excellent local tumor control and safety.
On the other hand, an appropriate benchmark is necessary to determine the optimal dose fractionation schedule to obtain local tumor control while preserving renal function after hypofractionated RT, including PBT and SBRT, for RCC. Siva et al. stated in another systematic review that BED calculations may not be reliable for extremely hypofractionated RT, given that preclinical models do not account for such high doses per fraction (11). Shibamoto et al. suggested that correction of the errors associated with using the BED, estimated at 5-20%, is necessary when used for SBRT (27). Thus, estimation methods alternative to the linear-quadratic model for determining local tumor control after SBRT for RCC, as well as other diseases, need to be determined via large-scale analyses, to validate the efficacy of RT for RCC as a primary treatment.
The major limitations of the study are its retrospective nature, the heterogeneity among the patient and tumor characteristics, use of a wide variety of dose fractionation schedules, and absence of a standardized follow-up schedule. Furthermore, aspects of the PBT plans such as the treatment margins and management of respiratory movement were not completely unified. Future prospective studies can provide more refined treatment results for PBT. Moreover, it is necessary to compare the outcomes of PBT with those of other treatments including SBRT to understand the indications for PBT for RCC patients ineligible for surgery.
Conclusion
As the first multi-institutional retrospective study of PBT for RCC patients in Japan, our results revealed that PBT yields acceptable OS and local control rates with a low risk of toxicity. PBT may be an optional local treatment for RCC patients who cannot undergo surgery.
Conflicts of Interest
The Authors declare that there are no conflicts of interest with regard to the present study.
Authors’ Contributions
Conception: NF and HI. Acquisition of data: TA, HW, TO, YS, HI, and SS. Analysis and interpretation of data: NF, HI, TA, HW, TO, YS, and HI. Writing: NF. Review and revision: HI, SS, and HS. Supervision: HS.
References
- 1.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. Available at: https://ganjoho.jp/reg_stat/statistics/dl/index.html#incidence4pref [Last accessed on 2nd June 2020]
- 2.Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman M, Gallagher TH, Gore JL, Hancock SL, Harrison MR, Kim W, Kyriakopoulos C, LaGrange C, Lam ET, Lau C, Michaelson MD, Olencki T, Pierorazio PM, Plimack ER, Redman BG, Shuch B, Somer B, Sonpavde G, Sosman J, Dwyer M, Kumar R. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(6):804–834. doi: 10.6004/jnccn.2017.0100. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Zagoria RJ, Hawkins AD, Clark PE, Hall MC, Matlaga BR, Dyer RB, Chen MY. Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol. 2004;183(1):201–207. doi: 10.2214/ajr.183.1.1830201. [DOI] [PubMed] [Google Scholar]
- 5.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113(10):2671–2680. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponsky LE, Crownover RL, Rosen MJ, Rodebaugh RF, Castilla EA, Brainard J, Cherullo EE, Novick AC. Initial evaluation of Cyberknife technology for extracorporeal renal tissue ablation. Urology. 2003;61(3):498–501. doi: 10.1016/s0090-4295(02)02442-1. [DOI] [PubMed] [Google Scholar]
- 7.Teh BS, Bloch C, Paulino AC, Shen S, Hinckley L, Baskin D, Butler EB, Amato R. Pathologic complete response in renal cell carcinoma brain metastases treated with stereotactic radiosurgery. Clin Genitourin Cancer. 2007;5(5):334–337. doi: 10.3816/CGC.2007.n.013. [DOI] [PubMed] [Google Scholar]
- 8.Ponsky L, Lo SS, Zhang Y, Schluchter M, Liu Y, Patel R, Abouassaly R, Welford S, Gulani V, Haaga JR, Machtay M, Ellis RJ. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117(1):183–187. doi: 10.1016/j.radonc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Siva S, Jackson P, Kron T, Bressel M, Lau E, Hofman M, Shaw M, Chander S, Pham D, Lawrentschuk N, Wong LM, Goad J, Foroudi E. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose-response relationship. Radiother Oncol. 2016;118(3):540–546. doi: 10.1016/j.radonc.2016.01.027.. [DOI] [PubMed] [Google Scholar]
- 10.Correa RJM, Louie AV, Zaorsky NG, Lehrer EJ, Ellis R, Ponsky L, Kaplan I, Mahadevan A, Chu W, Swaminath A, Hannan R, Onishi H, Teh BS, Muacevic A, Lo SS, Staehler M, Siva S. The emerging role of stereotactic ablative radiotherapy for primary renal cell carcinoma: a systematic review and meta-analysis. Eur Urol Focus. 2019;5(6):958–969. doi: 10.1016/j.euf.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Siva S, Pham D, Gill S, Corcoran NM, Foroudi F. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int. 2012;110(11 Pt B):E737–743. doi: 10.1111/j.1464-410X.2012.11550.x. [DOI] [PubMed] [Google Scholar]
- 12.Kasuya G, Tsuji H, Nomiya T, Makishima H, Haruyama Y, Kobashi G, Ebner DK, Hayashi K, Omatsu T, Kishimoto R, Yasuda S, Igarashi T, Oya M, Akakura K, Suzuki H, Ichikawa T, Shimazaki J, Kamada T, Working Group for Genitourinary Tumors Updated long-term outcomes after carbon-ion radiotherapy for primary renal cell carcinoma. Cancer Sci. 2018;109(9):2873–2880. doi: 10.1111/cas.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royce TJ, Efstathiou JA. Proton therapy for prostate cancer: A review of the rationale, evidence, and current state. Urol Oncol. 2019;37(9):628–636. doi: 10.1016/j.urolonc.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Apisarnthanarax S, Bowen SR, Combs SE. Proton beam therapy and carbon ion radiotherapy for hepatocellular carcinoma. Semin Radiat Oncol. 2018;28(4):309–320. doi: 10.1016/j.semradonc.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Leroy R, Benahmed N, Hulstaert F, Van Damme N, De Ruysscher D. Proton therapy in children: a systematic review of clinical effectiveness in 15 pediatric cancers. Int J Radiat Oncol Biol Phys. 2016;95(1):267–278. doi: 10.1016/j.ijrobp.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89(2):292–302. doi: 10.1016/j.ijrobp.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Yaes RJ, Patel P, Maruyama Y. On using the linear-quadratic model in daily clinical practice. Int J Radiat Oncol Biol Phys. 1991;20(6):1353–1362. doi: 10.1016/0360-3016(91)90249-4. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Common terminology criteria for adverse events (CTCAE) Version 4.03. US Department of Health and Human Services, National Cancer Institute 2010. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf [Last accessed on 26th May, 2020]
- 20.Siva S, Louie AV, Warner A, Muacevic A, Gandhidasan S, Ponsky L, Ellis R, Kaplan I, Mahadevan A, Chu W, Swaminath A, Onishi H, Teh B, Correa RJ, Lo SS, Staehler M. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124(5):934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 21.Chang JH, Cheung P, Erler D, Sonier M, Korol R, Chu W. Stereotactic ablative body radiotherapy for primary renal cell carcinoma in nonsurgical candidates: initial clinical experience. Clin Oncol. 2016;28(9):e109–114. doi: 10.1016/j.clon.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Sun MR, Brook A, Powell MF, Kaliannan K, Wagner AA, Kaplan ID, Pedrosa I. Effect of stereotactic body radiotherapy on the growth kinetics and enhancement pattern of primary renal tumors. AJR Am J Roentgenol. 2016;206(3):544–553. doi: 10.2214/AJR.14.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funayama S, Onishi H, Kuriyama K, Komiyama T, Marino K, Araya M, Saito R, Aoki S, Maehata Y, Nonaka H, Tominaga L, Muramatsu J, Nakagomi H, Kamiyama M, Takeda M. Renal cancer is not radioresistant: slowly but continuing shrinkage of the tumor after stereotactic body radiation therapy. Technol Cancer Res Treat. 2019;18:1533033818822329. doi: 10.1177/1533033818822329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beitler JJ, Makara D, Silverman P, Lederman G. Definitive, high-dose-per-fraction, conformal, stereotactic external radiation for renal cell carcinoma. Am J Clin Oncol. 2004;27(6):646–648. doi: 10.1097/01.coc.0000145289.57705.07. [DOI] [PubMed] [Google Scholar]
- 25.Svedman C, Karlsson K, Rutkowska E, Sandström P, Blomgren H, Lax I, Wersäll P. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol. 2008;47(8):1578–1583. doi: 10.1080/02841860802123196. [DOI] [PubMed] [Google Scholar]
- 26.Svedman C, Sandstrom P, Pisa P, Blomgren H, Lax I, Kälkne KM, Nilsson S, Wersäll P. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45(7):870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 27.Shibamoto Y, Miyakawa A, Otsuka S, Iwata H. Radiobiology of hypofractionated stereotactic radiotherapy: what are the optimal fractionation schedules. J Radiat Res. 2016;57(Suppl 1):i76–i82. doi: 10.1093/jrr/rrw015. [DOI] [PMC free article] [PubMed] [Google Scholar]