Abstract
Malignancy as an etiological factor involved in priapism pathogenesis is rare. Malignant priapism (MP) can arise as a result of penile tumor invasion, either from primary penile tumors or from metastatic penile tumors, or due to hematological malignancies. Non-urological penile metastases are associated with significant worse prognosis compared to urological penile metastases, the appearance of priapism in such cases affecting even more the prognosis and the survival of these patients. Patients diagnosed with hematological malignancies and priapism present significant higher survival rates compared to those who develop MP in the context of a non-hematological malignancy, this being related to the fact that hematological malignancies are more sensitive to chemo- and radiotherapy. Most malignant priapism cases are ischemic; therefore the management should be based on the initial steps of the IP therapeutic protocol. Considering the trigger factor that has led to the priapic event specific oncologic treatment can be added as well.
Keywords: Malignant priapism, penile tumors, hematological malignancy, review
Priapism is a urological emergency that requires urgent evaluation and treatment in order to avoid the appearance of irreversible structural changes at the level of the cavernosal tissue that will lead to definitive erectile dysfunction. Compared to other urological pathologies that need immediate treatment its incidence is significantly lower, estimated to range between 0.5 and 1 cases/100,000 males/year (1,2).
Considering the physiopathological mechanisms responsible for the priapism onset, the clinical display, as well as the therapeutic approach used for its management, priapism presents three subtypes: ischemic priapism (IP), non-ischemic priapism (NIP) and recurrent priapism (RP) (3).
IP is the most common type, accounting for approximately 95% of all priapism cases, a high percentage being idiopathic. Over time, several factors involved in IP pathogenesis have been identified: benign or malignant hematological disorders (more frequently sickle cell disease (SCD), glucose-6-phosphate dehydrogenase deficiency and leukemia), vasoactive erectile agents, recreational drugs, alcohol abuse, neurological pathologies (cauda equina syndrome, autonomic neuropathy, spinal cord injury/ tumors), metabolic disorders (amyloidosis, Fabry disease), antihypertensive medication (4), antidepressants, antipsychotics, anxiolytic drugs, antagonists of the alpha-adrenergic receptors and also malignancy. NIP is a less frequent type of priapism usually related to perineal/pelvic trauma, whereas RP is a form of ischemic priapism and has the same risk factors, but it is more frequently associated with hematological disorders (5-7).
Malignancy as an etiological factor involved in priapism pathogenesis is rare, but nevertheless this should be considered in patients without an obvious risk factor that could explain priapism onset. The first mention of malignant priapism (MP) dates back to the beginning of the 20th century, when in 1938 Peacock described this type of priapism as a persistent erection, without any identifiable trigger factors that could lead to sexual stimulation. The mechanism proposed for this type of priapism was the invasion of cavernosal sinusoids and their efferent veins by malignant cells (5,8).
MP Pathogenesis
MP can arise as a result of penile tumor invasion, either from primary penile tumors or from metastatic penile tumors, or due to hematological malignancies. The incidence of MP associated to primary penile tumors is extremely small, more frequently MP developing in the context of penile metastasis (9-11). Despite the fact that the penis has a well-developed lymphovascular network through which it is connected with the majority of the pelvic organs, the incidence of penile metastasis is quite small. Therefore, the appearance of penile metastases can be explained by the dissemination of tumor cells and their implantation at the level of the penile tissues either by arterial spread or by a retrograde venous or lymphatic route. Tumor cell dissemination can also occur during surgical instrumentation, but this possibility is very small. MP may as well be the result of a nearby pelvic or perineal tumor that extends and invades the penis and the corpora cavernosa. This scenario can be encountered especially in malignancies that affect the prostate, bladder, urethra, testicles and the anorectal region (5,12,13).
It is generally accepted that MP is an ischemic type of priapism, due to the fact that the main physiopathological mechanism implies direct cavernosal tumor invasion which affects the cavernosal sinusoids and their emerging veins, eventually causing their obstruction with the appearance of cavernosal venous stasis that leads to erection. Another possible mechanism for ischemic MP, but significantly less frequently encountered, is the tumoral infiltration of the neurologic pathways involved in the penile tumescence and detumescence process (5,12).
Nevertheless, there have also been reported cases of high-flow (non-ischemic) MP. A possible mechanism that can lead to high-flow MP is represented by the development of an arterio-cavernosal fistula, which appears as a result of a penile tumor process that erodes the cavernosal arteries and their branches during its evolution (5). In 1998, Dubocq reported the case of a high-flow MP in a patient with bladder cancer, who had developed priapism three years after radical cystectomy and urethrectomy. The etiological factor that led to the appearance of priapism in this case was a penile metastasis. The author reported that color Doppler ultrasound examination revealed an increased flow in both cavernosal arteries, followed by its reversal during diastole. In this case the presence of an arterial fistula was not mentioned; in fact the metastasis was located in the distal part of the penis which makes us believe that the mechanism previously described did not apply in this case (14).
In terms of priapism associated to hematological malignancies, several mechanisms that could explain priapism pathogenesis have been proposed. The main mechanism through which hematological malignancies can lead to priapism is a significantly increased leukocytosis with leucostasis that determines hyperviscosity and leucocytes aggregation, favoring the formation of microtrombs that can obstruct the cavernosal sinusoids and the efferent veins, therefore leading to cavernosal congestion (15-17). Another plausible hypothesis that can lead to microvascular obstruction is represented by the fact that malignant cells, such as leukemic cells, associate with an increased cytokine and adhesion molecule production, activating the endothelial cells and determining a phenomenon known as cellular sequestration. Therefore, this phenomenon can severely affect small caliber vessels, obstructing them and determining cavernosal blood stasis (5,8). Other possible mechanisms, more or less feasible, for the pathogenesis of priapism associated to hematological malignancies are: the dissemination of hematologic malignant cells to penile tissues using the same routes as in penile metastases from other solid non-hematological tumors; hematological malignant cells infiltration at the level of the sacral nerves or at the level of the central nervous system, severely interfering with the erection pathways, and also an increased intra-abdominal pressure due to the presence of an important splenomegaly (usually found in malignant hematological pathologies) that may affect the venous return, therefore determining cavernosal congestion (5,17,18).
Clinical Assessment of Priapism Patients
The management of this pathology depends on the type of priapism whether it is ischemic or non-ischemic, therefore a proper medical history assessment and clinical examination should be immediately performed. Other important tools that can reveal the type of priapism are imaging investigations and cavernosal blood gas analysis. Anamnesis should focus on several key elements like priapism duration, pain intensity, the presence of previous priapism episodes, history of recent perineal or genital trauma, medication or recreational drugs, the status of the erectile function prior to the priapic episode, history of associated pathologies that might explain the appearance of a priapism episode (especially hematologic and metabolic disorders, malignancies, neurologic conditions) (3,11,19).
Physical examination is very important because it allows us to differentiate between the two types of priapism, IP versus NIP (IP is characterized by increased cavernosal rigidity accompanied by local pain, the spongiosum and the penile glans being soft, whereas in NIP the corpus cavernosum is engorged, but not rigid and the pain is absent), and it can also reveal signs of recent perineal/genital trauma that might explain the priapism, as well as its type. A rigorous penile examination can unveil the presence of skin lesions, penile masses, ulcerations or indurated nodules, these findings being suggestive for primary or secondary penile tumors, which would explain priapism onset. Other findings that could raise the suspicion of an unknown malignant process possibly linked with the current priapic event are the presence of palpable, enlarged lymph nodes, hepatosplenomegaly, marked weight loss, rectal bleeding, hematuria, urethrorrhagia and even the presence of irritative or obstructive urinary symptoms (12,20).
In patients with suspicion of malignancy, the investigation protocol should also include the assessment of tumor markers, bone marrow studies, as well as imaging investigations like thoracic, abdominal and pelvic computer tomography evaluation. Patients with hematological malignancies usually present modified laboratory investigations, frequently associating leucocytosis, anemia, thrombocytopenia and also coagulation disorders, such as a markedly increased prothrombin time (5).
Cavernosal blood gas analysis is an investigation that clearly shows the type of priapism. Therefore, in IP cases it reveals a significantly decreased oxygen partial pressure with increased carbon dioxide partial pressure, as well as acidosis (pO2 <30 mmHg, pCO2 >60 mmHg, pH <7.25), whereas in NIP the partial pressure of oxygen and carbon dioxide are within normal limits, as well as the value of the cavernosal pH (pO2 >90 mmHg, pCO2 <40 mmHg, pH of 7.4) (19,21).
Color Doppler ultrasound (CDU) is a fast diagnostic method that has proven its efficiency in terms of differentiating between the two types of priapism. In IP, CDU reveals a low or even absent blood flow in the cavernosal arteries, as well as in the corpuscavernous, whereas in NIP the blood flow is normal or significantly increased (3,22). Other imaging investigations, such as CT scan, magnetic resonance imaging (MRI) and internal pudendal/penile arteriography can also prove useful, but not in the initial phase of the evaluation process. CT examination may be used at a later stage in order to scan for priapism etiological factors, especially if we suspect an underlying malignancy. MRI is very useful because it provides high quality images of the penile tissues, therefore offering essential information about the viability of the cavernosal smooth muscle fibers and it also allows us to appreciate the patient’s outcome in terms of regaining a normal erectile function (10,23). Internal pudendal arteriography is usually used in NIP cases in order to detect an arterio-cavernosal fistula. This procedure also allows the treatment of priapism etiological factor by super-selective embolization (6,21). In order to provide a successful maneuver of embolization, the most efficient embolization materials are byproducts inducing a temporary embolization (such as autologous blot clot, gelatinous sponge or absorbable gel) or permanent embolization (cyanoacryl, metallic coils). Initially most authors prefer an embolization using temporary materials in order to prevent the development of erectile dysfunction; however, in such cases the rate of recurrence might reach 40% (21).
General Principles of Priapism Management
The majority of priapism cases are ischemic, therefore immediate treatment is necessary in order to avoid its complications. Treatment aims to achieve penile detumescence and relieve local pain, but this may prove to be very challenging. The first-line treatment is represented by corporal aspirative drainage with or without cavernosal irrigation using a saline solution. The success rate attributed to this procedure is estimated to be up to 30% (24). If the aspirative drainage does not prove useful, the current guidelines recommend symphatomimetic agents injected directly in the corpora cavernosum: phenylephrine (200 μg of phenylephrine can be injected every three to five minutes, up to a total dosage of 1mg within one hour), etilephrine (2.5 mg diluted in 1-2 ml saline solution), epinephrine (2 ml out of a dilution of 1/100,000; it can be injected every four minutes for a period of time of maximum 20 minutes), norepinephrine (20-80 μg), methylene blue (50-100 mg) (24-26). These maneuvers can be repeated for up to an hour, but if penile detumescence is not achieved then we must consider performing other procedures (3).
Second-line treatment options include cavernosal shunts and penile prosthesis implantation. The shunt technique, either proximal or distal, consists in creating a fistula that permits the drainage of the blood, which has stagnated in the cavernous bodies. Distal shunts (performed either percutaneous or open) are more popular among surgeons due to the fact that they associate a significantly lower rate of complications compared to proximal shunt procedures and also because they are easier to perform (3,19). The success rate attributed to these procedures is estimated to range between 50% and 70% in terms of achieving penile detumescence, but it is worth mentioning that these techniques can increase the risk of erectile dysfunction due to the fact that they alter the integrity of the erectile tissues (21).
The immediate implant of a penile prosthesis can be directly recommended as a first-line approach for patients diagnosed with IP that lasts more than 72 h, especially for those patients who are sexually active and also to avoid complications such as penile shortening and penile curvature (25). This treatment option can also be considered for cases where the priapism duration is between 24 and 36 h, especially for patients who want to maintain a normal sexual life (1). The risk of overtreatment in such cases can be avoided by using MRI examination, this imaging investigation having a great sensitivity in terms of detecting the presence of necrotic tissue at the level of cavernous smooth muscle fibers, or by biopsies that can be taken from the cavernosal smooth muscle during shunt procedures (1,27). In patients for whom shunt procedures have been previously performed, the implantation of a penile prothesis should be done several weeks after the shunt procedure in order to reduce the risk of infection. According to literature, this period of time varies between 14 and 21 days (28).
NIP is less common, being usually a complication of perineal trauma. Considering the fact that this type of priapism does not require emergency treatment, the first-line approach consists of conservative measures such as observation (periodic clinical and CDU assessment), perineal ice packs and compression, as well as androgen deprivation therapy (gonadotropin releasing hormone agonists and anti-androgens) that aims at reducing the frequency of nocturnal penile erections, which favor the persistence of the arterio-cavernosal fistula (25,29). If the conservative treatment fails, the next step implies angiography with selective fistula embolisation using either temporary agents (autologous blood clot or absorbable gel foam) or permanent materials such as metallic coils or synthetic agents (ethylene-vinyl alcohol and N-butyl-cyanoacrylate) (21,23,30).
Discussion
Priapism associated to malignancy, either hematological or non-hematological, is a rare condition that should be considered in patients with apparently idiopathic priapism, because in some cases the priapism can be the first clinical manifestation of an unknown underlying malignant process. The true incidence of MP is unknown due to its rarity, which also explains the lack of information regarding this subject, the majority of the published articles on this topic being case reports. Nevertheless, researchers have tried to appreciate its incidence based on the existing case reports. A study published in 2019 reported a rate of MP within a group of patients that presented with idiopathic IP of 3.5% (8). According to the existing data most MP cases occur in the context of non-hematological penile metastases, followed by hematological malignancies (5).
Regarding the non-hematological malignancies that lead to penile metastases the most common are urogenital tumors, accounting for up to 70% of penile metastases, followed by tumors originating in the gastrointestinal tract, the percentage of penile metastases associated to these types of tumors being approximately 20% (11,31-33). The first documented case of a penile metastasis dates back to 1870, being reported by Eberth and up to 2011 only 394 cases have been documented (12,32). A 2016 review that focused on MP and penile metastases reported that penile metastases are more frequently associated with urogenital cancers, followed by rectal and colon cancer. By the time the article was published, the authors had identified a total number of 512 cases of penile metastases that had been published in the literature. After analyzing these cases, the authors reported that penile metastases were more frequent in patients with primary tumors located in the bladder and prostate (30.6% respectively 29.6%), followed by rectosigmoid and kidney tumors (14% and 6.6%). Other primary tumors that have led to penile metastases have also been identified, but their incidence was less than 5% (lower gastro-intestinal tumors 3.9%, testicular malignancy 2.9%, lung cancer 2.5%, upper gastro-intestinal neoplasia 2.1%, bone tumors 1.1%). The incidence of hematological malignancies that led to penile metastases was less than 1% (34).
A similar review was published by Cocci and his colleagues, but the number of patients that have been analyzed in this study was significantly smaller compared to the previous study (69 patients versus 512 patients). Therefore, out the 69 patients with penile metastases 33 patients (47.8%) presented urological primary cancers, the majority being prostate and bladder cancers (17 and 13 patients), followed by two kidney cancer cases and a patient with seminal vesicle cancer. The remaining 36 patients (52.2%) presented primary tumors located in the lungs, gastrointestinal track, skin, testicles, thyroid and also hematological malignancies. Out of these 69 patients only ten presented priapism, the ratio between urological and non-urological penile metastases being 1:1. In terms of survival the authors have reported that the patients with urological penile metastases were associated with an increased survival compared with the non-urological penile metastases patients (18 months versus 11 months). A similar observation occurred when comparing the patients with penile metastases that had developed priapism. Therefore, the median survival period in the urological penile metastases group associated with priapism was 30 months, whereas in non-the urological group this period was 15 months. The authors stated that non-urological penile metastases associate with a significant worse prognosis compared to urological penile metastases and that the appearance of priapism in such cases affects even more the prognosis and the survival of these patients (11).
In general the prognosis of the patients diagnosed with malignant priapism due to penile metastases is poor, because the presence of metastases represents an advanced-stage disease, but it also depends on the type of primary tumor, being reported that MP associated to penile metastases originating from the lower digestive tract has better survival rates compared to penile tumors that originate from other primary sites. Furthermore, it has been noticed that patients with hematological malignancies and priapism present significantly higher survival rates compared to those who develop MP in the context of a non-hematological malignancy, this being related to the fact that hematological malignancies are more sensitive to chemo- and radiotherapy and also to the fact that this type of malignancy, in general, associates with a better prognosis (8,35). The current data regarding the overall survival period in patients with penile metastases range between 14 and 18 months, but the average survival is less than one year. In fact, Lin reported in a review that included all the available penile metastases cases up to 2011 that more than 60% of the patients have died within the first six months after being diagnosed with penile metastases (11,32). However, isolated cases of patients with penile metastases that have survived for up to seven and nine years have been reported; the primary tumors originating from the prostate and from the rectosigmoid (9,36).
Considering the fact that most MP cases are ischemic, the management should be based on the initial steps of the IP therapeutic protocol. Therefore, MP cases associated to penile tumors (either primary or metastases) should first be managed with cavernosal aspiration with or without cavernosal irrigation. In case of failure intracavernosal injections with symphatomimetic agents, especially phenylepherine, can be used. If these procedures prove to be unsuccessful then we are facing a real problem, because normally the next step involves performing a cavernosal shunt and/or a penile prosthesis implantation, but in patients with priapism due penile tumors these procedures associate high risks of tumor cell dissemination due to cutaneous seeding (5,8). The next steps could include options such as tumor excision, partial or total penectomy. The patients who present persistent local pain that does not respond to analgesic drugs can be managed with dorsal nerve section, as well as with total penile amputation. The management of these patients should consider the disease stage, the tumor size and its location, the presence of other possible associated clinical manifestations that could occur as a result of the penile tumor (local pain, local infection, ulceration, necrosis, urinary obstructive symptoms), the patient age, his general status and especially his prognosis. Such patients usually require a multidisciplinary approach, because in most cases the treatment is palliative, its purpose being to improve the patient's quality of life (12,20,37,38). Chemotherapy, radio- or brachytheraphy can be considered, but these therapeutic options have shown that they do not improve patient survival. High-flow MP is extremely rare, usually appearing as a result of tumor invasion that creates an arterio-cavernosal fistula. These cases should be managed with internal pudendal arteriography and selective embolisation (9,17).
According to literature, the incidence of priapism among patients with leukemia ranges between 1% and 5%, approximately half of these patients being diagnosed with chronic myeloid leukemia (CML), but there have also been reported cases that have occurred in the context of other hematological malignancies such as acute myeloid leukemia, chronic lymphocytic leukemia and also acute lymphocytic leukemia. Usually, the appearance of a priapic event in this type of patients was noticed to be much more common in undiagnosed patients or in those who had been diagnosed with a hematological malignancy but who either did not receive a proper treatment or did not follow the treatment protocol (5,35).
The management of priapism associated with hematological malignancies must include the general steps that are recommended for the IP treatment and also specific treatment for the hematological malignancy. Therefore, in cases of patients known with malignant hematological disorders who for various reasons are not under adequate treatment, the management of the priapic event requires in addition to the classic treatment of IP the initiation of a proper systemic treatment for the hematological malignancy, whereas in patients with apparently idiopathic IP the possibility of an underlying hemotalogical malignancy should be investigated and if this is confirmed, then systemic treatment should be initiated as soon as possible, being shown that systemic treatment in such patients prevents the recurrence of priapsism (17).
If the initial steps of IP management (cavernosal aspirative drainage with or without intracavernosal injections with symphatomimetic agents) prove ineffective, the next step is to perform cavernosal shunts or penile prosthesis implantation. The surgical approach is possible in patients with hematological malignant priapism, in contrast to patients with MP associated to non-hematological malignancies for which the surgical approach is questionable due to the risk of tumor cell dissemination (5,18).
Systemic therapy includes cytoreductive drugs such as hydroxycarbamide and tyrosine-kinase inhibitors (TKI). The introduction of TKI such as imatinimb, nilotinib, dasatinib has significantly improved the management of hematological malignancies (especially CML), these agents controlling the disease and preventing its unwanted complications. Another option that could be considered is leukapheresis, but its popularity has decreased with the introduction of TKI (35,39). This procedure is a blood filtrating process that separates and removes the white blood cells from the circulating blood and it is recommended especially in patients with acute leukemia who present hyperleucocytosis, the American Society of Apheresis reporting that this procedure can reduce the white blood cell count up 60% (40). Leukapheresis should be considered as an alternative for chemotherapeutic agents in patients for whom this type of treatment proves to be intolerable, especially in the initial phases, and also in patients who develop symptoms as a result of hyperleukocytosis and leukostasis, this procedure significantly reducing the degree of tissue infiltration and blood hyperviscosity, therefore relieving patients from acute symptoms (41).
Several authors have tried over the years to clarify the management of priapism in patients with hematological malignancies, most articles emphasizing on the association of specific IP measures and cytoreductive therapy. Nevertheless, there have been reported several patients for whom specific hematological treatment has proven successful without the use of cavernosal aspirative drainage or without other procedures that are usually applied in IP, but the number of these cases is small (42,43).
A 2012 article that focused on the pathogenesis and management of priapism in patients with CML recommended the use of high dose of hydroxycarbamide and TKI with or without leukapheresis in addition to specific IP treatment in order to reduce hyperleukocytosis and viscosity. The authors suggested that hydroxycarbamide treatment should be initiated in patients with IP suspected of CML with a dosage ranging from 2 to 6 g per day until the white blood cell count is reduced sufficiently, reporting that this treatment could decrease the white blood count with up to 60% in 24 to 48 hours. They also stated that TKI treatment should start immediately after the diagnosis of CML is confirmed. Therefore, considering the CML phase the patients should receive 400 mg imatinib per day in the chronic phase, between 600 and 800 mg per day in the accelerated phase and 800 mg per day in blast crisis (35).
In order to standardize the treatment of such cases, Chisick and colleagues have proposed a two-phase algorithm. They recommend starting the treatment with intravenous hydration and allopurinol, hydroxycarbamide in doses that range between 50 and 100mg/kg/day and a multidisciplinary assessment. The next phase consists in specific IP treatment to which the authors have recommended adding the following measures: periodic assessment of the white cell blood count (recommended every six hours), prophylactic anticoagulants, orally administrated sympathomimetic agents (terbutaline, etilferine, metaraminol, phenylephrine), cytoreductive therapy and leukapheresis (40).
It is worth mentioning the fact that CML has a bimodal age distribution more frequently affecting children of ages between 5 and 10 years and also younger males between 20 and 50 years (35). In these patients, the penile prosthesis approach should be considered the first choice, especially if the priapism exceeds 36-48 hours. There have been debates regarding whether the implant should be performed early or at a later time and the majority of published reports favor an early implant using a malleable prosthesis that can be changed fallowing several months with an inflatable penile prosthesis. This recommendation is based on the fact that an early implant associates with significantly lower complications (infections, urethral injury, cavernosal and albuginea erosions) compared to a delayed implant and also because it is easier to apply it before the complete onset of the fibrotic tissue (6). The preference for a malleable prosthesis as a first option is due to the fact that being a semi-rigid device it acts as an expander within the corpora cavernosa, preventing penile contracture and deformity and also protecting itself from these structural changes that can appear during the weeks that fallow the priapism onset. An inflatable prosthesis can also be used as a first choice, but in order to prevent cavernosal contracture, penile shrinkage and deformity this device must be cycled daily starting several days after its implantation, which creates great discomfort especially in the first days following surgery (44,45).
Considering all these aspects that we have presented we can say that MP is a complex pathology, which proves to be a real challenge for both patients and physicians. These cases require a multidisciplinary assessment in order to establish the etiological factor and a proper treatment that could provide a good outcome, as well as preventing further priapic events.
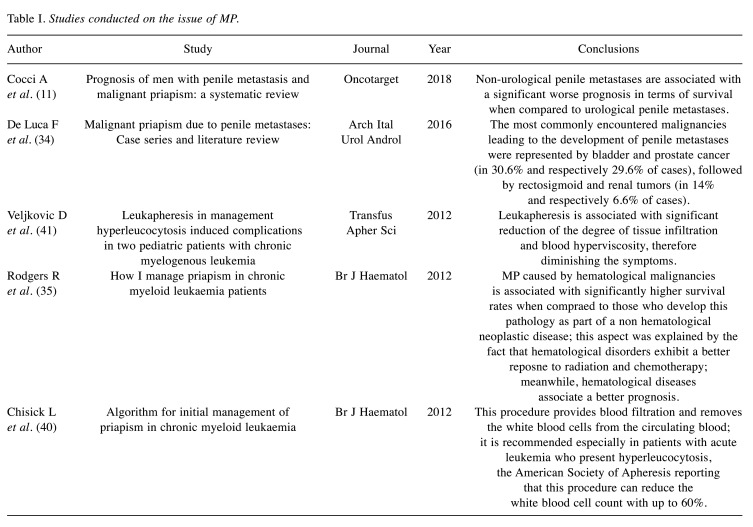
The largest studies conducted on the issue of MP are summarized in Table I.
Table I. Studies conducted on the issue of MP.
Conclusion
Malignancy in spite of being a rare cause for priapism, should be considered in all patients with apparently idiopathic priapism, therefore a proper assessment that could identify the presence of an unknown malignancy is mandatory. The prognosis of MP patients is poor, non-urological penile metastases associating a significant worse prognosis compared to urological penile metastases. On the other hand MP associated to hematological disorders associate with higher survival rates, due to their chemo and radio-sensitivity. The management in such cases is similar to the protocol applied in non-oncological patients to which specific oncological treatment that targets the trigger which has led to the priapism onset is added.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
D. Marcu: Conception, wrote the article, and approved the final version. L. Iorga, N. Bacalbasa, I. Balescu and D. Mischianu: Discussed and revised the article, approved the final version. O.G. Bratu: conception, wrote the article, discussed and revised the article, approved the final version.
References
- 1.Muneer A, Alnajjar HM, Ralph D. Recent advances in the management of priapism. F1000Res. 2018;7:37. doi: 10.12688/f1000research.12828.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson MJ, Kristinsson S, Ralph O, Chiriaco G, Ralph D. The surgical management of ischaemic priapism. Int J Impot Res. 2020;32(1):81–88. doi: 10.1038/s41443-019-0197-9. [DOI] [PubMed] [Google Scholar]
- 3.Zacharakis E, Garaffa G, Raheem AA, Christopher AN, Muneer A, Ralph DJ. Penile prosthesis insertion in patients with refractory ischaemic priapism: early vs delayed implantation. BJU Int. 2014;114(4):576–581. doi: 10.1111/bju.12686. [DOI] [PubMed] [Google Scholar]
- 4.Diaconu CC. Midaortic syndrome in a young man: case presentation. Cor et Vasa. 59:e171–173. doi: 10.106/j.crvasa.2016.04.007. [DOI] [Google Scholar]
- 5.Ralph O, Shroff N, Johnson MJ, Alnajjar HM, Ralph D. Malignancy: A Rare, Important and Poorly Understood Cause of Priapism. Sex Med Rev. 2020 doi: 10.1016/j.sxmr.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AL, Sharlip ID. Standard operating procedures for priapism. J Sex Med. 2013;10(1):180–194. doi: 10.1111/j.1743-6109.2012.02707.x. [DOI] [PubMed] [Google Scholar]
- 7.Scherzer ND, Reddy AG, Le TV, Chernobylsky D, Hellstrom WJG. Unintended Consequences: A Review of Pharmacologically-Induced Priapism. Sex Med Rev. 2019;7(2):283–292. doi: 10.1016/j.sxmr.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 8.James JM, Hallerstrom M, Alnajjar HM, Frederick JT, Skrodzka M, Chiriaco G, Muneer A, Ralph DJ. Which patients with ischaemic priapism require further investigation for malignancy. Int J Impot Res. 2020;32(2):195–200. doi: 10.1038/s41443-019-0141-z. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed H, Elsamra S, Sigman M. Case of malignant priapism and review of the literature. Med Health R I. 2012;95(1):14–15. [PubMed] [Google Scholar]
- 10.Salonia A, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Vardi Y, Wespes E, Hatzimouratidis K. European Association of Urology guidelines on priapism. Eur Urol. 2014;65(2):480–489. doi: 10.1016/j.eururo.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Cocci A, Hakenberg OW, Cai T, Nesi G, Livi L, Detti B, Minervini A, Morelli G, Carini M, Serni S, Gacci M. Prognosis of men with penile metastasis and malignant priapism: a systematic review. Oncotarget. 2018;9(2):2923–2930. doi: 10.18632/oncotarget.23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitley CA, Mosier AD, Keylock J, Nguyen D. Malignant priapism secondary to adenocarcinoma of the prostate. BMJ Case Rep. 2010 doi: 10.1136/bcr.07.2009.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Mufarrej F, Kamel MH, Mohan P, Hickey D. Tricorporal priapism postradical cystoprostatectomy: first sign of recurrent urogenital malignancy. Int J Urol. 2006;13(4):460–462. doi: 10.1111/j.1442-2042.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 14.Dubocq FM, Tefilli MV, Grignon DJ, Pontes JE, Dhabuwala CB. High flow malignant priapism with isolated metastasis to the corpora cavernosa. Urology. 1998;51(2):324–326. doi: 10.1016/s0090-4295(97)00607-9. [DOI] [PubMed] [Google Scholar]
- 15.Jameel T, Mehmood K. Priapism - An unusual presentation in chronic myeloid leukaemia: Case report and review of the literature. Biomedica. 2009;25:197–199. [Google Scholar]
- 16.Mallik AU, Chowdhury GM, Shameem IA. Successful management of priapism secondary to leukemia – A case report. Bangladesh J Urol. 2010;13(2):80–82. [Google Scholar]
- 17.Sun HH, Zhang JH, DeWitt-Foy M, Waldron M, Mukherjee S, Montague DK. Urologic Management of Priapism Secondary to Chronic Myeloid Leukemia. Urology. 2019;125:24–28. doi: 10.1016/j.urology.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Shaeer OK, Shaeer KZ, AbdelRahman IF, El Haddad MS, Selim OM. Priapism as a result of chronic myeloid leukemia: case report, pathology, and review of the literature. J Sex Med. 2015;12(3):827–834. doi: 10.1111/jsm.12812. [DOI] [PubMed] [Google Scholar]
- 19.Capece M, Gillo A, Cocci A, Garaffa G, Timpano M, Falcone M. Management of refractory ischemic priapism: current perspectives. Res Rep Urol. 2017;9:175–179. doi: 10.2147/RRU.S128003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CL, Su B, Kuo HC. The dreadful and the deadly erection - Malignant priapism from renal cell carcinoma. Urol Sci. 2014;25:152–154. doi: 10.1016/j.urols.2014.01.001. [DOI] [Google Scholar]
- 21.Shigehara K, Namiki M. Clinical management of priapism: A review. World J Mens Health. 2016;34(1):1–8. doi: 10.5534/wjmh.2016.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metawea B, El Nashar AR, Gad-Allah A, Abdul-Wahab M, Shamloul R. Intracavernous papaverine/phentolamine-induced priapism can be accurately predicted with color Doppler ultrasonography. Urology. 2005;66(4):858–860. doi: 10.1016/j.urology.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Ingram AR, Stillings SA, Jenkins LC. An update on non-ischemic priapism. Sex Med Rev. 2020;8(1):140–149. doi: 10.1016/j.sxmr.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Huang YC, Harraz AM, Shindel AW, Lue TF. Evaluation and management of priapism: 2009 update. Nat Rev Urol. 2009;6(5):262–271. doi: 10.1038/nrurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAU Guidelines on priapism. Available at: https://uroweb.org/guideline/priapism/?type=pocket-guidelines.
- 26.Muneer A, Ralph D. Guideline of guidelines: priapism. BJU Int. 2017;119(2):204–208. doi: 10.1111/bju.13717. [DOI] [PubMed] [Google Scholar]
- 27.Ralph DJ, Borley NC, Allen C, Kirkham A, Freeman A, Minhas S, Muneer A. The use of high-resolution magnetic resonance imaging in the management of patients presenting with priapism. BJU Int. 2010;106(11):1714–1718. doi: 10.12688/f1000research.12828.1. [DOI] [PubMed] [Google Scholar]
- 28.Tausch TJ, Zhao LC, Morey AF, Siegel JA, Belsante MJ, Seideman CA, Flemons JR. Malleable penile prosthesis is a cost-effective treatment for refractory ischemic priapism. J Sex Med. 2015;12(3):824–826. doi: 10.1111/jsm.12803. [DOI] [PubMed] [Google Scholar]
- 29.Levey HR, Segal RL, Bivalacqua TJ. Management of priapism: an update for clinicians. Ther Adv Urol. 2014;6(6):230–244. doi: 10.1177/1756287214542096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudnall M, Reed-Maldonado AB, Lue TF. Advances in the understanding of priapism. Transl Androl Urol. 2017;6(2):199–206. doi: 10.21037/tau.2017.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaux A, Amin M, Cubilla AL, Young RH. Metastatic tumors to the penis: a report of 17 cases and review of the literature. Int J Surg Pathol. 2011;19(5):597–606. doi: 10.1177/1066896909350468. [DOI] [PubMed] [Google Scholar]
- 32.Lin YH, Kim JJ, Stein NB, Khera M. Malignant priapism secondary to metastatic prostate cancer: a case report and review of literature. Rev Urol. 2011;13(2):90–94. [PMC free article] [PubMed] [Google Scholar]
- 33.Bolocan A, Paduraru DN, Nitipir C, Hainarosie R, Pituru SM, Diaconu C, Suceveanu A, Pantea Stoian A. Mixed adenoneuroendocrine carcinoma of the gastrointestinal tract – features, diagnosis, management and prognostics. Rom Biotech Lett. 2018;23(6):14193–14202. [Google Scholar]
- 34.De Luca F, Zacharakis E, Shabbir M, Maurizi A, Manzi E, Zanghi A, De Dominicis C, Ralph D. Malignant priapism due to penile metastases: Case series and literature review. Arch Ital Urol Androl. 2016;88(2):150–152. doi: 10.4081/aiua.2016.2.150. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers R, Latif Z, Copland M. How I manage priapism in chronic myeloid leukaemia patients. Br J Haematol. 2012;158(2):155–164. doi: 10.1111/j.1365-2141.2012.09151.x. [DOI] [PubMed] [Google Scholar]
- 36.Cherian J, Rajan S, Thwaini A, Elmasry Y, Shah T, Puri R. Secondary penile tumours revisited. Int Semin Surg Oncol. 2006;3:33. doi: 10.1186/1477-7800-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcu D, Spinu D, Mischianu D, Socea B, Oprea I, Bratu O. Oncological follow-up after radical prostatectomy. Rom J Mil Med. 2017;120(3):39–42. [Google Scholar]
- 38.Popescu R, Bratu O, Spinu D, Marcu D, Farcas C, Dinu M, Mischianu D. Neuroendocrine differentiation in prostate cancer – a review. Rom J Mil Med. 2015;118(3):16–19. [Google Scholar]
- 39.Kumar M, Garg G, Sharma A, Pandey S, Singh M, Sankhwar SN. Comparison of outcomes in malignant vs. non-malignant ischemic priapism: 12-year experience from a tertiary center. Turk J Urol. 2019;45(5):340–344. doi: 10.5152/tud.2019.75044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chisick L, Seftel M, Kumar R. Algorithm for initial management of priapism in chronic myeloid leukaemia. Br J Haematol. 2012;159(2):250–251. doi: 10.1111/bjh.12015. [DOI] [PubMed] [Google Scholar]
- 41.Veljkovic D, Kuzmanovic M, Micic D, Serbic-Nonkovic O. Leukapheresis in management hyperleucocytosis induced complications in two pediatric patients with chronic myelogenous leukemia. Transfus Apher Sci. 2012;46(3):263–267. doi: 10.1016/j.transci.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Ponniah A, Brown CT, Taylor P. Priapism secondary to leukemia: effective management with prompt leukapheresis. Int J Urol. 2004;11(9):809–810. doi: 10.1111/j.1442-2042.2004.00872.x. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A, Seth T, Gupta A. Successful use of terbutaline in persistent priapism in a 12-year-old boy with chronic myeloid leukemia. Pediatr Hematol Oncol. 2009;26(1):70–73. doi: 10.1080/08880010802435146. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Salamanca JI, Mueller A, Moncada I, Carballido J, Mulhall JP. Penile prosthesis surgery in patients with corporal fibrosis: a state of the art review. J Sex Med. 2011;8(7):1880–1889. doi: 10.1111/j.1743-6109.2011.02281.x. [DOI] [PubMed] [Google Scholar]
- 45.Tsambarlis PN, Chaus F, Levine LA. Successful placement of penile prostheses in men with severe corporal fibrosis following vacuum therapy protocol. J Sex Med. 2017;14(1):44–46. doi: 10.1016/j.jsxm.2016.11.304. [DOI] [PubMed] [Google Scholar]