Abstract
Background/Aim: The aim of this study was to elucidate the clinical impact of coagulation disorders on outcomes after curative resection of pancreatic ductal adenocarcinoma. Patients and Methods: Preoperative coagulation activity in 135 patients, who had undergone curative resections for pancreatic ductal adenocarcinoma was retrospectively evaluated and the impact on survival outcomes analyzed. Results: A prolonged prothrombin time-international normalized ratio (PT-INR) (≥1.1) was detected in 23/135 patients (17%). Univariate analysis that showed prolonged PT-INR was associated with worse relapse-free (hazard ratio=1.79, p=0.044) and overall (hazard ratio=2.18, p=0.004) survival. Multivariate analyses showed prolonged PT-INR, large tumor (>30 mm), and lymph node metastasis were independent predictors of poor overall survival. Conclusion: Prolonged PT-INR may be a predictor of poor prognosis in patients with pancreatic ductal adenocarcinoma who have undergone curative resection. Coagulation disorders may be a therapeutic target for improving outcomes of pancreatic ductal adenocarcinoma.
Keywords: Pancreatic ductal adenocarcinoma, coagulation activity, coagulation disorders, PT-INR, prognosis
Pancreatic ductal adenocarcinoma (PDAC) comprises 85% of pancreatic cancer cases and remains one of the most common and aggressive malignancies, with a 5-year survival rate of approximately 20% despite improvements in achieving macroscopically curative resection with surgical procedures and adjuvant chemotherapy (1). Several clinical trials have shown that neoadjuvant therapies can prolong survival in patients with PDAC because such therapies can down-stage PDAC and thus improve the curative resection rate. However, it remains to be determined which regimen is the most suitable for PDAC according to each patient’s clinical, genetic, and performance status (2). To improve selection of treatment strategies for PDAC and its outcomes, prognostic biomarkers that can be measured easily and reliably are needed to optimally evaluate tumor aggressiveness. A close association between cancer biology and coagulation disorders that has an impact on tumor progression has been reported (3). Hypercoagulability is more likely to occur in patients with pancreatic cancer than in those with other types of cancer (4). Patients with pancreatic cancer are predisposed to developing venous thromboembolic events (VTEs) (5). VTEs in patients with cancer are caused by hypercoagulable states and associated with a poor prognosis (6). Pancreatic resection is also associated with VTEs (7). In patients with hepatocellular carcinoma, coagulation disorders are associated with various complications and poor prognosis (8). Increased fibrinogen, fibrin split products, and D-dimer levels have been associated with worse survival outcomes in patients with breast and colorectal cancer (9-11). In patients with lung cancer, a prolonged prothrombin time-international normalized ratio (PT-INR) was found to be associated with worse survival outcomes (12). However, the clinical impact of coagulation disorders in PDAC is still unclear. Therefore, the purpose of this study was to evaluate the prognostic significance of coagulation disorders in patients with PDAC who had undergone curative resection.
Patients and Methods
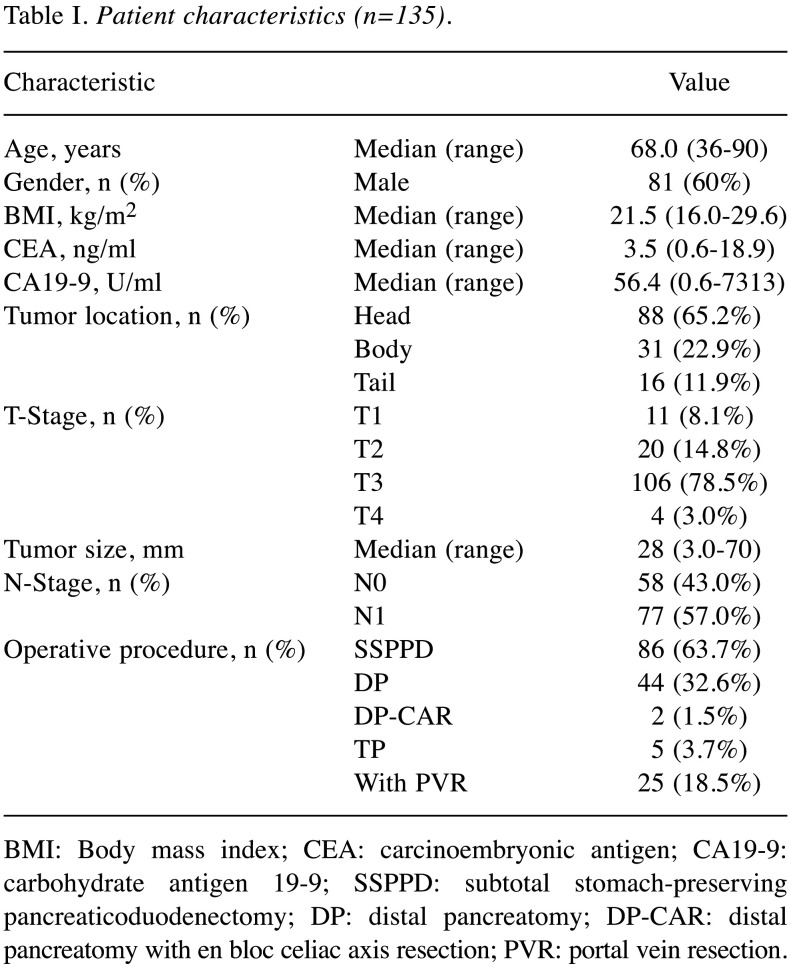
Patients. Between October 2010 and March 2019, 157 consecutive patients underwent pancreatectomy for pancreatic cancer at the Department of Gastroenterological Surgery, Kumamoto University. PDAC was histologically confirmed in 144 of them. Nine of these 144 patients were excluded because they were taking anticoagulant therapy. Finally, this study cohort consisted of 135 patients. This study was approved by the Kumamoto University Hospital Ethics Committee (2020-No.1291) and informed consent was obtained from each patient according to the Institutional Review Board protocols. The enrolled patient’s characteristics are shown in Table I.
Table I. Patient characteristics (n=135).
BMI: Body mass index; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; SSPPD: subtotal stomach-preserving pancreaticoduodenectomy; DP: distal pancreatomy; DP-CAR: distal pancreatomy with en bloc celiac axis resection; PVR: portal vein resection.
Surgical strategy and postoperative follow-up. At our Institution, the routine surgical strategy for PDAC is pancreatic resection with D2 dissection of the lymph nodes according to tumor location or tumor size, as described previously (13). Gemcitabine and fluorouracil-based chemotherapy was administered as adjuvant chemotherapy from 2004 to 2012, and S-1 after 2013. All patients were monitored either until January 2020 or their death, whichever came first. They were followed-up at 3 -monthly intervals by our hospital or affiliated hospitals. The mean follow-up period was 25 months.
Stage of cancer and cut-off for blood tests. The tumors were staged according to the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer staging manual. Postoperative complications were graded according to the Clavien-Dindo classification (14). In this report, postoperative complications were defined as those scoring 3 or more in that classification. Blood tests were performed within the month before pancreatic resection. The cut-off value for each clotting factor was based on normal values used at our Institution. For clinical factors for which normal values had not been established, median values were used.
Statistical analysis. Comparisons between groups were examined using the Mann-Whitney U-test for continuous variables, and the chi-squared or Fisher’s exact test for categorical variables. Continuous variables are presented as the median with range and categorial variables as number with percentage. Relapse-free survival (RFS) rate and overall survival (OS) rate were calculated using the Kaplan-Meier method and compared using the log-rank test. The prognostic factors for RFS and OS after pancreatic resection were investigated using Cox regression models. p-Values are two-sided, and p<0.05 was considered to denote statistical significance. JMP (version 12; SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
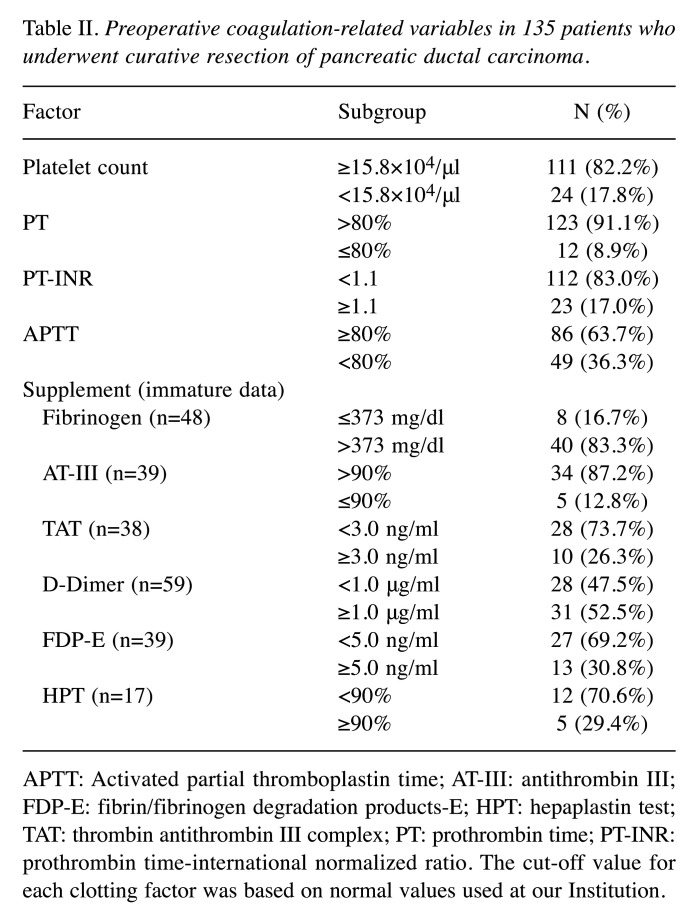
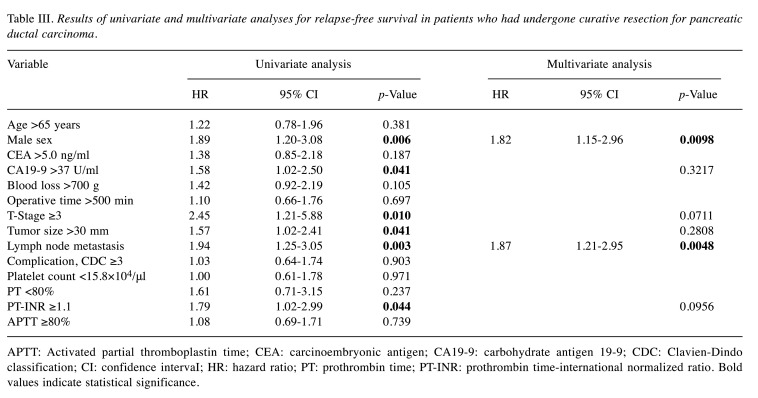
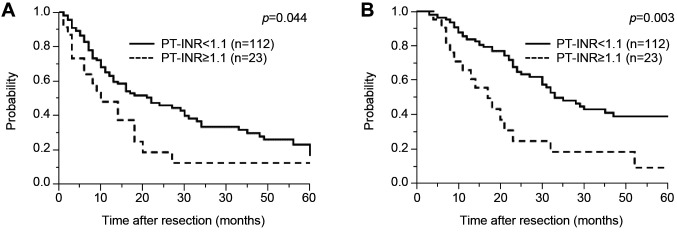
Abnormalities in the coagulation-related variables of platelet count, prothrombin time, PT-INR, and activated partial thromboplastin time were detected in 17.8%, 8.9%, 17%, and 36.3% of patients, respectively (Table II). According to univariate Cox regression analysis, male sex [hazard ratio (HR)=1.89, 95% confidence interval (CI)=1.20-3.08; p=0.006), high carbohydrate antigen 19-9 (CA19-9) concentration (HR=1.58, 95% CI=1.02-2.50; p=0.041), T-stage ≥3 (HR=2.45, 95% CI=1.21-5.88; p=0.001), large tumor size (HR=1.57, 95% CI=1.02-2.41; p=0.041), lymph node metastasis (HR=1.94, 95% CI=1.25-3.05; p=0.003), and prolonged PT-INR (HR=1.79, 95% CI=1.02-2.99; p=–0.044) were significantly associated with shorter RFS (Table III). Survival curve analysis identified that prolonged PT-INR was significantly associated with worse RFS compared with normal PT-INR, the median RFS in patients with prolonged PT-INR being significantly shorter than that in patients with normal PT-INR (11 and 22 months, respectively; p=0.044) (Figure 1A).
Table II. Preoperative coagulation-related variables in 135 patients who underwent curative resection of pancreatic ductal carcinoma.
APTT: Activated partial thromboplastin time; AT-III: antithrombin III; FDP-E: fibrin/fibrinogen degradation products-E; HPT: hepaplastin test; TAT: thrombin antithrombin III complex; PT: prothrombin time; PT-INR: prothrombin time-international normalized ratio. The cut-off value for each clotting factor was based on normal values used at our Institution.
Table III. Results of univariate and multivariate analyses for relapse-free survival in patients who had undergone curative resection for pancreatic ductal carcinoma.
APTT: Activated partial thromboplastin time; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; CDC: Clavien-Dindo classification; CI: confidence intervaI; HR: hazard ratio; PT: prothrombin time; PT-INR: prothrombin time-international normalized ratio. Bold values indicate statistical significance.
Figure 1. Kaplan-Meier analysis of relapse-free (A) and overall (B) survival according to prothrombin time-international normalized ratio (PTINR) in patients who underwent curative resection for pancreatic ductal adenocarcinoma (PDAC).
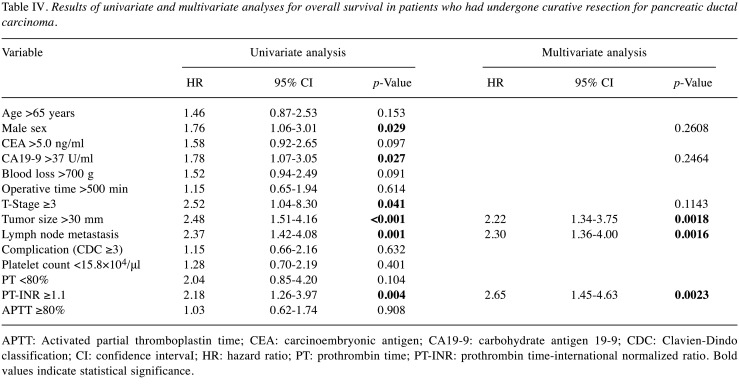
According to multivariate Cox regression analyses, male sex (HR=1.86, 95% CI=1.15-2.96, p=0.0098) and lymph node metastasis (H=1.87, 95% CI=1.21-2.95; p=0.0048) were independent predictors of shorter RFS. For OS, male sex (HR=1.76, 95% CI=1.06-3.01; p=0.029), high CA19-9 concentration (HR=1.78, 95% CI=1.07-3.05; p=0.027), T stage ≥3 (HR=2.52, 95% CI=1.04-8.30; p=0.041), large tumor (HR=2.48, 95% CI=1.51-4.16; p<0.001), lymph node metastasis (HR=2.37, 95% CI=1.42-4.08; p=0.001), and prolonged PT-INR (HR=2.18, 95% CI=1.26-3.97; p=0.004) were significantly associated with shorter OS according to univariate Cox regression analysis (Table IV). According to multivariate Cox regression analyses, large tumor (HR=2.22, 95% CI=1.34-3.75; p=0.0018), lymph node metastasis (HR=2.3, 95% CI=1.36-4.00; p=0.0016), and prolonged PT-INR (HR=2.65, 95% CI=1.45-4.63; p=0.002) were independent predictors of worse OS.
Table IV. Results of univariate and multivariate analyses for overall survival in patients who had undergone curative resection for pancreatic ductal carcinoma.
APTT: Activated partial thromboplastin time; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; CDC: Clavien-Dindo classification; CI: confidence intervaI; HR: hazard ratio; PT: prothrombin time; PT-INR: prothrombin time-international normalized ratio. Bold values indicate statistical significance.
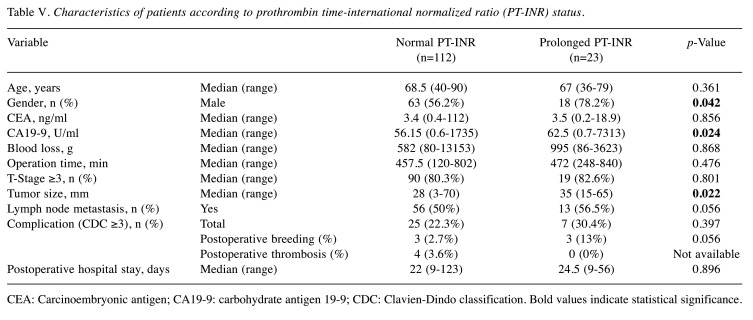
Patients with prolonged PT-INR had significantly worse OS than those with normal PT-INR in the survival curve analysis, the median OS in patients with prolonged PT-INR being significantly shorter than that of patients with normal PT-INR (17 vs. 33 months, respectively; p=0.003) (Figure 1B). Interestingly, when other patient characteristics were compared between patients with normal and prolonged PT-INR, male sex, CA19-9, and large tumor size were found to be significantly associated with prolonged PT-INR (Table V). However, prolonged PT-INR was not significantly associated with surgical outcomes such as intraoperative blood loss and operative time. Postoperative bleeding events requiring transfusion of red blood cells such as rupture of a pseudoaneurysm (n=1), bleeding from drain (n=3), bleeding from anastomosis (n=1), anemia progression (n=1) occurred in six patients (three with normal PT-INR and three with prolonged PT-INR). Postoperative thrombosis was detected in four patients (portal thrombosis in two, deep venous thrombosis in one, and renal infarction in one), all of whom had normal PT-INR. Although prolonged PT-INR was not significantly associated with postoperative bleeding events and thrombosis (p=0.056 and p=not available), bleeding complications in three patients with prolonged PT-INR, namely bleeding from a drain, bleeding from anastomosis, and anemia progression in one patient each, may have been related to their coagulation disorders (Table V).
Table V. Characteristics of patients according to prothrombin time-international normalized ratio (PT-INR) status.
CEA: Carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; CDC: Clavien-Dindo classification. Bold values indicate statistical significance
Discussion
In this study, coagulation disorders characterized by prolonged PT-INR were associated with worse outcomes in patients with PDAC who had undergone curative resection. Thus, we have here identified prolonged PT-INR as a novel marker of poor prognosis in patients with PDAC. The coagulation disorders that are often detected in patients with cancer are usually subclinical, even when coagulation tests are abnormal (15). The PT is a simple test for detecting and diagnosing bleeding or excessive clotting disorders and the international normalized ratio (INR) is a means of standardizing the PT.
Although coagulation disorders in individuals with cancer are multifactorial in origin, several studies have reported the important role of coagulation activity in cancer progression (16,17). Vitamin K, a fat-soluble vitamin, is necessary for the blood coagulation cascade. Vitamin K activates coagulation factors II, VII, IX, X, which promote coagulation. In vitamin K deficiency, the blood concentration of protein induced by vitamin K absence II (PIVKA-II), a precursor of factor II, increases (18). PIVKA-II has been used as a tumor marker, especially for hepatocellular carcinoma. Although patients with malignant tumors other than hepatocellular carcinoma rarely have a high serum PIVKA-II concentration, a high PIVKA-II level has been reported in association with lung, stomach, colon, and ovarian cancer (19-22). The serum concentration of PIVKA-II has also been reported to be higher in patients with pancreatic cancer than in those with benign pancreatic diseases; thus, it may be useful as a tumor biomarker (23). Vitamin K deficiency may be associated with prognosis of patients with pancreatic cancer.
Furthermore, coagulation disorders associated with cancer are reportedly caused by activation of tissue factor (TF) in tumor tissue and activation of MET oncogene (4). TF is recognized as playing an important role in this coagulation process. It is a transmembrane receptor for factor VII/VIIa (FVII/VIIa). The TF:FVIIa complex is called the ‘extrinsic’ pathway because an exogenous agent is required for activation of clotting factors in plasma (24). The TF:FVIIa complex is the key initiator of the coagulation protease cascade, activating both FIX to FIXa and FX to FXa (24). The PT is determined by adding exogenous TF to plasma and is used to assess the activity of the extrinsic pathway. TF and its ligand FVIIa have been shown to play a role in tumor metastasis. The TF:FVIIa complex has been reported to influence tumor metastasis through tumor angiogenesis, inhibition of apoptosis and induces intracellular changes such as cell survival (25). The TF:FVIIa complex has been shown to be an independent risk factor for hepatic metastasis in patients with colon cancer and to play a role in ovarian cancer invasion and metastasis (26-28). Thus, several studies have recently reported on the involvement of the TF:FVIIa complex in cancer progression. To our knowledge, although TF is not known to be associated with progression of pancreatic cancer, TF in patients with pancreatic cancer may act to contribute to prolonged PT-INR, leading to worse prognosis. The underlying mechanism for prolonged PT-INR in patients with PDAC and worse outcomes requires elucidation by further investigation.
The first limitation of this study is that it was retrospective. A second limitation is the validity of cut-off values for coagulation factors. We based the cut-off value for each clotting factor on normal values for our Institution. Other institutions may have different cut-off values, which might lead to different results. A third limitation is that some coagulation factors, such as D-dimers and fibrinogen, were not measured in all study patients and are possibly prognostic markers. A large prospective study is required to further investigate the role of coagulation factors as prognostic factors and their cut-off values.
In conclusion, a prolonged PT-INR might be a simple and novel predictor of prognosis in patients who have undergone curative resection of PDAC. Preoperative normalization of coagulation activity may be an alternative strategy for improving the prognosis of patients with PDAC.
Conflicts of Interest
All Authors of this article have no conflicts of interest to declare.
Authors’ Contributions
Conceptualization: Kazuki Matsumura. Methodology: Kazuki Matsumura, Hiromitsu Hayashi. Formal analysis and investigation: Kazuki Matsumura, Hiromitsu Hayashi. Data curation: Kazuki Matsumura, Norio Uemura, Fumimasa Kitamura, Yusuke Nakao, Toshihiko yusa, Rumi Itoyama. Writing original draft preparation: Kazuki Matsumura. Writing, review and editing: Hiromitsu Hayashi. Supervision: Hideo Baba. All Authors approved the final draft of the article.
Acknowledgements
The Authors thank Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this article.
References
- 1.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLOS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyman GH, Khorana AA. Cancer, clots and consensus: New understanding of an old problem. J Clin Oncol. 2009;27(29):4821–4826. doi: 10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5(11):655–663. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 5.Godinho J, Casa-Nova M, Moreira-Pinto J, Simões P, Paralta Branco F, Leal-Costa L, Faria A, Lopes F, Teixeira JA, Passos-Coelho JL. ONKOTEV score as a predictive tool for thromboembolic events in pancreatic cancer – a retrospective analysis. Oncologist. 2020;25(2):e284–e290. doi: 10.1634/theoncologist.2019-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iguchi T, Sugimachi K, Mano Y, Kono M, Kagawa M, Nakanoko T, Uehara H, Sugiyama M, Ota M, Ikebe M, Morita M4, Toh Y. The preoperative prognostic nutritional index predicts the development of deep venous thrombosis after pancreatic surgery. Anticancer Res. 2020;40(4):2297–2301. doi: 10.21873/anticanres.14195. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XP, Cheng SQ. ASO Author Reflections: Preoperative hypercoagulability predicts poor prognosis in hepatocellular carcinoma patients with microvascular invasion after hepatectomy. Ann Surg Oncol. 2019;26:806–807. doi: 10.1245/s10434-019-07986-5. [DOI] [PubMed] [Google Scholar]
- 9.Oberhoff C, Rollwagen C, Tauchert AM, Hoffmann O, Winkler UH, Schindler AE. Perioperative development of a thrombogenic risk profile in patients with carcinoma of breast: A cause of increased thrombosis. Eur J Gynaecol Oncol. 2000;21(6):560–568. [PubMed] [Google Scholar]
- 10.Mei Y, Liu H, Sun X, Li X, Zhao S, Ma R. Plasma fibrinogen level may be a possible marker for the clinical response and prognosis of patients with breast cancer receiving neoadjuvant chemotherapy. Tumour Biol. 2017;39(6):1010428317700002. doi: 10.1177/1010428317700002. [DOI] [PubMed] [Google Scholar]
- 11.Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31(8):388–394. doi: 10.1093/jjco/hye075. [DOI] [PubMed] [Google Scholar]
- 12.Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Prognostic significance of coagulation assays in lung cancer. Respir Med. 2013;107(3):451–457. doi: 10.1016/j.rmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda Y, Sakoda K, Kinoshita T, Yasui K, Shimada H, Katoh H. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19(3):230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falanga A. Thrombophilia in cancer. Semin Thromb Hemost. 2005;31(1):104–110. doi: 10.1055/s-2005-863812. [DOI] [PubMed] [Google Scholar]
- 16.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: Implications for tumour progression. Biosci. Rep. 2013;33(5):e00064. doi: 10.1042/BSR20130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruf W, Rothmeier AS, Graf C. Targeting clotting proteins in cancer therapy - progress and challenges. Thromb Res. 2016;140:S1–S7. doi: 10.1016/S0049-3848(16)30090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong R, Wang N, Yang Y, Ma L, Du Q, Zhang W, Tran AH, Jung H, Soh A, Zheng Y, Zheng S. Review on vitamin K deficiency and its biomarkers: Focus on the novel application of PIVKA-II in clinical practice. Clin Lab. 2018;64(4):413–424. doi: 10.7754/Clin.Lab.2017.171020. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Inoue T, Fukusato T. Protein induced by vitamin K absence or antagonist II-producing gastric cancer. World J Gastrointest Pathophysiol. 2010;1(4):129–136. doi: 10.4291/wjgp.v1.i4.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasu M, Soma T, Fukushima H, Kudo K, Matsubara O. Hepatoid carcinoma of the lung with production of alpha-fetoprotein and abnormal prothrombin: An autopsy case report. Mod Pathol. 1997;10(10):1054–1058. [PubMed] [Google Scholar]
- 21.Miyashita K, Nagasaka A, Nakanishi M, Kudo T, Wakahama O, Nishikawa S, Higuchi A, Sato H. An alpha-fetoprotein and PIVKA-II producing carcinoma of the colon: report of a case. Nippon Shokakibyo Gakkai Zasshi. 2000;97(12):1480–1486. [PubMed] [Google Scholar]
- 22.Senzaki H, Kiyozuka Y, Mizuoka H, Yamamoto D, Ueda S, Izumi H, Tsubura A. An autopsy case of hepatoid carcinoma of the ovary with PIVKA-II production: Immunohistochemical study and literature review. Pathol Int. 1999;49(2):164–169. doi: 10.1046/j.1440-1827.1999.00840.x. [DOI] [PubMed] [Google Scholar]
- 23.Tartaglione S, Pecorella I, Zarrillo SR, Granato T, Viggiani V, Manganaro L, Marchese C, Angeloni A, Anastasi E. Protein induced by Vitamin K absence II (PIVKA-II) as a potential serological biomarker in pancreatic cancer: A pilot study. Biochem Med (Zagreb) 2019;29(2):020707. doi: 10.11613/BM.2019.020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackman N. The role of tissue factor and factor VIIa in Hemostasis. Anesth Analg. 2009;108(5):1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versteeg HH, Spek CA, Peppelenbosch MP, Richel DJ. Tissue factor and cancer metastasis: The role of intracellular and extracellular signaling pathways. Mol Med. 2004;10(1-6):6–11. doi: 10.2119/2003-00047.versteeg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Versteeg HH, Spek CA, Slofstra SH, Diks SH, Richel DJ, Peppelenbosch MP. FVIIa:TF Induces cell survival via G12/G13-dependent Jak/STAT activation and BclXL production. Circ Res. 2004;94(8):1032–1040. doi: 10.1161/01.RES.0000125625.18597.AD. [DOI] [PubMed] [Google Scholar]
- 27.Seto S, Onodera H, Kaido T, Yoshikawa A, Ishigami S, Arii S, Imamura M. Tissue factor expression in human colorectal carcinoma: Correlation with hepatic metastasis and impact on prognosis. Cancer. 2000;88(2):295–301. doi: 10.1002/(sici)1097-0142(20000115)88:2<295::aid-cncr8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Ma Z, Zhang T, Wang R, Cheng Z, Xu H, Li W, Wang Y, Wang X. Tissue factor–factor VIIa complex induces epithelial ovarian cancer cell invasion and metastasis through a monocyte-dependent mechanism. Int J Gynecol Cancer. 2011;21(4):616–624. doi: 10.1097/IGC.0b013e3182150e98. [DOI] [PubMed] [Google Scholar]