Abstract
Background: Nuchal-type fibroma is a rare benign tumor arising from the connective tissue. Our aim was to present our experience via two cases of this tumor and a comprehensive review of the literature. Patients and Methods: We report a case of a 23-year-old female with a mass located in the posterior neck and the upper back and a case of a 50-year-old male with a mass located in the posterior neck, which were proved to be nuchal-type fibromas in the histopathological examination. We also searched the PubMed/Medline database for published cases of nuchal-type fibromas. Results and Discussion: Nuchal-type fibroma is a rare benign tumor arising from the connective tissue, usually in the posterior neck, which affects different ages, with most patients being male. It is a poorly circumscribed tumor consisting of hypocellular, thick, dense and haphazardly arranged collagen bundles with entrapped adipocytes, nerve fibers and muscle fascicles and a few scattered spindle cells, which are CD34 positive. Its excision is curative, and the recurrence risk is generally low. However, patients with Gardner’s syndrome may experience recurrence more frequently. Conclusion: Nuchal-type fibroma should be included in the differential diagnosis of subcutaneous, soft-tissue masses, especially when these involve the posterior neck.
Keywords: Nuchal, fibroma, tumor, CD34, review
Nuchal-type fibroma is a rare benign tumor arising from the connective tissue (1). It is usually located in the posterior neck (2-18) and affects different ages (5), with most patients being male (1-3,5-13,17,19-29). Since the first description of nuchal-type fibroma by Enzinger and Weiss in 1988 (1), over than 100 cases have been reported in the literature (1-33).
Herein, we report a case of a 23-year-old female with a mass located in the posterior neck and the upper back and a case of a 50-year-old male with a mass located in the posterior neck. Both cases were proved to be nuchal-type fibromas in the histopathological examination. We present a review of all the reported cases of this entity in the literature.
Patients and Methods
Cases presentation
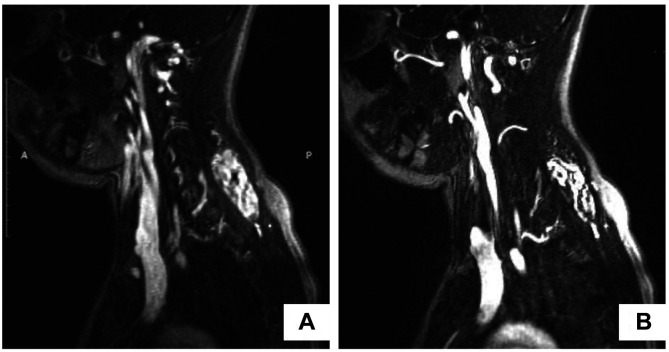
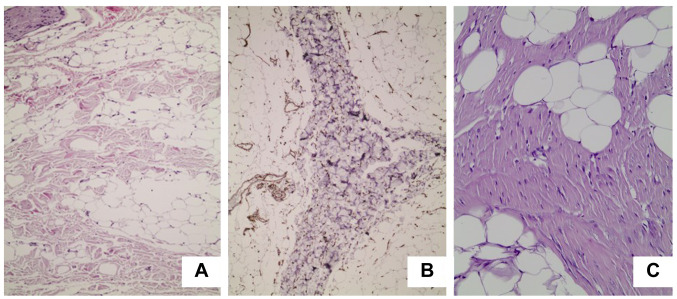
Case 1. A 23-year-old female was admitted to our department with a painless, firm, relatively fixed mass 5cm in diameter that was located in the posterior neck and the upper back. The mass originally appeared six months earlier and had reportedly increased in size during the last two months. The patient underwent a magnetic resonance imaging (MRI) scan, which revealed the presence of a well-demarcated tumor 4.8cm in diameter, between the left trapezius muscle and the left splenius muscle, and a small lipoma 2cm in diameter, located superficially to the deeper lesion (Figure 1A). Both lesions demonstrated high signal intensity in T1 weighted MRI sequence, with heterogeneous contrast enhancement for the larger tumor and homogeneous contrast enhancement for the smaller tumor (Figure 1B). The patient underwent surgical excision of the tumors under general anesthesia. Her postoperative course was uneventful, and she was discharged from the hospital the next day. The macroscopic pathological examination of the surgical specimens revealed the presence of a poorly circumscribed, firm, yellow-white mass with fatty and fibrous areas which was 5 cm×2.5 cm×0.5 cm in dimensions for the larger lesion (Figure 2) and a well circumscribed, soft, yellow, fatty mass 2 cm in diameter for the smaller lesion. The histological examination of the larger tumor showed the presence of hypocellular dense collagen bundles with areas of entrapped adipocytes and nerve fibers. A few spindle cells without atypia were identified within the collagen bundles (Figure 3a). Immunohistochemistry demonstrated that these spindle cells were stained positive for CD34 (Figure 3b). The aforementioned histopathological features of the tumor supported the diagnosis of a nuchal-type fibroma. As far as the smaller tumor is concerned, it was a typical lipoma. No recurrence has been detected up to this point, thirty months after the lesion’s excision.
Figure 1. Magnetic resonance imaging (MRI) findings. A: MRI scan (T1 weighted sequence) showing two tumors with high signal intensity located in the posterior neck and the upper back; B: MRI scan (T1 weighted sequence) showing heterogeneous contrast enhancement of the larger tumor and homogeneous contrast enhancement of the smaller tumor.
Figure 2. Macroscopic view of the surgical specimen.
Figure 3. Microscopic view of the surgical specimen. A: Thick, haphazardly arranged collagen fibers with entrapped adipocytes and a few spindle cells (hematoxylin and eosin stain, 100× original magnification). B: Spindle cells stained positive for CD34 (100× original magnification). C: Microscopic view of the surgical specimen. Thick, haphazardly arranged collagen fibers with entrapped adipocytes and a few spindle cells (hematoxylin and eosin stain, 200× original magnification).
Case 2. A 50-year-old male was also admitted to our department with a painless, firm, movable mass 2.5 cm in diameter that was located in the posterior neck. The mass had made its appearance two years earlier and had become larger during the last six months. The patient underwent surgical excision of the tumor under local anesthesia, with the clinical diagnosis of neck lipoma. The macroscopic pathological examination of the surgical specimen revealed the presence of a poorly circumscribed, firm, yellow-white mass with fatty and fibrous areas which was 2.5 cm×2.2 cm×1.2 cm in dimensions. The histological examination of the tumor showed the presence of dense collagen bundles mixed with adipocytes. A few spindle cells without atypia were identified within the collagen bundles (Figure 3C). Immunohistochemistry revealed that these spindle cells produced CD34. All these histopathological features set the diagnosis of a nuchal-type fibroma. There was no recurrence twenty-two months after the lesion’s excision.
Literature search. An extensive search of the PubMed Index was performed for published cases of nuchal-type fibromas using the following terms: “nuchal”, “neck”, “cervical”, “nuchal-type” and “fibroma”. Results were filtered for publications in English, concerning human subjects. All references from the identified publications were searched for other non-indexed cases.
Out of fifty tree publications, thirty-two manuscripts were finally included in this review (Figure 4). Some publications (n=6) could not be electronically retrieved and were excluded. Fifteen were also excluded as they were earlier reviews of the literature.
Figure 4. Macroscopic view of the surgical specimen.
Results and Discussion
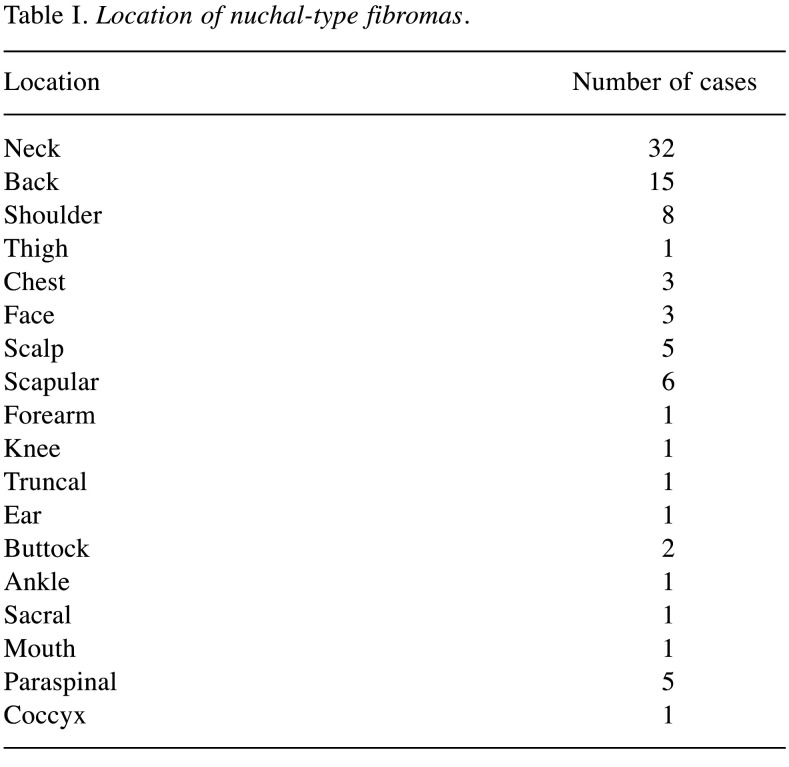
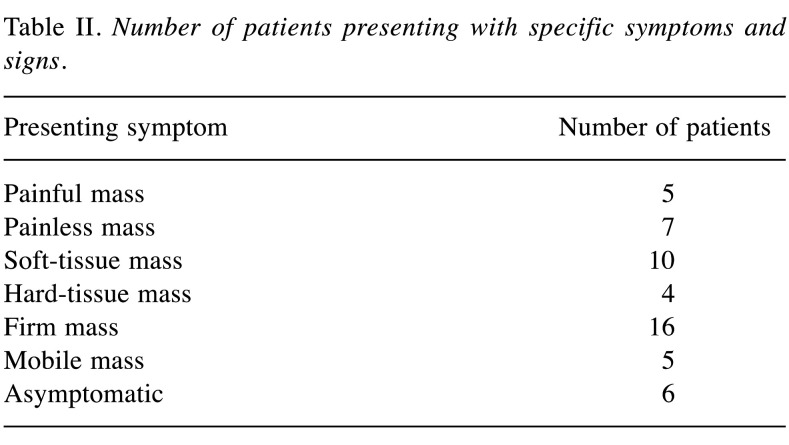
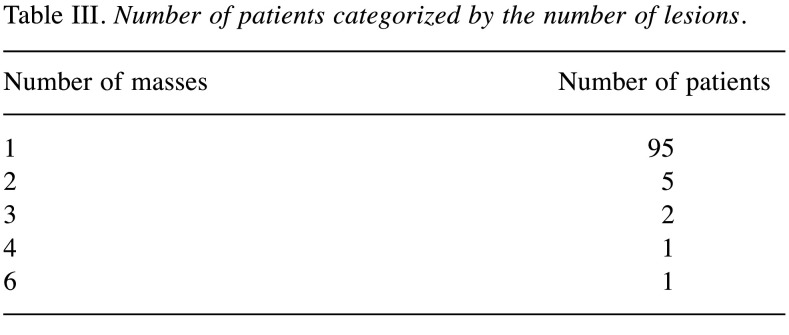
We were able to identify 107 cases of nuchal-type fibroma in the existing literature, including the present cases (2-33). Most patients were male (1-3,5-13,17,19-29). In particular, there were 82 (76.6%) male and 25 (23.4%) female patients (2-9,11-22,24-26,30,31,33). Patient age in the reported cases varied greatly, ranging from 3 months to 80 years, with a mean age of 43.7 years (1-33). Most nuchal-type fibromas arose in the posterior neck (2-9,11-18,23). However, other anatomic sites of origin were also reported less frequently, such as the back (3,5,20-22) chest (20,21), shoulder (5,19,22,27,30,32), scapula (2,5,19), thigh (21), buttock (25,31), scalp (3,21,26), sacral region (28), mouth floor (33), eyebrow (29) and coccyx (23) (Table I). A nuchal-type fibroma usually presents itself as a subcutaneous, soft-tissue, firm mass (3,5,6,8,12-19,21,25,29,32) that can be fixed or mobile (6,8,11,14,16-28) and painless or mildly tender (1,3,4,7,14,16-19,21,24-28,31,33) (Table II). A wide range of tumor sizes was reported, with tumor diameter ranging from 0.7 cm to 20 cm (1-5,7,9-11,13-16,18-24,27-29,31-33). Most patients, specifically 95 out of 107 (88.8%), presented with a single nuchal-type fibroma (1-13,16-25,27,28,30-33). Cases with 2 (5,14,15,21,22), 3 (21), 4 (21) or even 6 fibromas (26) have also been reported (Table III).
Table I. Location of nuchal-type fibromas.
Table II. Number of patients presenting with specific symptoms and signs.
Table III. Number of patients categorized by the number of lesions.
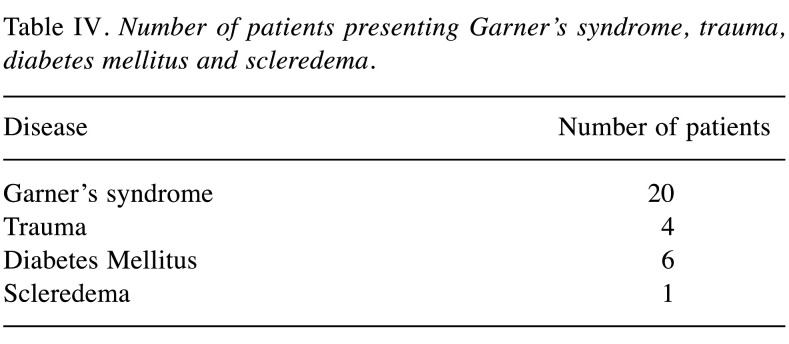
There was an association between the appearance of nuchal-type fibromas and Gardner’s syndrome (5,7,20-22,25). Twenty out of the 107 (18.7%) reported patients had Gardner’s syndrome (5,7,20-22,25,29). However, the majority of the patients, specifically 87 out of the 107 cases (81.3%), had no medical history of this syndrome (1-6,8-19,21,23,24,26-28,30,31,33). It is noteworthy that out of the 12 patients with multiple fibromas (5,10,14,15,21,22,26,29), 6 (50.0%) had Gardner’s syndrome (5,21,22). Moreover, the majority of patients with nuchal-type fibromas and Gardner’s syndrome were male [13/20 (65.0%)]. Another interesting finding is that patients with Gardner’s syndrome were younger (mean age: 15.6 years, range: 3 months - 60 years) (5,7,20-22,25,29) than patients without (mean age: 40 years, range: 6 months - 74 years) (1-6,8-19,21,23,24,26-28,30,31,33). Furthermore, in 6 cases nuchal-type fibroma was accompanied by diabetes mellitus (4,5,33) and in 6 cases the fibroma reportedly presented at a site of previous trauma (2,6,12,22,27,28). Table IV describes the coexistence of nuchal-type fibroma with other diseases.
Table IV. Number of patients presenting Garner’s syndrome, trauma, diabetes mellitus and scleredema.
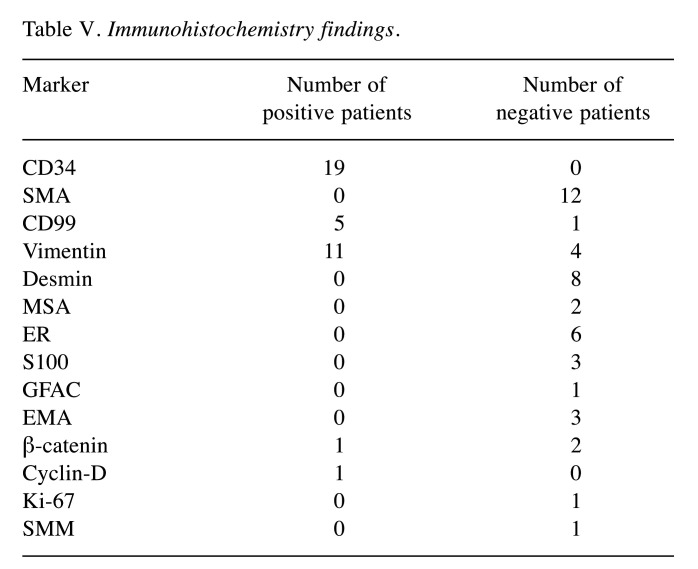
In regard to the histopathological examination, there is a remarkable homogeneity of the findings among the various reported cases. The macroscopic examination of nuchal-type fibromas uniformly demonstrated circumscribed and firm masses (5,8,9,20-22,28,32,33) of usually yellow and/or white color, corresponding to fatty and fibrous areas, respectively (3-5,8-10,21,22,24,25,32). Microscopically, the specimen consisted of hypocellular, thick, dense and haphazardly arranged collagen bundles. Specifically, elastic fibers, entrapped nerve fibers, adipose tissue and muscle fascicles were present within the collagen bundles (2-9,11,13-15,17-22,24-27,30-32). There are a few elastic fibers (3,5,8,11,20,22,24,25,30) and often entrapped nerve fibers, adipose tissue and muscle fascicles within the collagen bundles (3,5,7,8,12-15,18-22,24-27,30,32). Sparsely scattered spindle cells could also be detected among the collagen fibers; the cells resembled fibroblasts with no evidence of atypia (2,3,5,7,13,21,22,24-27,30,31). The immunohistochemical examination showed that these spindle cells were CD34 positive (1,8,12,16,20-22,25,27-29,33) and smooth muscle actin (SMA) (5,16,18-21,25,33), desmin (5,18,21) and S100 negative in all cases (5,19). However, as for vimentin (3,5,16,21,22,25) and CD99 (8,12,25), the literature is more controversial, with reports suggesting that nuchal-type fibromas may or may not express these markers (16,21) (Table V).
Table V. Immunohistochemistry findings.
Only a few reports focus on the findings of imaging studies in nuchal-type fibromas (7,9,10,12,17-19,23,24,28,29,31-33). Two articles reported that a nuchal-type fibroma was depicted as a hypoechoic mass during a diagnostic ultrasound (17,29). Moreover, another article reported a nuchal-type fibroma with high density and two articles reported well-circumscribed masses on computed tomography (9,29,33). In addition, there are also a few reports of patients with this type of tumor that underwent MRI scans. The respective lesions demonstrated low signal intensity on T1, T2, turbo inversion recovery magnitude (TIRM) and short inversion time inversion recovery (STIR) weighted MRI sequences (7,19,23,24,29,31) with one tumor displaying homogeneous enhancement (31) and another tumor no enhancement after intravenous contrast administration (7). However, there was a case in which the tumor had mixed signal intensity in T1 and T2 weighted MRI sequences (32).
Data regarding patient surveillance were available in 51 out of the 107 reported cases (3,5,11,16,17,21,22,24,25,27,32). Thirteen patients with available follow-up data suffered from Gardner’s syndrome (5,21,22,25) while the remaining 38 did not (3,5,11,16,17,24,27,32). Recurrence was reported in 9 out of 51 cases (17.6%) (5,21). Interestingly, 4 out of 13 cases (30.8%) of fibroma in patients with Gardner’s syndrome recurred as desmoid tumors (21), whereas only 5 out of the 38 cases (13.2%) without Gardner’s syndrome experienced recurrence (5).
Conclusion
Our patients had typical nuchal-type fibromas. The only exception was perhaps the fact that the lesion in the 23-year-old female patient demonstrated high signal intensity in T1 weighted images of MRI scan with heterogeneous contrast enhancement. In conclusion, nuchal-type fibroma is a rare benign tumor arising from the connective tissue, usually in the posterior neck. It is a poorly circumscribed tumor consisting of hypocellular, thick, dense and haphazardly arranged collagen bundles with entrapped adipocytes, nerve fibers and muscle fascicles and a few scattered spindle cells, which are CD34 positive. Its excision is curative, and the recurrence risk is generally low. However, patients with Gardner’s syndrome may experience recurrence more frequently. Nuchal-type fibroma should be included in the differential diagnosis of subcutaneous, soft-tissue masses, especially when these involve the posterior neck. Indications of the existence of a nuchal-type fibroma may be the location in the posterior neck and a compatible imaging study, especially when the mass is presented in a male patient or a patient with Gardner syndrome. Nevertheless, histological examination combined with immunohistochemistry is needed for definitive diagnosis.
Conflicts of Interest
All the Authors declare that there are no conflicts of interest regarding this study.
Authors’ Contributions
IDK, CD and EAA designed the study. IDK, TF and CD wrote the article. IDK, TF, CD and NG collected the data. EAA performed the surgical operations. GL and IP performed the histopathological evaluations. CD and NG critically revised the manuscript and offered scientific advice. AG and VEG revised the manuscript. EAA was the supervisor.
References
- 1.Enzinger FM, Weiss SW. Benign tumors and tumorlike lesions of fibrous tissue. In: Soft Tissue Tumors, 2nd Ed. Enzinger FM (ed.) St Louis, Mosby. 1988;In:pp 102–135. [Google Scholar]
- 2.Lister DM, Graham-Brown RA, Burns DA, Richardson RA, Milward TM. Collagenosis nuchae – A new entity. Clin Exp Dermatol. 1988;13(4):263–264. doi: 10.1111/j.1365-2230.1988.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran K, Allen PW, MacCormac LB. Nuchal fibroma. A clinicopathological study of nine cases. Am J Surg Pathol. 1995;19(3):313–317. [PubMed] [Google Scholar]
- 4.Abraham Z, Rozenbaum M, Rosner I, Naschitz Y, Boss Y, Rosenmann E. Nuchal fibroma. J Dermatol. 1997;24(4):262–265. doi: 10.1111/j.1346-8138.1997.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 5.Michal M, Fetsch JF, Hes O, Miettinen M. Nuchal-type fibroma: A clinicopathologic study of 52 cases. Cancer. 1999;85(1):156–163. doi: 10.1002/(sici)1097-0142(19990101)85:1<156::aid-cncr22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41(1):39–41. doi: 10.1046/j.1440-0960.2000.00386.x. [DOI] [PubMed] [Google Scholar]
- 7.Dawes LC, La Hei ER, Tobias V, Kern I, Stening W. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70(11):824–826. doi: 10.1046/j.1440-1622.2000.01958.x. [DOI] [PubMed] [Google Scholar]
- 8.Diwan AH, Horenstein MG. Dermatofibrosarcoma protuberans association with nuchal-type fibroma. J Cutan Pathol. 2004;31(1):62–66. doi: 10.1046/j.0303-6987.2004.0129.x. [DOI] [PubMed] [Google Scholar]
- 9.Tsunemi Y, Saeki H, Tamaki K. Nuchal fibroma clearly visualized by computed tomography: A case report. Int J Dermatol. 2005;44(8):703–704. doi: 10.1111/j.1365-4632.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S, Nakano H, Kaneko T, Hanada K. A Japanese case of nuchal-type fibroma. J Dermatol. 2005;32(11):931–932. doi: 10.1111/j.1346-8138.2005.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 11.Abe M, Nagai Y, Okada E, Aoyama K, Tamura A, Ishikawa O. Case of nuchal fibroma. J Dermatol. 2007;34(7):498–500. doi: 10.1111/j.1346-8138.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Núñez A, Tardío JC, Castellano-Megias VM, Romero-Maté A, Borbujo J. Nuchal-type fibroma associated with lipoma and traumatic neuroma. J Eur Acad Dermatol Venereol. 2007;21(10):1420–1422. doi: 10.1111/j.1468-3083.2007.02223.x. [DOI] [PubMed] [Google Scholar]
- 13.Karonidis A, Rigby HS, Orlando A. Collagenosis nuchae: A case report of a rare and often misdiagnosed condition. J Plast Reconstr Aesthet Surg. 2007;60(3):320–323. doi: 10.1016/j.bjps.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 14.Lee SE, Kim YC, Kim SC. Nuchal fibroma presenting as two posterior neck masses. J Dermatol. 2007;34(4):262–263. doi: 10.1111/j.1346-8138.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc KG Jr, Wenner M, Davis LS. Multiple nuchal fibromas in a 2-year-old without Gardner syndrome. Pediatr Dermatol. 2011;28(6):695–696. doi: 10.1111/j.1525-1470.2011.01415.x. [DOI] [PubMed] [Google Scholar]
- 16.Döngel I, Yazkan R, Duman L, Oztürk O, Kapucuoğlu FN. Huge inflammatory myofibroblastic tumor of pleura with concomitant nuchal fibroma. Ann Thorac Surg. 2013;96(4):1461–1464. doi: 10.1016/j.athoracsur.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 17.Møller M, Sørensen FB. Fibroma nuchae i nakkeregionen. Ugeskr Laeger. 2014;176:50–51. [Google Scholar]
- 18.Alsaleh N, Amanguno H. Nuchal Fibroma: A rare entity of neck masses. Gulf J Oncolog. 2015;1(18):10–12. [PubMed] [Google Scholar]
- 19.Hameed M, Benevenia J, Blacksin M, Aisner SC. Nuchal fibroma of the shoulder involving skeletal muscle: A radiographic and clinicopathological study. A case report. J Bone Joint Surg Am. 1998;80(11):1684–1686. doi: 10.2106/00004623-199811000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Diwan AH, Graves ED, King JA, Horenstein MG. Nuchal-type fibroma in two related patients with Gardner’s syndrome. Am J Surg Pathol. 2000;24(11):1563–1567. doi: 10.1097/00000478-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Wehrli BM, Weiss SW, Yandow S, Coffin CM. Gardner-associated fibromas (GAF) in young patients: A distinct fibrous lesion that identifies unsuspected Gardner syndrome and risk for fibromatosis. Am J Surg Pathol. 2001;25(5):645–651. doi: 10.1097/00000478-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Michal M, Boudova L, Mukensnabl P. Gardner’s syndrome associated fibromas. Pathol Int. 2004;54(7):523–526. doi: 10.1111/j.1440-1827.2004.01660.x. [DOI] [PubMed] [Google Scholar]
- 23.Shin JB, Son SW, Kim IH. Nuchal-type fibroma of the coccyx. Ann Dermatol. 2008;20(1):41–44. doi: 10.5021/ad.2008.20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sraj SA, Lahoud LE, Musharafieh R, Taha A. Nuchal-type fibroma of the ankle: A case report. J Foot Ankle Surg. 2008;47(4):332–336. doi: 10.1053/j.jfas.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Linos K, Sedivcová M, Cerna K, Sima R, Kazakov DV, Nazeer T, Glazyrin A, Valerian BT, Carlson JA. Extra nuchal-type fibroma associated with elastosis, traumatic neuroma, a rare APC gene missense mutation, and a very rare MUTYH gene polymorphism: A case report and review of the literature. J Cutan Pathol. 2011;38(11):911–918. doi: 10.1111/j.1600-0560.2011.01745.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim do H, Kim TH, Sung NH, Shin H, Lee AY, Lee SH. Multiple nuchal-type fibromas on the scalp: A case report. Ann Dermatol. 2015;27(2):194–196. doi: 10.5021/ad.2015.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CC, Lai CS, Lin CH, Lin YN, Chu SC, Chai CY, Lee CH. Extra nuchal-type fibroma associated with repetitive blunt trauma during religious activities. Trauma Case Rep. 2016;4:16–20. doi: 10.1016/j.tcr.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JS, Mun JH. Asymptomatic solitary mass on the sacral region. Clin Exp Dermatol. 2019;44(5):535–539. doi: 10.1111/ced.13760. [DOI] [PubMed] [Google Scholar]
- 29.Kiessling P, Dowling E, Huang Y, Ho ML, Balakrishnan K, Weigel BJ, Highsmith WE Jr, Niu Z, Schimmenti LA. Identification of aggressive Gardner syndrome phenotype associated with a de novo APC variant, c.4666dup. Cold Spring Harb Mol Case Stud. 2019;5(2):a003640. doi: 10.1101/mcs.a003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller PI, Kunte C, Sander CA, Kind P. Nuchales fibrom. Hautarzt. 1997;48(10):759–761. doi: 10.1007/s001050050657. [DOI] [PubMed] [Google Scholar]
- 31.Lee GK, Suh KJ, Lee SM, Lee SJ. Nuchal-type fibroma of the buttock: Magnetic resonance imaging findings. Jpn J Radiol. 2010;28(7):538–541. doi: 10.1007/s11604-010-0459-4. [DOI] [PubMed] [Google Scholar]
- 32.Gong Y, Zhao X, Wu DI, Liu J. Nuchal-type fibroma of the shoulder: A case report and review of the literature. Oncol Lett. 2016;11(6):4152–4154. doi: 10.3892/ol.2016.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poisson M, Ferri J, Schlund M. Mouth floor tumour in an elderly woman. J Stomatol Oral Maxillofac Surg. 2019;120(1):75–76. doi: 10.1016/j.jormas.2018.10.009. [DOI] [PubMed] [Google Scholar]