Abstract
Background/Aim: Development of malignant tumors is preceded by molecular biological events. Our aim was to establish an assay panel by using miRNAs and other genes for the rapid screening of potential carcinogens or chemopreventive agents. Materials and Methods: Six male and 6 female CBA/Ca mice received 20 mg/bwkg 7,12-dimethylbenz(α)anthracene (DMBA) intraperitoneally, and 24 h later RNA was isolated from parenchymal organs. Expression of miR-330, miR-29a, miR-9-1, miR-9-3 and mTORC1 was analysed by real time polymerase chain reaction and compared to non-treated controls. Results: DMBA caused significant alterations in the expression of the studied genes. The most profound changes were the strongly elevated miR-9-3 and mTORC1 expressions in female mice in all organs studied. Conclusion: miR-9-3 and mTORC1 expression in female mice were found to be the most suitable biomarkers for rapid identification of possible carcinogenic effects.
Keywords: Gene expression, microRNA, carcinogenicity, 7, 12-dimethylbenz(α)anthracene, DMBA, miR-330, miR-29a, miR-9-1, miR-9-3, mTORC1
According to estimates, in 2018 there were 18.1 million new malignant cancer cases and 9.6 million cancer deaths (1). According to WHO data, 30-50 % of all malignant tumors could be prevented (2), and early diagnosis would usually increase the chance of successful treatment.
The development of most malignant tumors is preceded by molecular biological events such as changes in gene expression patterns. During this period clinical symptoms do not appear, so, it is difficult to identify any signs of the carcinogenic process. Identification of tumor development based on mutations, chromosome aberrations and/or the phenotypic appearance of the disease is costly, time-consuming and often delayed. Therefore, development of comprehensive biomarker assays to detect the harmful effects of carcinogenic substances as early as possible is of great interest.
Conventional carcinogenecity examination methods - like the Ames test, that is applicable to detect mutations - are based on genotoxicity and show mutagenicity at chromosome or gene levels (3). However, changes in gene expression can signal damages caused by a much wider range of carcinogenic compounds (as well as by ionizing radiation) both in cell cultures and in vivo animal models. Within this, mapping of the effects of microRNAs (miRNAs) provides the possibility to recognize details of relevant regulatory pathways and the functions of mutually regulated miRNAs, as well as the modifications and the physiological processes reflected by their patterns of expression, even as biomarkers. Biomarkers providing early (24-48 h) identification of possibly tumorigenic agents may play an important role in the deeper understanding of carcinogenic processes. Finally, using them as tools for primary, secondary and tertiary prevention they can open up new possibilities in further reduction of cancer incidence or mortality rates.
The miRNAs are small, 19-22 nucleotide length single stranded RNA molecules. Via their binding to the corresponding 3’ UTR of mRNAs, they influence the translation process and protein synthesis. miRNAs regulate the expression of approximately 30-50% of the human genes (4,5), thereby influencing important biological processes like cell cycle (6), differentiation or apoptosis (7). It is noteworthy, that many miRNAs are present in fragile genomic regions and in malignant tissues they mostly display altered expression patterns (8,9). Certain miRNAs are direct targets of oncoproteins or tumor suppressor proteins, for example the p53 tumor suppressor protein regulates the expression of miR-34 through a p53-responsive element upstream of the miR-34 locus (10,11). Others directly regulate the expression of oncogenes and/or tumor suppressor genes, such as miR-199a that regulates the MET proto-oncogene (12). Furthermore, several microRNAs, e.g. miR-134, are involved in the proliferation of tumor cells, in apoptosis, invasion and metastasis (13). Evidence based on experimental studies and clinical histological sample analysis suggests that miRNAs in the serum are relatively stable and could serve as potential biomarkers for the detection of different tumors and other diseases (14).
A wide variety of potentially carcinogenic and anti-carcinogenic agents are known. 7,12-dimethylbenz(α)anthracene (DMBA) is a commonly occuring environmental polycyclic aromatic hydrocarbon that, acting as a mutagene, is capable of exerting a carcinogenic effect, that can be tested both in cell culture and in vivo animal models (15,16). DMBA is activated by cytochrome P450 metabolizing enzymes (17,18), and then it may participate in the early stages of carcinogenesis, i.e. in the initiation and the promotion (19). Previous studies have demonstrated that DMBA affects the expression of H-RAS, C-MYC and TP53 genes even 24-48 h after treatment, which can thus be used as early biomarkers (20).
Our aim was to establish an assay panel by using miRNAs and other genes, that can be used for the rapid screening of potential carcinogens, or anticarcinogenic substances. Therefore, in the present study experimental animals were exposed to DMBA and the expression levels of miR-330, miR-29a, miR-9-1, miR-9-3 were examined. These miRNAs have already been reported to exhibit altered expression in several malignant tumors and through different signaling pathways they play a role in the regulation of oncogenes or tumor suppressor genes, cell growth, proliferation, invasion, migration, metastasis or apoptosis (21-26). Besides the mentioned microRNAs, we also examined the expression of the mTORC1 gene. Activating mutations in mTORC1 have been identified in a wide range of human cancers and as one of the central regulators of cell growth, it has a key role in the development of malignant tumors, cardiovascular diseases, obesity or diabetes (27,28). Our hypothesis was that the altered expression patterns of the studied miRNAs and mTORC1, as biomarkers, may indicate early damages caused by DMBA. In order to explore this, parenchymal organs of CBA/CA mice, i.e. the spleen, liver and kidneys were examined after exposure to DMBA. Thus, we can further understand the supposed roles of these miRNAs and mTORC1 in tumorigenesis and we can evaluate their potential use as early biomarkers by developing an assay panel for identification of early signs of carcinogenic processes.
Materials and Methods
During the test we used two groups of 6-8 weeks old CBA/Ca mice. Both the control and the DMBA treated group consisted of 12 mice (6 males and 6 females). The DMBA treated group received 20 mg/kg/bw DMBA intraperitoneally disolved in 0.1 ml corn oil (Sigma), while the control group received 0.1 ml corn oil only. Following a 24 h exposure to DMBA, the mice were sacrificed by cervical dislocation, the liver, kidneys and spleen were removed and RNA was isolated from them as described below. Mice received humane care in accordance with the appropriate guidelines concerning laboratory animals, and the experiment was conducted according to the current ethical permission (Ethical permission no.: BA02/2000-79/2017).
RNA isolation. Total cellular RNA was isolated using TRIZOL reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The quality of RNA was examined by NanoDrop absorption photometry and for RT-PCR process only RNA fractions with A >2.0 at 260/280 nm were used.
Reverse transcription polymerase chain reaction (RT-PCR). One-step PCR, including reverse transcription and target amplification, was performed using Kapa SYBR FAST One-step qPCR kit (Kapa Biosystems, Wilmington, MA, USA) in a 96-well plate, on a LightCycler 480 qPCR (Roche Diagnostics, Indianapolis, IN, USA) platform.
The temperature program was set as follows: 5 minutes incubation at 42˚C followed by a 3 min incubation at 95˚C, then 45 cycles were performed (95˚C – 5 s, 56˚C – 15 s, 72˚C – 5 s) and a fluorescent reading was made at the end of each cycle. In each run melting curve analysis (95˚C – 5 s, 65˚C – 60 s, 97˚C ∞) was performed to strengthen the specificity of the amplification. The reaction mixture was included: 10 μl KAPA SYBR FASTqPCR Master Mix, 0.4 μl KAPA RT Mix, 0.4 dUTP, 0.4 μl primers, 5 μl miRNA template supplemented with sterile double-distilled water to a total volume of 20 μl.
Primer sequences of the mTORC1 gene and of the examined miRNAs (miR-330, miR-29a, miR-9-1, miR-9-3) and the internal control (mouse U6 gene) are summarized in Table I. Primers were synthetized by Integrated DNA Technologies (Leuven, Belgium), sequences are from previous publications (29,30).
Table I. Primer sequences (5’-3’) of the mTORC1 gene, the examined miRs (miR-330, miR-29a, miR-9-1, miR-9-3) and the internal control gene (mouse U6).
Calculations and statistical analysis. Relative miRNA expression levels were calculated and compared using the 2-ΔΔCT method. During the statistical analysis distributions and variances were tested using the Kolmogorov-Smirnov Levene’s test, and T-probe to compare averages. IBM SPSS 21 (Armonk, NY, USA) statistical software was used for calculations and analysis. We determined the level of statistical significance at p-value<0.05.
Results
Changes in miRNA and mTORC1 gene expression in the liver after DMBA treatment. Twenty-four hours after treatment, DMBA-induced changes could be observed in the expression of the examined miRNAs and the mTORC1 gene.
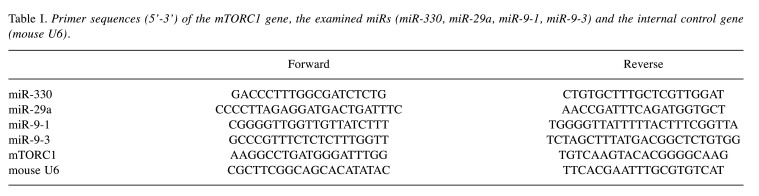
In comparison with the control group, we found statistically significant (p<0.05) changes in the expression of miR-29a, miR-9-1, miR-9-3 and mTORC1 in the liver of DMBA-treated female mice. DMBA treatment resulted in an increase in the expression of miR-29a, miR-9-3 and mTORC1, and in a decrease in the expression of miR-9-1, as it is shown in Figure 1. Compared to controls the biggest difference was observed for miR-9-3 (329%).
Figure 1. Gene expression results after 24 h in the liver of females compared to non-treated mice. In case of miR-29a and mTORC1, p<0.05, as well as in miR-9-1 and miR-9-3, p<0.001. *p<0.05, ***p<0.001.
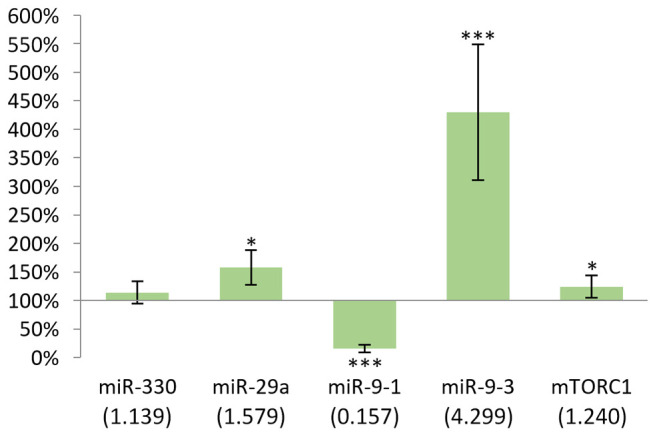
In males, the changes in miR-330, miR-9-1 and miR-9-3 were statistically significant (p<0.05). While miR-330 showed an increased expression, miR-9-1 and miR-9-3 showed decreased expression (Figure 2). Compared to controls the biggest difference was observed for miR-330 (184%).
Figure 2. Gene expression results after 24 h in the liver of males compared to non-treated mice. In case of miR-330, miR-9-1 and miR-9-3, p<0.05. *p<0.05.
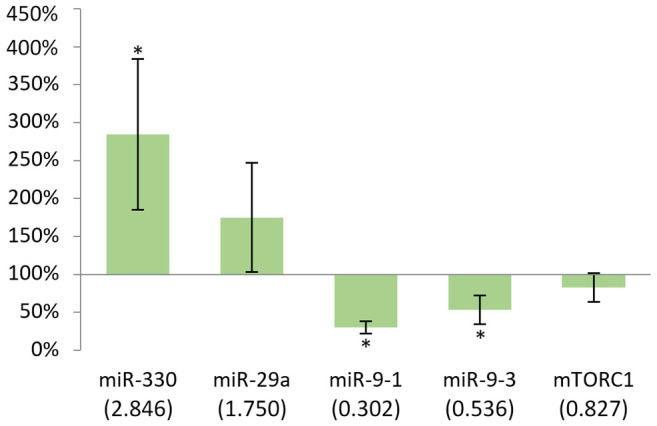
Changes in miRNA and mTORC1 gene expression in the spleen following DMBA treatment. In the spleen of female mice, DMBA induced a statistically significant change in the expression (p<0.05) of miR-9-3 and mTORC1 genes compared with the control group (Figure 3).
Figure 3. Gene expression results after 24 h in the spleen of females compared to non-treated mice. In case of miR-9-3 and mTORC1, p<0.001. ***p<0.001.
As it is shown in Figure 3, both the examined miRNAs and mTORC1 showed an increased expression as the result of DMBA treatment, and the biggest difference was found in miR-9-3 (130%).
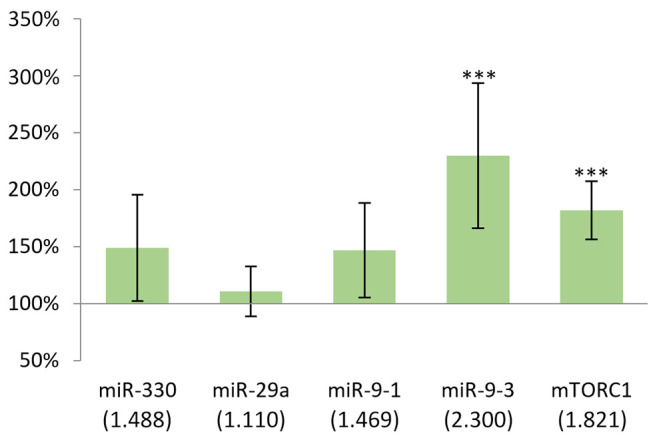
The results on the expression of miR-330 and miR-29a were statistically significant in the spleen of males (p<0.05). While miR-330 showed increased expression, miR-29a showed decreased expression upon DMBA treatment (Figure 4). The strongest effect was observed for miR-330 (456%).
Figure 4. Gene expression results after 24 h in the spleen of males compared to non-treated mice. In case of miR-330, p<0.05, while in case of miR-29a, p<0.001. *p<0.05, ***p<0.001.
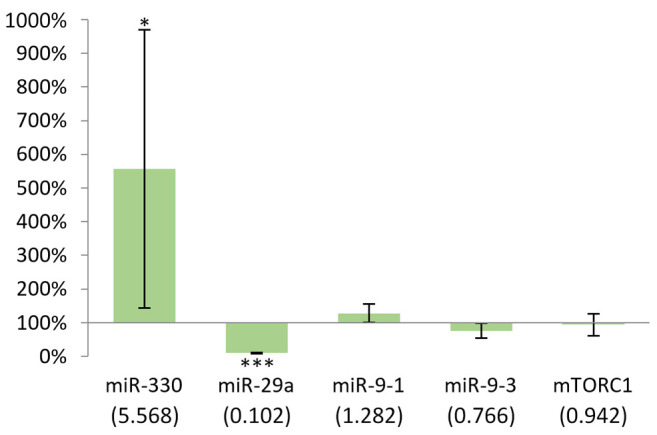
Changes in miRNA and mTORC1 gene expression in the kidney following DMBA treatment. Figure 5 illustrates that DMBA treatment lead to statistically significant changes in the expression (p<0.05) of mTORC1 and miR-330, miR-9-1 and miR-29a in the kidney of females. We found an elevated expression of both miRNAs and mTORC1 as a result of DMBA treatment, compared with the control group. The difference was particularly strong in the case of miR-9-1 (332%).
Figure 5. Gene expression results after 24 h in the kidney of females compared to non-treated mice. In case of miR-9-1, p<0.05, as well as in miR-330, miR-9-3 and mTORC1, p<0.001. *p<0.05, ***p<0.001.

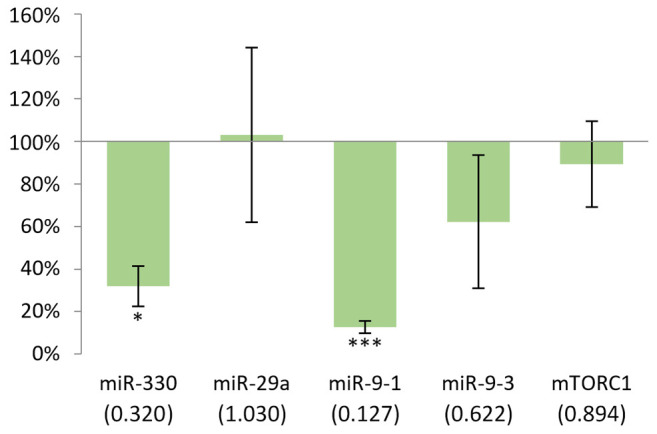
With the exception of miR-29a, decreased expressions were observed in the kidney of males, particularly miR-9-1 where the difference was the highest (-88%). However, statistically significant results were found only in the cases of miR-330 and miR-9-1 (Figure 6).
Figure 6. Gene expression results after 24 h in the kidney of males compared to non-treated mice. In case of miR-330, p<0.05, while in miR-9-1, p<0.001. *p<0.05, ***p<0.001.
Discussion
miR-330: As a result of DMBA treatment a smaller increase in the expression of miR-330 tumor suppressor was observed in the spleen and liver of female mice than in male mice. This may be due to the high expression of the proapoptic Phosphatase and tensin homolog (PTEN) tumor suppressor gene in the absence of testosterone (31), the gene product of which reduces the oncogenic effect of MYC proteins highly expressed due to DMBA treatment (19). The decrease in the oncogenic effect of the MYC protein family is known regarding the Inhibitor of growth protein 4 (ING4) (32), however, miR-330 inhibits the expression of ING4 gene and – from this point of view – it may even have a tumor promoting effect (33).
The increase in the expression of miR-330 tumor suppressor, measured in the kidney of female mice, is consistent with the observation, that it counteracts the estrogen-induced risk increase for renal cell carcinoma (RCC) (34). However, it is known that in DMBA-induced hypercholesterolemia (35) the levels of low-density lipoprotein (LDL) cholesterol increase, which may consequently lead to decreased expression of the LDL receptor gene (LDLR), whereas LDLR and miR-330 cross-regulate each other’s expression (36).
Based on our results and literature data, it can be concluded that the expression levels of miR-330 could be used as a biomarker mainly in male animals. However, according to literature data due to the multifaceted effects of sex hormones and because of the regulation by a negative feedback mechanism, the use of miR-330 expression as a biomarker is questionable.
miR-29a: DMBA increases the levels of transforming growth factor beta 1 (TGF-β1) which in turn, decreases the expression of miR-29a (37,38). However, in addition to the oncogenic effect of TGF-β1, the opposite effect (observed e.g. in hepatocytes) as tumor suppressor has also been described (39,40). For example, Zhang et al. have found in a mouse model that spontaneous tumor formation occurs in the absence of TGF-β1 (41). The various manifestations of the effects of TGF-β1 are explained by the ability of miRNAs to regulate TGF-β1 levels (42), which in the case of miR-29a tumor suppressor presumably occurs in the form of mutual cross-regulation. This shows that regulatory factors other than miR-29a may have a stronger effect on the TGF-β1-mediated effects of DMBA.
According to data from the literature, miR-29a is involved in a wide range of cellular biological processes which are still not fully explored. Considering that a significant decrease in miR-29a expression was observed only in the spleen of males, and that the mechanism of regulation is complex, apparently contradictory and is still unexplored, the expression of miR-29a, as a biomarker, is not relevant in the animal model we describe in this article.
miR-9-1 and miR-9-3: According to literature data, miR-9 inhibits the progression of hepatocellular carcinoma (HCC) as tumor suppressor, and furthermore, the expression of miR-9-1 has been shown to be significantly reduced in HCC tissues and negatively correlated with the overall survival of HCC (43,44). It is known, that silencing of miR-9-1 enhances the expression of oncogenes induced by the RUNX1-RUNX1T1 transcription factor complex regulated by miR-9-1 (23). Furthermore, the miR-9 decrease has been shown to be an early biomarker for the development of various malignancies, such as breast cancer (45). Acting as transcription factors, MYC and MYCN oncoproteins directly bind to a MYC binding site in the miR-9-3 locus (with an increased expression due to DMBA) and may increase miR-9 expression in tumor cells, that (in this case acting as promoter of oncogenes) induces a further increase of c-MYC through the amplification of E-cadherin, thus promoting the development of hepatocellular carcinoma (46,24).
This finding is supported by the fact that we found significant difference between miR-9-1 and miR-9-3 expression in the liver tissue of female subjects.
Twenty-four hours after DMBA treatment expression of miR-9-3 was increased in each tested organs of female mice, despite the protective effect of estrogen, while that of miR-9-1 was decreased in the liver. As it could be expected, miR-9-3 can be well used as a biomarker in the case of female mice according to our own study, as well. Thus, by studying miR-9-3, and extending the experimental design by complementing DMBA treatment with presumably chemopreventive agents, new molecular epidemiological relationships are likely to be discovered.
mTORC1: Mutations in mTOR can be found in a wide range of human malignant tumorous diseases, in agreement with the proven tumor promoting effect of mTOR (27,47). The activation of mTOR signaling induces several oncogenic processes, including promotion of the growth, survival and proliferation of cancerous cells (48). mTORC1 mediates cell proliferation stimulated by growth factors such as insulin-like growth factor (IGF) (28). In DMBA-induced breast tumors high levels of transforming growth factor α (TGFα), IGF-1 and probably IGF-2 at the mRNA levels can be related to the effect of estrogen (49). The potential oncogenic role of mTORC1 has also been demonstrated in our model. We also found that due to the effect of DMBA, mTORC1 expression increased in female mice, in contrast to expected decrease due to the PTEN gene expression (31), as PTEN is an inhibitor of mTORC1. This inhibition lacks a direct negative feedback (28), so a more distant negative feedback regulatory mechanism could exist.
Despite our expectations, mTORC1 gene - whose cancer promoting mechanisms are supported by literature data - has not shown significant changes in male mice. However, the expression of mTORC1 gene showed significant increase in the liver, spleen and kidneys of females as a result of DMBA treatment. Therefore, expression of the mTORC1 gene in females in the model used in the present study appears to be applicable for testing carcinogens.
In summary, consistent and significant changes toward the same direction in all the three studied organs were observed in the case of miR-9-3 and mTORC1 genes in female mice. The expression patterns were somewhat different in males which may be due to sex-specific hormonal differences (such as the effects of estrogen and testosterone) that require further investigation. Thus, the interpretation of miR-330, miR-29a and miR-9-1 gene expression changes seems to be rather complex, while miR-9-3 and mTORC1 genes in females were found to be suitable for the rapid screening of potential carcinogens. The same model can be used later to study chemopreventive effects as well by the simultaneous use of DMBA and supposed chemopreventive agents, measuring to what extent these agents are able to reduce/prevent the DMBA-induced gene expression changes.
While gene expressions are sensitive biomarkers, and in contrast to classic genotoxicity tests they are able to detect minor/early signs of carciongenic exposures, sometimes they might be influenced by potential confounders. Our results also emphasize that early carcinogenecity testing requires a careful selection of the test system, since hormonal differences can substantially influence gene expression patterns. In order to create a robust assay panel designed for our purposes, additional genes need probably to be tested.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
Study design and conception: AT, LS, RM, IK; Experiments: AT, LS, RM, NG, TV, AD, RD; Data analysis and interpretation: AT, LS, IK; Manuscript preparation: AT, IK; Critical reviewing and revising the manuscript: DM, FB, BN, IK.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, Comparative Risk Assessment collaborating group (Cancers) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 3.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1-2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38:S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 6.Bueno MJ, Malumbres M. MicroRNAs and the cell cycle. Biochim Biophys Acta. 2011;1812(5):592–601. doi: 10.1016/j.bbadis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Bueno MJ, Pérez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7(20):3143–3148. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174(4):1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent. Growth Cancer Res. 2007;67(18):8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 11.He C, Xiong J, Xu X, Lu W, Liu L, Xiao D, Wang D. Functional elucidation of MiR-34 in osteosarcoma cells and primary tumor samples. Biochem Biophys Res Commun. 2009;388(1):35–40. doi: 10.1016/j.bbrc.2009.07.101. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Lee UJ, Kim MN, Lee E-J, Kim JY, Lee MY, Choung S, Kim YJ, Choi Y-C. MicroRNA miR-199a* regulates the MET Proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2) J Biol Chem. 2008;283(26):18158–18166. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 13.Pan JY, Zhang F, Sun CC, Li SJ, Li G, Gong FY, Bo T, He J, Hua RX, Hu WD, Yuan ZP, Wang X, He QQ, Li DJ. miR-134: A human cancer suppressor. Mol Ther Nucleic Acids. 2017;6:140–149. doi: 10.1016/j.omtn.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 15.Bosland MC, Prinsen MK. Induction of dorsolateral prostate adenocarcinomas and other accessory sex gland lesions in male Wistar rats by a single administration of N-methyl-N-nitrosourea, 7,12-dimethylbenz(a)anthracene, and 3,2’-dimethyl-4-aminobiphenyl after sequential treatment with cyproterone acetate and testosterone propionate. Cancer Res. 1990;50(3):691–699. [PubMed] [Google Scholar]
- 16.El-Sohemy A, Archer MC. Inhibition of N-methyl-N-nitrosourea- and 7,12-dimethylbenz[a] anthracene-induced rat mammary tumorigenesis by dietary cholesterol is independent of Ha-Ras mutations. Carcinogenesis. 2000;21(4):827–831. doi: 10.1093/carcin/21.4.827. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Lauer FT, Dunaway S, Burchiel SW. Cytochrome P450 1B1 is required for 7,12-dimethylbenz(a)-anthracene (DMBA) induced spleen cell immunotoxicity. Toxicol Sci. 2005;86(1):68–74. doi: 10.1093/toxsci/kfi176. [DOI] [PubMed] [Google Scholar]
- 18.Lindhe O, Granberg L, Brandt I. Target cells for cytochrome p450-catalysed irreversible binding of 7,12-dimethylbenz[a]anthracene (DMBA) in rodent adrenal glands. Arch Toxicol. 2002;76(8):460–466. doi: 10.1007/s00204-002-0367-1. [DOI] [PubMed] [Google Scholar]
- 19.Budán F, Varjas T, Nowrasteh G, Prantner I, Varga Z, Ember E, Cseh J, Gombos K, Pázsit E, Gobel G, Bauer M, Gracza T, Arany I, Perjési P, Ember I, Kiss I. Early modification of c-myc, Ha-ras and p53 expressions by chemical carcinogens (DMBA, MNU) In Vivo. 2009;23(4):591–598. [PubMed] [Google Scholar]
- 20.Ember I, Kiss I, Pusztai Z. Effect of 7,12-dimethylbenz(a) anthracene on onco/suppressor gene action in vivo: a short-term experiment. Anticancer Res. 1998;18(1A):445–447. [PubMed] [Google Scholar]
- 21.Cheng CW, Yu JC, Hsieh YH, Liao WL, Shieh JC, Yao CC, Lee HJ, Chen PM, Wu PE, Shen CY. Increased cellular levels of microRNA-9 and microRNA-221 correlate with cancer stemness and predict poor outcome in human breast cancer. Cell Physiol Biochem. 2018;48(5):2205–2218. doi: 10.1159/000492561. [DOI] [PubMed] [Google Scholar]
- 22.Wang LQ, Kwong YL, Kho CS, Wong KF, Wong KY, Ferracin M, Calin GA, Chim CS. Epigenetic inactivation of miR-9 family microRNAs in chronic lymphocytic leukemia—implications on constitutive activation of NFĸB pathway. Mol Cancer. 2013;12:173. doi: 10.1186/1476-4598-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu L, Shi J, Liu A, Zhou L, Jiang M, Fu H, Xu K, Li D, Deng A, Zhang Q, Pang Y, Guo Y, Hu K, Zhou J, Wang Y, Huang W, Jing Y, Dou L, Wang L, Xu K, Ke X, Nervi C, Li Y, Yu L. A minicircuitry of microRNA-9-1 and RUNX1-RUNX1T1 contributes to leukemogenesis in t(8;21) acute myeloid leukemia. Int J Cancer. 2017;140(3):653–661. doi: 10.1002/ijc.30481. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JY, Zhang Q, Wang DD, Yan W, Sha HH, Zhao JH, Yang SJ, Zhang HD, Hou JC, Xu HZ, He YJ, Hu JH, Zhong SL, Tang JH. MiR-29a: a potential therapeutic target and promising biomarker in tumors. Biosci Rep. 2018;38(1) doi: 10.1042/BSR20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tréhoux S, Lahdaoui F, Delpu Y, Renaud F, Leteurtre E, Torrisani J, Jonckheere N, Van Seuningen I. Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochim Biophys Acta. 2015;1853(10 Pt A):2392–2403. doi: 10.1016/j.bbamcr.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Nakashima A, Guo L, Coffman K, Tamanoi F. Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer. Oncogene. 2010;29(18):2746–2752. doi: 10.1038/onc.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MN. mTOR-what does it do? Transplant Proc. 2008;40(10 Suppl):S5–8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Shor B, Cavender D, Harris C. A kinase-dead knock-in mutation in mTOR leads to early embryonic lethality and is dispensable for the immune system in heterozygous mice. BMC Immunol. 2009;10:28. doi: 10.1186/1471-2172-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30(45):15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.55 Ma J, Fan Y, Zhang J, Feng S, Hu Z, Qiu W, Long K, Jin L, Tang Q, Wang X, Zhou Q, Gu Y, Xiao W, Liu L, Li X, Li M. Testosterone-dependent miR-26a-5p and let-7g-5p act as signaling mediators to regulate sperm apoptosis via targeting PTEN and PMAIP1. Int J Mol Sci. 2018;19(4) doi: 10.3390/ijms19041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger PL, Frank SB, Schulz VV, Nollet EA, Edick MJ, Holly B, Chang TT, Hostetter G, Kim S, Miranti CK. Transient induction of ING4 by Myc drives prostate epithelial cell differentiation and its disruption drives prostate tumorigenesis. Cancer Res. 2014;74(12):3357–3368. doi: 10.1158/0008-5472.CAN-13-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Feng Y, Sun L, Qu L, Sun C. Roles of microRNA-330 and its target gene ing4 in the development of aggressive phenotype in hepatocellular carcinoma cells. Dig Dis Sci. 2017;62(3):715–722. doi: 10.1007/s10620-016-4429-2. [DOI] [PubMed] [Google Scholar]
- 34.Moyad MA. Obesity, interrelated mechanisms, and exposures and kidney cancer. Semin Urol Oncol. 2001;19(4):270–279. [PubMed] [Google Scholar]
- 35.Iqbal J, Minhajuddin M, Beg ZH. Suppression of 7,12-dimethylbenz[alpha]anthracene-induced carcinogenesis and hypercholesterolaemia in rats by tocotrienol-rich fraction isolated from rice bran oil. Eur J Cancer Prev. 2003;12(6):447–453. doi: 10.1097/01.cej.0000102802.33147.4b. [DOI] [PubMed] [Google Scholar]
- 36.Zhang GM, Wang MY, Liu YN, Zhu Y, Wan FN, Wei QY, Ye DW. Functional variants in the low-density lipoprotein receptor gene are associated with clear cell renal cell carcinoma susceptibility. Carcinogenesis. 2017;38(12):1241–1248. doi: 10.1093/carcin/bgx098. [DOI] [PubMed] [Google Scholar]
- 37.Johnson AT, Kaufmann YC, Luo S, Todorova V, Klimberg VS. Effect of glutamine on glutathione, IGF-I, and TGF-beta 1. J Surg Res. 2003;111(2):222–228. doi: 10.1016/s0022-4804(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 38.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44(4):237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhurst RJ. TGF-beta antagonists: why suppress a tumor suppressor. J Clin Invest. 2002;109(12):1533–1536. doi: 10.1172/JCI15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986;46(5):2330–2334. [PubMed] [Google Scholar]
- 41.Zhang Y, Wen G, Shao G, Wang C, Lin C, Fang H, Balajee AS, Bhagat G, Hei TK, Zhao Y. TGFBI deficiency predisposes mice to spontaneous tumor development. Cancer Res. 2009;69(1):37–44. doi: 10.1158/0008-5472.CAN-08-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki HI. MicroRNA control of TGF-β signaling. Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19071901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Cheng J, Zeng Z, Wang Y, Li X, Xie Q, Jia J, Yan Y, Guo Z, Gao J, Yao M, Chen X, Lu F. Comprehensive profiling of novel microRNA-9 targets and a tumor suppressor role of microRNA-9 via targeting IGF2BP1 in hepatocellular carcinoma. Oncotarget. 2015;6(39):42040–42052. doi: 10.18632/oncotarget.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Chong CC, Chen GG, Lai PB. A seven-microRNA expression signature predicts survival in hepatocellular carcinoma. PLoS One. 2015;10(6):e0128628. doi: 10.1371/journal.pone.0128628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orangi E, Motovali-Bashi M. Evaluation of miRNA-9 and miRNA-34a as potential biomarkers for diagnosis of breast cancer in Iranian women. Gene. 2019;687:272–279. doi: 10.1016/j.gene.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 46.Morin PJ. Beta-catenin signaling and cancer. Bioessays. 1999;21(12):1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Grabiner BC, Nardi V, Birsoy K, Possemato R, Shen K, Sinha S, Jordan A, Beck AH, Sabatini DM. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4(5):554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruggeri BA, Klurfeld DM, Kritchevsky D, Frick KK. Steady-state mRNA expression for growth factors in DMBA-induced rat mammary tumors. Cancer Lett. 1990;55(2):89–93. doi: 10.1016/0304-3835(90)90016-q. [DOI] [PubMed] [Google Scholar]