Abstract
Background/Aim: Fluorescence imaging has been shown to improve intra-operative detection of liver metastasis. The present study aimed to determine whether humanized anti-TAG-72 antibody (huCC49) conjugated to a near-infrared dye provides selective labeling of colorectal- cancer liver metastasis in orthotopic mouse models. Materials and Methods: Humanized anti-TAG-72 (huCC49) was conjugated to IRDye800CW (huCC49-IR800). Orthotopic liver-metastasis nude-mouse models (n=5) were established with the human colon-cancer LS174T cell-line. Three weeks later, mice were administered huCC49-IR800 and intra-vital imaging was performed 48 h later. The mean tumor-to-liver ratio (TLR) was calculated. Results: Intra-vital imaging demonstrated clear tumor margins with minimal liver fluorescence 48 h after administration of 50 μg huCC49-IR800 with mean TLR=7.53 (SD±2.76). Conclusion: Anti-TAG-72 monoclonal antibody conjugated to IRDye800 provides distinct and bright labeling of colorectal tumors in orthotopic nude-mouse models of liver metastasis. TAG-72 may be a useful target for intra-operative imaging of colorectal cancer liver metastasis in the clinic.
Keywords: TAG-72, fluorescent antibody, colorectal cancer orthotopic, liver metastasis, nude mice, infrared fluorescence imaging, fluorescence-guided surgery
Colorectal cancer (CRC) remains one of the most common cancers and causes of cancer-related deaths worldwide (1,2). A total of 1.2 million people in the United States are diagnosed with CRC yearly and 600,000 people die from it annually in the United States (3). The presence or absence of metastasis, most commonly to the liver, is one of the most important prognostic factors associated with CRC (2). It has been previously found that 25-30% of patients diagnosed with CRC will develop liver metastasis (1,3). Synchronous presentation, defined as liver metastasis found at the time of CRC diagnosis, is associated with significantly worse outcomes (2,4). For patients who are appropriate surgical candidates, the gold standard for treatment of hepatic metastasis is surgical resection (2,4). Patients diagnosed with CRC with resectable liver metastasis have a 5-year overall survival (OS) of 50-58%, demonstrating the importance of complete resection of liver metastasis for surgical candidates (1,4).
Given the prognostic importance of liver-metastasis resection, researchers have evaluated ways to improve both detection and resection, including genetic-reporter labeling and antibody-based fluorescence-guided surgery (FGS). Murakami et al. demonstrated improved disease-free survival (DFS) and OS after FGS of a colon-cancer liver metastasis derived from a green-fluorescent protein (GFP)-expressing human cell line, in a nude-mouse model (5). Yano et al. showed that in situ GFP labeling with a telomerase-dependent adenovirus enhanced FGS of liver metastasis in a patient-derived orthotopic xenograft (PDOX) nude-mouse model, resulting in improved resection and outcomes (6). Hiroshima et al. developed liver-metastasis nude-mouse models using a human colon-cancer cell line and randomized mice to bright-light surgery vs. FGS with a near-infrared fluorescent anti-CEA antibody (7). Hiroshima et al. demonstrated that the FGS arm had significantly less residual tumor as well as significantly increased DFS and OS (7). In addition to CEA, other novel targets have been identified for FGS. Park et al. studied insulin-like growth-factor 1 receptors (IGF-1R) which are overexpressed in certain colorectal carcinomas (8). IGF-1R fluorescent antibodies allowed liver metastasis in nude-mouse models to be easily visualized with non-invasive imaging distinct from normal liver tissue (8).
Tumor-associated-glycoprotein (TAG)-72 is a surface antigen glycoprotein that has been found overexpressed in over 80% of colorectal carcinomas and is rarely overexpressed in normal colonic epithelium (9). TAG-72 becomes overexpressed during epithelial-cell transformation in the tumorigenesis of colon cancer (10). Given the consistent over-expression of TAG-72, it has been identified as a marker of CRC that can be used in clinical practice for diagnosis of malignancy (11). Our laboratory showed that patient-derived tumors maintain expression of TAG-72 in 3-dimensional histoculture, as they do in vivo; therefore, tumors can be readily screened for their potential to express TAG-72 (12).
Previous studies have demonstrated that a humanized anti-TAG-72 monoclonal antibody (huCC49) targets and provides specific fluorescence imaging of primary colorectal tumors and intra-peritoneal regional metastasis in orthotopic nude-mouse models when conjugated to near-infrared fluorophores (13,14). In the present study, we utilized the huCC49 monoclonal antibody conjugated to a near-infrared fluorophore for bright and specific labeling of CRC liver metastasis in orthotopic nude-mouse models.
Materials and Methods
Animals. Female and male athymic nude mice (nu/nu) aged 4-6 weeks purchased from The Jackson Laboratory (Bar Harbor, ME, USA), were used for this study. Mice were maintained in a biosafety room and fed a standard laboratory-approved autoclaved diet. Prior to surgical procedures, mice were anesthetized with an intraperitoneal injection of a xylazine and ketamine cocktail reconstituted in phosphate-buffered saline (PBS). Post-procedure pain was treated with subcutaneous buprenorphine. At the conclusion of the study, mice were euthanized with CO2 inhalation, which was confirmed with cervical dislocation. All studies were approved by the San Diego Veterans Administration Medical Center Institutional Animal Care and Use Committee (IACUC, animal use protocol A17-020).
Antibody conjugation. An Amicon 3 ml stirred cell (Millipore, Burlington, MA) on a stir table attached to a flow-through reservoir chamber was used for near-infrared dye conjugation to the TAG-72 antibody. One ml of plasma-grade water (Fisher Scientific, Waltham, MA, USA) was added to the stirred cell and 15 ml of plasma water was added to the supply reservoir. The plasma water was allowed to flow through the chamber using a light vacuum to maintain a consistent chamber fluid level. Once the supply reservoir and chamber were empty, 5 mg of the humanized anti-TAG-72 CC49 monoclonal antibody was added to the chamber. The suspension was dialyzed under basic-pH conditions. IRDye-800CW-NHS (LI-COR Biosciences, Lincoln, NE, USA) was dissolved in conjugation buffer to a concentration of 10 mg/ml and added at a 10:1 molar ratio. The mixture was placed on a stirrer for one h at room temperature, protected from light. Post-conjugation dialysis was performed and the dialyzed antibody-dye conjugate was removed and filtered through a sterile low-protein-binding syringe filter (0.2 μm) (Pall Corporation, Port Washington, NY, USA). Protein concentration and degree of labeling were determined using spectrophotometry at 280 nm and 780 nm, as per the dye-manufacturer’s protocol. Antibody-dye-conjugate purity was assessed by high-performance liquid size-exclusion chromatography (Superdex200) (GE Healthcare Life Sciences, Chicago, IL, USA).
Tumor establishment. Human colon-cancer cell-line LS174T (American Type Culture Collection, Manassas, VA, USA) was used for this study. Cells (1×106), suspended in a cold Matrigel and PBS mixture, were injected into the bilateral flanks of nude mice (n=2). Tumors were allowed to grow until 5 mm in diameter and were then resected and divided into small pieces for implantation. For orthotopic liver implantation, the abdomen of the mouse (n=5) was sterilized with a 70% ethanol solution. A small incision was made at the upper midline of the abdomen through the skin and peritoneum. The left lobe of the liver was identified and a small, 1 mm incision was made through the liver parenchyma. A 1 mm tumor fragment was placed inside the liver incision. The left lobe of the liver was carefully returned to anatomic position and the skin and peritoneum were closed with a 6-0 nylon suture (Ethicon Inc., Somerville, NJ, USA) (15,16). Tumors were allowed to grow for three weeks.
Imaging. Intra-vital imaging was performed on the Pearl Trilogy Small Animal Imaging System (LI-COR Biosciences), which is equipped for near-infrared imaging of 700 and 800 nm probes. Mice were administered 50 μg huCC49-IR800 reconstituted in PBS 48 h prior to imaging via tail vein injection. Laparotomy was performed to expose intra-abdominal organs for imaging. After intra-vital imaging, tumors were resected and cut in half to visualize the internal aspect of the tumor. Ex vivo tumor imaging was performed on the LI-COR Odyssey Infrared Imaging System model 9120 (LI-COR). Resected tumors were then sectioned from paraffin blocks and hematoxylin and eosin (H&E) staining was performed at the University of California San Diego Moore’s Cancer Center Histology Core. Paraffin sections were imaged on the Keyence BZ-X800 microscope to obtain bright-light images of H&E staining, to confirm specificity of the fluorescence signal (Keyence Corp., Itasca, IL, USA). Images were obtained using 2× and 10× objective microscopic lenses.
Statistical analysis. Imaging analysis was performed using Image Studio Software Small Animal Imaging Analysis (LI-COR) and statistical analysis was performed using SPSS version 24. The liver was set as the background and an area of interest around the tumor fluorescence was automatically provided by the system with a minimum of 250 pixels and at least 2.5 standard deviations from the background signal. Tumor-to-liver ratio (TLR) was calculated for each mouse by dividing maximum tumor fluorescence by maximum liver fluorescence. Mean TLR was determined for the entire cohort and presented as the mean with standard deviation.
Results
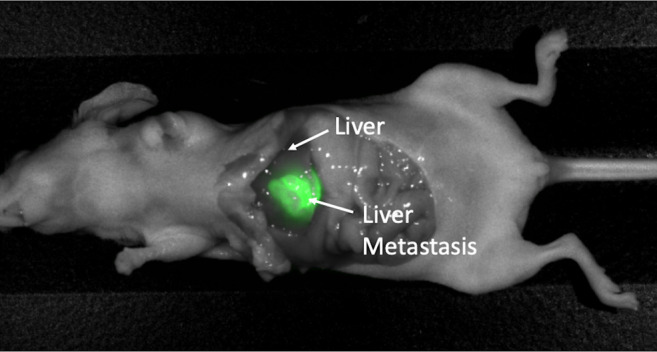
Intra-vital imaging on the LI-COR Pearl Trilogy Small Animal Imaging System demonstrated a very bright liver metastasis with clear margins in all mice with minimal liver fluorescence, 48 h after administration of 50 μg huCC49-IR800 (Figure 1). Tumors measured approximately 1 cm in diameter (Figure 1). In addition to minimal liver fluorescence, complete laparotomy demonstrated minimal fluorescence of all intra-abdominal organs, as demonstrated in Figure 2. All images were analyzed to determine the TLR which was 7.53 (SD±2.76) for the entire cohort of mice (n=5).
Figure 1. Intra-vital imaging of the LS174T colon-cancer cell-line orthotopic liver-metastasis mouse model. (a) Bright-light imaging. (b) Fluorescence imaging; the metastasis is very brightly labeled and tumor margins are distinct with minimal fluorescent-liver signal after administration of 50 μg huCC49-IR800. Representative image from n=5 mice. Mean TLR=7.53 (SD±2.76).
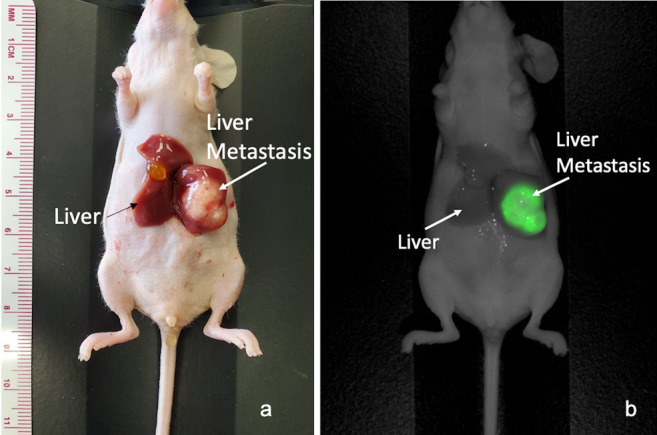
Figure 2. Intra-vital imaging of LS174T colon-cancer cell-line orthotopic liver-metastasis mouse model. Tumor is very brightly labeled and margins are distinctly fluorescently visualized after administration of 50 μg huCC49-IR800, with minimal liver or intra-abdominal-organ fluorescence signal.
After intra-vital imaging, tumors were resected for ex vivo analysis to determine tumor penetration of the huCC49-IR800 probe. Ex vivo bright light imaging of a halved tumor, with 1 cm diameter, with surrounding normal-liver tissue, can be seen in Figure 3a. Fluorescence imaging of the tumor section was performed using the LI-COR Odyssey imaging system, which demonstrated fluorescence signal throughout the entire tumor, with depth of penetration into the center of the tumor (Figure 3b).
Figure 3. Ex-vivo imaging of a tumor section. (a) Bright-light imaging of tumor with surrounding liver. Tumor measures approximately 1 cm in diameter. (b) Ex-vivo fluorescence imaging of a metastasis section demonstrating penetration of the huCC49-IR800 antibody throughout the entire depth of the metastasis. (c) H&E staining of liver metastasis visualized using a 2× objective lens. (d) H&E staining of liver metastasis using a 10× objective lens.
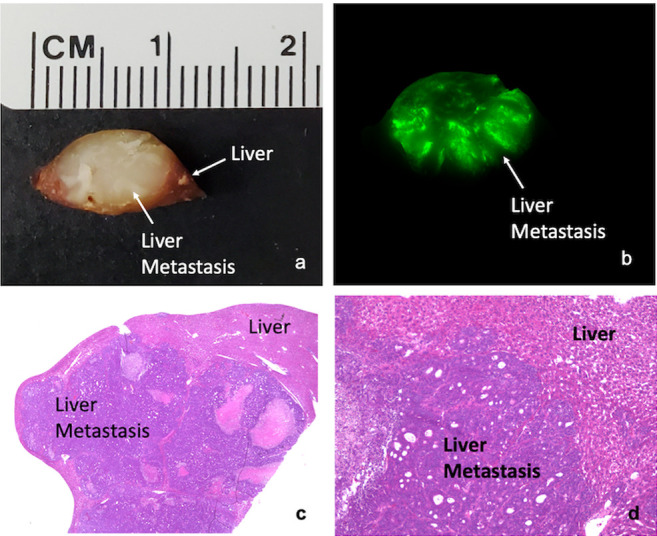
The excised tumor imaged on the LI-COR Odyssey was further sectioned from paraffin blocks, with subsequent H&E staining to confirm the histological presence of colorectal-cancer liver metastasis. Slides were imaged on the Keyence BZ-X800 microscope system with 2× (Figure 3c) and 10× objectives (Figure 3d), which demonstrated a clear demarcation of tumor and normal-liver histology, confirming that the fluorescence signal, seen in Figure 3b, is specific to the liver metastasis.
Discussion
The present study demonstrated that huCC49-IR800 brightly and specifically labeled liver metastasis in orthotopic mouse models of colon-cancer liver metastasis, with a mean TLR=7.53. Imaging demonstrated minimal background liver signal, which is essential for demarcation of tumor and liver parenchyma. Tumor sectioning demonstrated penetration of the antibody-fluorophore conjugate throughout the entire tumor, which is important for accurate imaging of larger tumors. In addition, histological imaging confirmed that the fluorescence signal is specific to the liver metastasis.
Previous studies have utilized antibody-based fluorescence imaging for selective detection of CRC liver metastasis in orthotopic nude-mouse models using an IGF-1 monoclonal antibody (8). Further studies have utilized fluorescent anti-CEA monoclonal antibodies for FGS of liver metastasis and demonstrated improved frequency of complete resection and decreased recurrence in nude-mouse models (7,17). Since TAG-72 has been identified as a promising target for fluorescence imaging of CRC (13) and is overexpressed in the majority of colorectal tumors (10), it is an ideal antigen to target liver metastasis. In addition, a previous study demonstrated detection of small regional metastasis with a humanized TAG-72 antibody (13), which is promising for detection of metastasis that may be undiagnosed upon pre-operative imaging. The present study serves as a proof-of concept that huCC49-IR800 specifically and brightly labels CRC liver metastasis with a minimal background liver signal in an orthotopic model. Future studies will be performed using huCC49-IR800 for FGS of CRC liver metastasis.
A previous study using an anti-CEA antibody conjugated to IR800 near-infrared dye for colon-cancer imaging in orthotopic mouse models, demonstrated a high liver-fluorescence signal, occasionally brighter than the tumor signal, which would make fluorescence visualization of liver tumors difficult (18). In the present study, huCC49-IR800 demonstrated very minimal liver signal and a high TLR, which makes it a very promising probe for identification of liver metastasis in patients with colon cancer. Since it is a humanized antibody, huCC49-IR800 can be used in clinical trials.
In the present study, we utilized a human colon-cancer cell-line LS174T, since this cell-line has been used in previous studies for fluorescence visualization with huCC49-IR800 (13). Future studies using huCC49-IR800 on patient-derived orthotopic xenografts of colon-cancer liver metastasis will serve as a bridge to the clinic.
The presence of liver metastasis at the time of diagnosis of CRC significantly impacts prognosis and overall survival (2-4). Surgical resection of CRC liver metastasis remains a mainstay of treatment, but with poor outcome. With the transition to minimally- invasive surgical techniques, such as laparoscopic and robotic platforms, the surgeon loses tactile sensation that can improve detection of tumor margins. The use of intra-operative fluorescence imaging can assist with identification of occult tumors otherwise invisible with the naked eye. The minimally-invasive platforms currently used in clinical settings are equipped with the technology to visualize 800 nm near-infrared fluorescence wavelengths, which adds to the translational potential of the huCC49-IR800 fluorescent probe in the present study.
Intra-operative fluorescence imaging can assist the surgeon in correctly identifying all metastatic tumors and improve the possibility of complete resection. Fluorescent, humanized, monoclonal antibody huCC49-IR800 has a very high TLR (mean TLR=7.53) and very minimal liver fluorescence at the time of imaging and can penetrate the entire depth of the tumor. Therefore, this near-infrared probe has the best potential of a fluorescent probe for translation to clinical CRC liver-metastasis labeling and FGS. The clinical potential of the present study is realistic due to the use of orthotopic models (19-22).
Conflicts of Interest
HN and RMH are non-salaried affiliates of AntiCancer Inc. This affiliation did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All other authors have nothing to disclose.
Authors’ Contributions
All Authors were involved in conception and design of the work. Drs. Hollandsworth, Amirfakhri and Mr. Filemoni were involved in the acquisition of the data. Drs. Hollandsworth, Amirfakhri, Yazaki, Hoffman and Bouvet were involved in analysis and interpretation of data. Drs. Hollandsworth and Turner were involved in drafting of the manuscript. All Authors were involved in critically revision the manuscript for important intellectual content. All Authors gave final approval of the version to be published. All Authors agree to be accountable for all aspects of the work.
Acknowledgements
This study was funded by VA Merit Review grant numbers 1 I01 BX003856-01A1 and 1 I01 BX004494-01 (MB) and NIH/NCI T32CA121938 (HH).
References
- 1.Engstrand J, Nilsson H, Strombery C, Jonas E, Freedman J. Colorectal cancer liver metastasis – a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazhirkarzar B, Khoshpouri P, Shaghaghi M, Ghasabeh MA, Pawlik TM, Kamel IR. Current state of the art imaging approaches for colorectal liver metastasis. HepatoBiliary Surg Nutr. 2020;9(1):35–48. doi: 10.21037/hbsn.2019.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siebenhüner AR, Guller U, Warschkow RL. Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastasis. BMC Cancer. 2020;20:246. doi: 10.1186/s12885-020-6710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lillemoe HA, Vauthey J. Surgical approach to synchronous colorectal liver metastasis: stage, combined or reverse strategy. HepatoBiliary Surg Nutr. 2020;9(1):25–34. doi: 10.21037/hbsn.2019.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami T, Hiroshima Y, Zhang Y, Bouvet M, Chishima T, Tanaka K, Endo I, Hoffman RM. Improved disease-free survival and overall survival after fluorescence-guided surgery of liver metastasis in an orthotopic nude mouse model. J Surg Oncol. 2015;112:119–124. doi: 10.1002/jso.23986. [DOI] [PubMed] [Google Scholar]
- 6.Yano S, Takehara K, Miwa S, Kishimoto H, Hiroshima Y, Murakami T, Urata Y, Kagawa S, Bouvet M, Fujiwara T, Hoffman RM. Improved resection and outcome of colon-cancer liver metastasis with fluorescence-guided surgery using in situ GFP labeling with a telomerase-dependent adenovirus in an orthotopic mouse model. PLOS. 2016;11(2):e0148760. doi: 10.1371/journal.pone.0148760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiroshima Y, Lwin TM, Murakami T, Mawy AA, Kuniya T, Chishima T, Endo I, Clary BM, Hoffman RM, Bouvet M. Effective fluorescence-guided surgery of liver metastasis using a fluorescent anti-CEA antibody. J Surg Oncol. 2016;114(8):951–958. doi: 10.1002/jso.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JY, Murakami T, Lee JY, Lee JY, Zhang Y, Hoffman RM, Bouvet M. Fluorescent-antibody targeting of insulin-like growth factor-1 receptor visualizes metastatic human colon cancer in orthotopic mouse models. PLoS One. 2016;11(1):e0146504. doi: 10.1371/journal.pone.0146504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guadagni F, Roselli M, Cosimelli M, Ferroni P, Spila A, Cavaliere F, Arcuri R, Carlini S, Mariotti S, Gandlfo HM, Casciani CU, Greiner JW, Schlom J. TAG-72 expression and its role in the biological evaluation of human colorectal cancer. Anticancer Res. 1996;16(4B):2141–2148. [PubMed] [Google Scholar]
- 10.Xu M, Real FX, Welt S, Schüssler MH, Oettgen HF, Old LJ. Expression of TAG-72 in normal colon, transitional mucosa, and colon cancer. Int J Cancer. 1989;44(6):985–989. doi: 10.1002/ijc.2910440607. [DOI] [PubMed] [Google Scholar]
- 11.Swiderska M, Choromańska B, Dąbrowska E, Konarzewska-Duchnowska E, Choromanska K, Szczurko G, Mysliwiec P, Dadan J, Ladny JR, Zwierz K. The diagnostics of colorectal cancer. Contemp Oncol (Pozn) 2014;18(1):1–6. doi: 10.5114/wo.2013.39995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guadagni F, Roselli M, Hoffman RM. Maintenance of expression of tumor antigen in three-dimensional in vitro human tumor gel-supported histoculture. Anticancer Res. 1991;11:534–546. [PubMed] [Google Scholar]
- 13.Hollandsworth HM, Amirfakhri S, Filemoni F, Hoffman RM, Molnar J, Yazaki PJ, Bouvet M. Humanized anti-tumor-associated glycoprotein-72 for submillimeter near-infrared detection of colon cancer in metastatic mouse models. J Surg Res. 2020;252:16–21. doi: 10.1016/j.jss.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou P, Xu S, Poviski SP, Wang A, Johnson MA, Martin EW, Subramaniam V, Xu R, Sun D. Near-infrared fluorescence labeled anti-TAG-72 monoclonal antibodies for tumor imaging in colorectal cancer xenograft mice. Mol Pharm. 2009;6(2):428–440. doi: 10.1021/mp9000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. PNAS. 1991;88(20):9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo TH, Kubota T, Watanabe M, Kurukawa T, Ishibiki K, Kitajima M, Moosa AR, Hoffman RM. Liver colonization competence governs colon cancer metastasis. PNAS. 1995;92(26):12085–12089. doi: 10.1073/pnas.92.26.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutowski M, Framery B, Boonstra MC, Garambois V, Quenet F, Dumas K, Scherninski F, Cailler F, Vahrmeijer AL, Pelegrin A. SGM-101: An innovative near-infrared dye-antibody conjugate that targets CEA for fluorescence-guided surgery. Surg Oncol. 2017;26(2):153–162. doi: 10.1016/j.suronc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 18.DeLong JC, Murakami T, Yazaki PJ, Hoffman RM, Bouvet M. Near-infrared-conjugated humanized anti-carcinoembryonic antigen antibody targets colon cancer in an orthotopic nude-mouse model. J Surg Res. 2017;218:139–143. doi: 10.1016/j.jss.2017.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: A bridge to the clinic. Invest New Drugs. 1999;17(4):343–359. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman RM. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman RM. Orthotopic is orthodox: Why are orthotopic-transplant metastatic models different from all other models. J Cell Biochem. 1994;56(1):1–3. doi: 10.1002/jcb.240560102. [DOI] [PubMed] [Google Scholar]
- 22.Tran Chau V, Liu W, Gerbé de Thoré M, Meziani L, Mondini M, O'Connor MJ, Deutsch E, Clémenson C. Differential therapeutic effects of PARP and ATR inhibition combined with radiotherapy in the treatment of subcutaneous versus orthotopic lung tumour models. Br J Cancer Jun. 2020;17 doi: 10.1038/s41416-020-0931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


