Abstract
Background/Aim: Neutralization of the acidic tumor microenvironment, which is associated with both progression and drug resistance of cancer cells, may be a new treatment option for progressing forms of cancer. We conducted a case-control study to investigate the effects of alkalization therapy, consisting of an alkaline diet with supplementary oral sodium bicarbonate, in patients with metastatic or recurrent pancreatic cancer (study registration no.: UMIN000036126). Patients and Methods: Thirty-six patients in the alkalization group (Karasuma Wada Clinic; alkalization therapy plus chemotherapy) were retrospectively compared to 89 patients in the control group (Kyoto University Hospital; chemotherapy only). Results: The median overall survival (OS) in the alkalization group was significantly longer than that in the control group (15.4 vs. 10.8 months; p<0.005). In the alkalization group, mean urine pH was significantly increased after alkalization therapy [6.38±0.85 (before) vs. 6.80±0.71 (after); p<0.05]. Furthermore, the median OS of patients with increased urine pH (pH>7.0 or ΔpH>1.0) in the alkalization group was significantly longer than that of the control group. Conclusion: Alkalization therapy may enhance the effects of chemotherapy in patients with advanced pancreatic cancer.
Keywords: Pancreatic cancer, alkalization therapy, urine pH, tumor microenvironment, alkaline diet, bicarbonate
Pancreatic cancer is frequently diagnosed when patients are already at advanced stages, and recurrence after surgery is also common. The effects of current standard chemotherapy regimens on advanced pancreatic cancer are very limited (1), and new treatment approaches are urgently needed. Recently, intervention of pH regulation in the tumor microenvironment was suggested to be a possible new treatment target (2,3). Several in vivo and in vitro studies have demonstrated that systemic buffering by bicarbonate administration is associated with neutralization of the acidic tumor microenvironment, and may lead to antitumor effects (4,5). Similar to alkalization effects of bicarbonate administration, the administration of trishydroxymethyl aminomethane (tris-base) buffer was reported to result in reduced tumor volumes and improved survival outcomes in mouse models of pancreatic cancer (6).
Normal cells usually generate adenosine triphosphate (ATP) via oxidative phosphorylation, whereas cancer cells are reported to produce ATP mainly using aerobic glycolysis (7). ATP production by glycolysis results in production of lactic acid, which generates protons. The extracellular and intracellular pH of cancer cells are regulated by acid-base transporters, such as Na+/H+ exchangers and monocarboxylate transporters, and the export of protons from cancer cells causes an acidic tumor microenvironment (3,8). A decrease in the extracellular pH of the tumor microenvironment is an important metabolic characteristic of cancer cells, that is associated with cancer progression and drug resistance (2,3). Therefore, neutralization of the acidic tumor microenvironment is thought to be associated with the antitumor effects of buffering therapy (4,5).
Recently, our group conducted a retrospective study investigating the effects of alkalization therapy, which comprised an alkaline diet (eating fruit and vegetables and limiting meat and milk) and bicarbonate, on the effects of chemotherapy in metastatic and recurrent pancreatic cancer patients. Our results showed that the median overall survival (OS) of patients with a urine pH of more than 7.0 was significantly improved compared to those with a urine pH of 7.0 or less (16.1 vs. 4.7 months; p<0.05) (9). We also reported that alkalization therapy resulted in a significant increase in urine pH compared to urine pH before alkalization therapy (6.85±0.74 vs. 6.39±0.92; p<0.05). However, it remains unclear whether alkalization therapy together with chemotherapy in advanced pancreatic cancer patients results in more favorable survival effects compared to standard chemotherapy only. Therefore, we conducted a case-control study to investigate the effects of alkalization therapy on chemotherapy outcomes in metastatic and recurrent pancreatic cancer patients, by comparing the alkalization group, in which patients were treated with alkalization therapy and chemotherapy, with the control group, in which patients were treated with chemotherapy only.
Patients and Methods
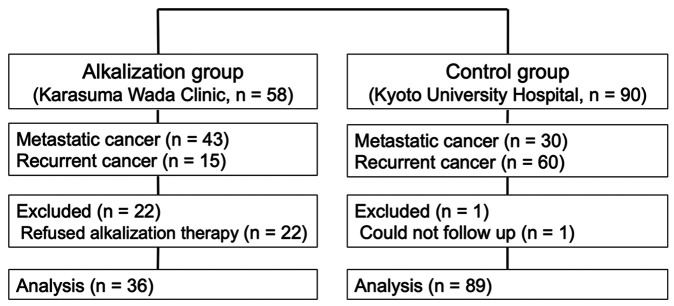
Study design. This case-control study was retrospectively conducted to investigate the effects of alkalization therapy on chemotherapy outcomes in advanced pancreatic cancer patients, using medical records from Karasuma Wada Clinic and the Department of Hepato-Biliary-Pancreatic Surgery and Transplantation at the Kyoto University Hospital. The alkalization group, which comprised patients with metastatic or recurrent pancreatic cancer who received alkalization therapy together with chemotherapy at the Karasuma Wada Clinic, was compared with the control group, which comprised patients who received only chemotherapy at the Kyoto University Hospital (Figure 1). Patients in the alkalization group included patients from our previous retrospective study (9). All procedures were performed in accordance with the ethical principles stated in the 1995 Declaration of Helsinki. This study was approved by the Ethics Committee of Graduate School of Medicine and Faculty of Medicine, Kyoto University and was registered with UMIN Clinical Trials (UMIN000036126). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology statement and its checklist (10).
Figure 1. Flowchart of the study. The flowchart shows the number of patients included in the alkalization and control group.
Alkalization group. Metastatic or recurrent pancreatic cancer patients who were treated with alkalization therapy together with standard chemotherapy were defined as the alkalization group. A total of 58 patients with metastatic or recurrent pancreatic cancer who were treated at the Karasuma Wada Clinic between April 1, 2015 and April 30, 2018 were recruited. Alkalization therapy was performed in principle in all patients as a routine treatment at the Karasuma Wada Clinic, as described below. Written informed consent was obtained from each patient. All patients were recommended to receive supplementary intravenous vitamin C (25-50 g/day every 1 or 2 weeks). Other interventional therapies were not recommended. In addition to the alkalization therapy, patients were allowed to undergo all appropriate concomitant chemotherapy treatments, which were administered at other hospitals. If patients insisted on not receiving alkalization therapy and visited our clinic less than 3 times, they were excluded from the study population. Therefore, 22 patients who did not follow the alkalization therapy were excluded from the study. Finally, the data of 36 pancreatic cancer patients who underwent alkalization therapy together with chemotherapy were collected 20 months after the end of the recruitment period, on December 31, 2019. Thirty-six patients in the alkalization group included 28 patients from our previous retrospective study (9).
Control group. Metastatic or recurrent pancreatic cancer patients who were treated with standard chemotherapy were defined as the control group. A total of 90 patients with metastatic or recurrent pancreatic cancer who were treated at the Department of Hepato-Biliary-Pancreatic Surgery and Transplantation of the Kyoto University Hospital between April 1, 2012 and March 31, 2016 were recruited. All patients in the control group received standard chemotherapy, and instructions on alkalization therapy were not provided. One patient in the control group was not followed and was excluded from the study. Finally, the data of 89 pancreatic cancer patients who were given only standard chemotherapy were collected 20 months after the end of the recruitment period on November 30, 2017.
Alkalization therapy. We defined alkalization therapy as a treatment consisting of an alkaline diet and bicarbonate therapy, which both have an alkalizing effect on the body. An alkaline diet was defined as food containing a large amount of vegetables and fruits, and few meat and dairy products, which was aimed to increase urine pH. In the alkalization group, all patients were instructed to take at least 400 g of fruits and vegetables per day and not to take meat and dairy products, but the actual diet was decided by the patients at their homes. Patients in the alkalization group were also instructed to take oral bicarbonate (3.0-5.0 g/day), in addition to the alkaline diet. All patients in the alkalization group recorded their daily meals during at least the first 4 weeks from the start of the alkaline diet, to confirm whether the meals were appropriate or not, and they were given advice according to their records. At every visit, a doctor or nurse provided patients with guidance regarding the alkaline diet and assessed whether patients had been following the alkaline diet appropriately and regularly. In contrast, all patients in the control group were not given any instructions regarding the alkaline diet.
Assessment procedures. The OS from the time of diagnosis of metastasis or recurrence after surgery in each group of patients was calculated, using the medical records at the Karasuma Wada Clinic and Kyoto University Hospital. In the alkalization group, urine pH data were also collected at the patients’ regular visits, which were at least once every 2 months, and up to twice a month.
Statistical analyses. Data were analyzed on March 31, 2020. In the alkalization group, mean urine pH values were calculated for each patient before and after the initiation of alkalization therapy. Urine pH data before alkalization therapy were from urine samples taken during the 6 months before the start of alkalization therapy. Urine pH data after alkalization therapy included all urine samples taken after the initiation of alkalization therapy. In the alkalization group, the mean urine pH of each patient was compared using the paired t-test comparing values between before and after alkalization therapy. The OS from the time of diagnosis or recurrence between the alkalization group and control group was compared using the Kaplan-Meier estimates. The OS of patients in the alkalization group who had a mean urine pH of more than 7.0 was compared with that of the control group. The OS of patients in the alkalization group who had a urine ΔpH of more than 1.0 was also compared to that of the control group. Urine ΔpH in the alkalization group was calculated by subtracting the mean urine pH before alkalization therapy from the mean urine pH after alkalization therapy. The standard deviations of mean dataset values were calculated and presented. All p-values were two-sided, and a p-value of less than 0.05 was considered to indicate a statistically significant difference between two groups. All statistical analyses were performed with EZR (version 1.32) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface that is a modified version of R (The R Foundation for Statistical Computing, Vienna, Austria) (11).
Results
Patient characteristics. The data of both groups were collected 20 months after the end of the patient recruitment period. Data from 36 patients (19 men and 17 women) in the alkalization group were collected on December 31, 2019. The mean age at diagnosis or recurrence was 64.5 (range=47-86) years. Twenty-five patients were clinical stage IV and 11 had recurrent disease. Twenty-seven out of the 36 patients took supplementary bicarbonate together with the alkaline diet. Thirty-two out of the 36 patients received intravenous vitamin C. Data from 89 patients (59 men and 30 women) in the control group were collected on November 30, 2017. The mean age at diagnosis or recurrence was 68.1 (range=44-84) years. Thirty patients were clinical stage IV and 59 had recurrent disease. Patient characteristics are shown in Table I. The number of patients with postoperative recurrence in the control group tended to be higher than that in the alkalization group.
Table I. Patient characteristics.
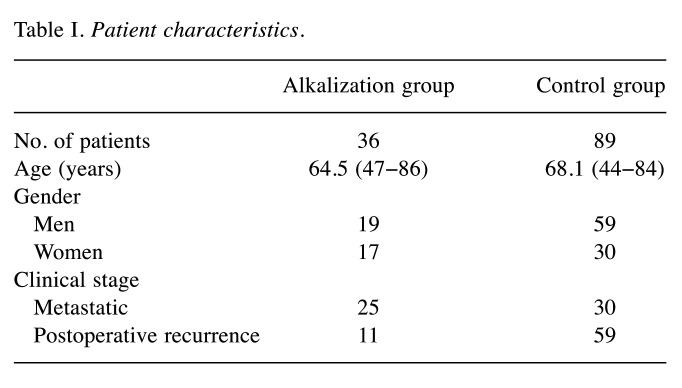
Urine pH analysis. The mean urine pH of the alkalization group, before starting alkalization therapy and after alkalization therapy are shown in Figure 2. After alkalization therapy, the mean urine pH was significantly increased compared with before alkalization therapy (6.38±0.85 vs. 6.80±0.71; p<0.05). Urine pH data of the control group were not collected in this study.
Figure 2. Effect of alkalization therapy on urine pH in the alkalization group. The mean urine pH before (n=36) and after initiation of alkalization therapy (n=36) is shown. The thick lines indicate the medians, the error bars indicate the maximum and minimum values, and the boxes indicate the values between the upper quartile and lower quartile.
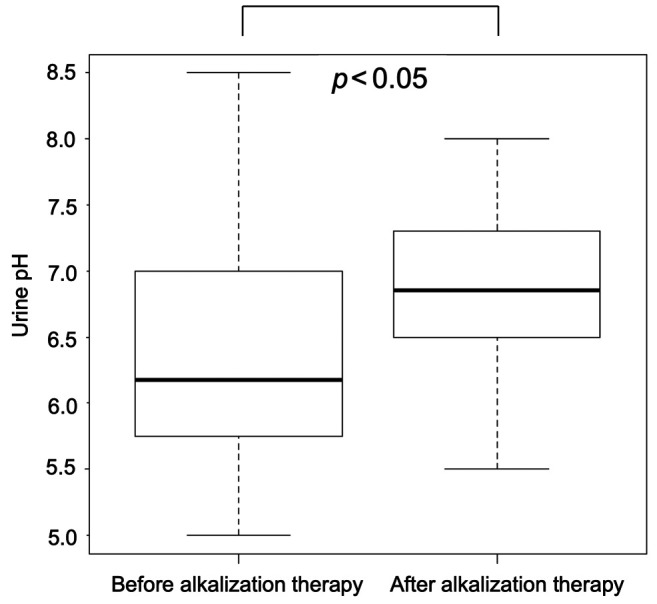
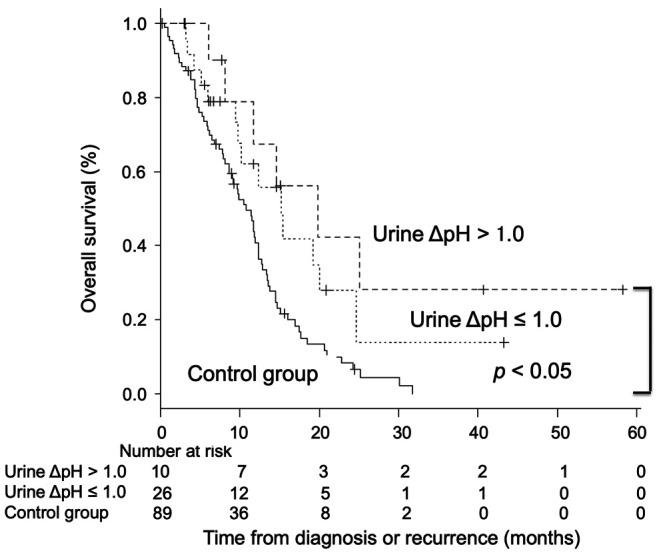
Overall survival. The median OS from the time of diagnosis or recurrence in the alkalization group was significantly longer than that of the control group [alkalization group: n=36, 15.4 months, 95% confidence interval (CI)=10.2−24.7 vs. control group: n=89, 10.8 months, 95% CI=8.7−12.3; p<0.005] in Figure 3. The median OS of patients with a urine pH of higher than 7.0 in the alkalization group was 25.1 months [n=13, 95% CI=5.2−not applicable (NA)] compared to 10.8 months for patients in the control group (n=89, 95% CI=8.7−12.3; p<0.005) (Figure 4). The median OS of patients with a urine ΔpH of greater than 1.0 in the alkalization group was 19.8 months (n=10, 95% CI=6.1−NA) as compared with 10.8 months for patients in the control group (n=89, 95% CI=8.7−12.3; p<0.05) (Figure 5). Twenty out of the 36 patients in the alkalization group have died as of December 31, 2019, whereas 71 out of 89 patients in the control group have died as of November 30, 2017.
Figure 3. Overall survival of the alkalization and control group. Kaplan- Meier curves of the overall survival from diagnosis or recurrence of patients in the alkalization group and the control group are shown.
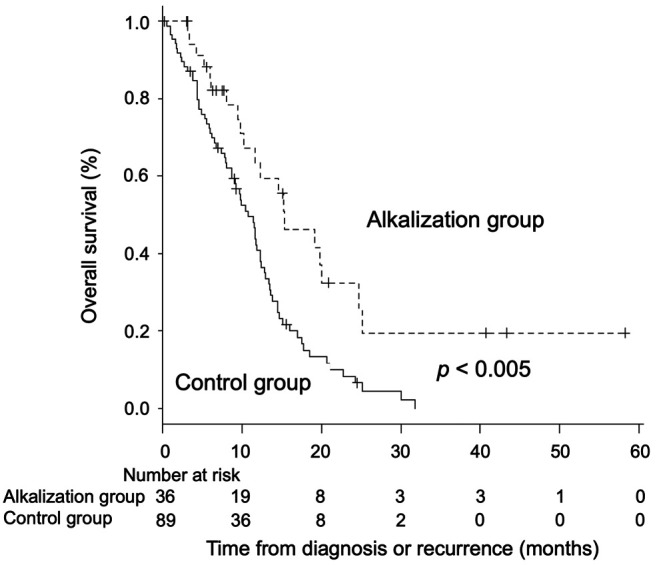
Figure 4. Association between overall survival and urine pH of patients. Kaplan-Meier curves of the overall survival from diagnosis or recurrence of patients with a urine pH of higher than 7.0 and 7.0 or lower are shown.
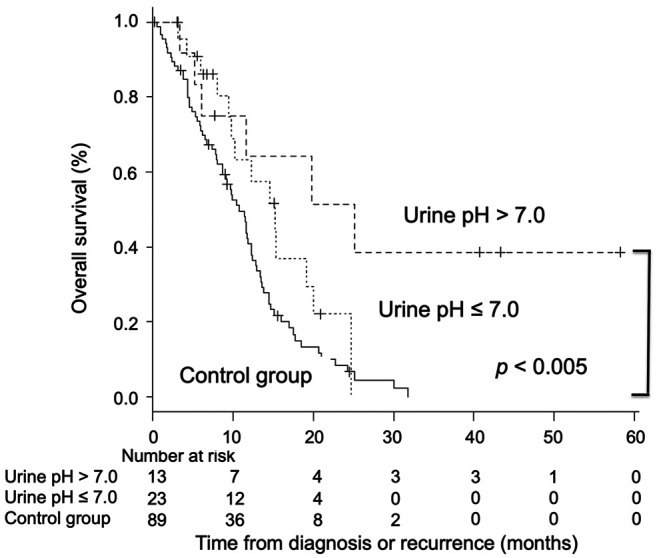
Figure 5. Association between overall survival and urine ΔpH of the patients. Kaplan-Meier curves of the overall survival from diagnosis or recurrence of the patients with a urine ΔpH of greater than 1.0 and 1.0 or less are shown.
Discussion
Our case-control study demonstrated that alkalization therapy, consisting of an alkaline diet and bicarbonate administration, significantly prolonged the median OS of metastatic or recurrent pancreatic cancer patients receiving chemotherapy, compared to those receiving chemotherapy only. A prospective study of European and American patient populations reported that the median OS of metastatic pancreatic cancer patients receiving standard chemotherapy was 11.1 months for patients receiving a combination regimen consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX), and 8.5 months for patients receiving nab-paclitaxel plus gemcitabine (12,13). The median OS of a Japanese patient population was 10.7 months in FOLFIRINOX-treated patients and 13.5 months in nab-paclitaxel plus gemcitabine-treated patients (14,15). In the present study, the median OS from diagnosis or recurrence in the alkalization group was 15.4 months, whereas the median OS in the control group was 10.8 months, which was similar to previous reports. Several in vivo and in vitro studies reported that the decrease in pH of the extracellular tumor microenvironment was associated with multichemotherapeutic drug resistance (3,5,16,17). Weak-base chemotherapeutic drugs become positively charged in the acidic tumor environment, which causes a reduction in their cell permeability, and hence their decreased cellular uptake and efficacy (18-20). Acidification of the tumor microenvironment was also shown to increase the number of exosomes containing chemotherapeutic drugs, which are then eliminated from cancer cells (21,22). An acidic tumor microenvironment is also thought to assist in the removal of chemotherapeutic agents from cancer cells, due to the increase in the activation and expression of the multidrug transporter p-glycoprotein (23,24). Therefore, alkalization therapy, which is expected to have an effect on neutralizing protons in the body, may be an adjuvant treatment to chemotherapy in pancreatic cancer patients, and may be particularly useful for advanced pancreatic cancer patients in whom current treatments have limited effects.
The mean urine pH after alkalization therapy was significantly higher than that before alkalization therapy, and the urine pH changes observed in these patients were similar to that of our previous studies. The alkalization group of this case-control study includes 28 advanced pancreatic cancer patients from our previous retrospective study, which investigated the effects of alkalization therapy on chemotherapy outcomes (9). We also previously demonstrated that an alkaline diet resulted in a significant increase in the urine pH of advanced lung cancer patients who were treated with epidermal growth factor receptor-tyrosine kinase inhibitor (n=11) (25). Bicarbonate has buffering effects on the human body, and was shown to increase urine pH levels in a clinical study, which demonstrated that the long-term consumption of bicarbonate (0.5 g/kg/day, i.e., 25 g/50 kg body weight) was safe and tolerable (26). It was reported that diet also affects the acid-base balance, which can be predicted through calculating net renal acid load, and fruits and vegetables have an alkalizing effect, and meat and dairy products have an acidifying effect on urine pH (27). In the present study, 27 out of 36 patients in the alkalization group had consumed bicarbonate at a dose of 3.0-5.0 g/day together with the alkaline diet, and demonstrated a significant increase in urine pH. Although the dose of bicarbonate in this study was lower than that of a previous study (26), its synergetic effects on urine alkalization together with the alkaline diet were observed. Therefore, we expect that alkalization therapy, which is a combination of an alkaline diet and bicarbonate administration, may have a stronger alkalizing effect than bicarbonate therapy on its own.
In this study, we also demonstrated that the median OS of patients with increased urine pH was significantly longer than that of the control group. Similarly, our previous study investigating the effects of alkalization therapy in patients with advanced pancreatic cancer showed that a urine pH of higher than 7.0, or a urine ΔpH of more than 1.0, was significantly associated with prolonged OS in advanced pancreatic cancer patients compared with a urine pH of 7.0 or lower, or a urine ΔpH of 1.0 or less (9). Therefore, an increase in urine pH after alkalization therapy may reflect the more favorable outcomes of patients with advanced pancreatic cancer undergoing chemotherapy. Although the association between pH of the tumor microenvironment and urine pH remains unclear, bicarbonate consumption has been reported to lead to an increase in pH levels of the tumor microenvironment, in mathematical models and computer simulation studies (28,29). In addition, it was reported that bicarbonate administration in mouse models of metastatic breast cancer increased the pH of tumor cells, resulting in the suppression of cancer progression (4). An increase in urine pH was also observed upon buffering therapy (tris-base buffer) in mouse models of pancreatic cancer, and was associated with more favorable outcomes (6). These studies support our hypothesis that the alkalization of urine pH may be associated with neutralization of the acidic tumor microenvironment; however, further investigations are required to clarify this point.
Intravenous vitamin C administration was reported to have some potential anticancer effects on pancreatic cancer in several in vivo and in vitro studies (30). Mouse models of pancreatic cancer xenografts have shown that gemcitabine therapy together with vitamin C administration has synergetic anticancer effects (31). The levels of dehydroascorbate, which is the oxidized form of vitamin C that may have anticancer effects through its inhibition of glyceraldehyde 3-phosphate dehydrogenase, is highly increased in oncogenic KRAS mutant cells, which are commonly found among pancreatic cancer cells and have high glycolytic metabolism (32). Phase I clinical studies demonstrated that intravenous vitamin C therapy together with chemotherapy in pancreatic cancer patients was safe (33,34). In our present study, 32 out of the 36 patients in the alkalization group received supplementary intravenous vitamin C, which may have also affected their treatment outcomes.
We acknowledge that this study has several limitations. First, this was a case-control study that compared patients of a single center (Karasuma Wada Clinic) with those of another single center (Kyoto University Hospital), and was not a prospective study. We recognize that there may be differences between the institutions in the patient characteristics, such as chemotherapy regimens depending on the time of patient recruitment. Propensity score matching was not performed in this study, because sample sizes were small. Second, although we showed that patients with increased urine pH in the alkalization group had more favorable survival outcomes, we could not demonstrate the association between urine pH and the tumor microenvironment, because it was difficult to analyze the extracellular pH of the tumor microenvironment of each patient in the actual clinical setting. We understand that an alkaline diet, which is high in vegetables and fruits, and low in meat and dairy products, may not only have an alkalizing effect but may have some potential effects on cancer metabolism, such as caloric restriction or anti-inflammatory effects, which may affect insulin levels or the microbiome. Third, we were unable to collect data on urine pH, or investigate the daily diets and diet preferences of the control patients. The control group may include patients who have diets that are similar to the alkaline diet, and hence urine pH analysis of the control group would be informative. Moreover, in the alkalization group, the timing of the initiation of alkalization therapy was not consistent and the details of the patients’ daily diet were not meticulously controlled. Therefore, a prospective randomized study is necessary to further clarify the effects of alkalization therapy.
Conclusion
We demonstrated that alkalization therapy together with chemotherapy may improve the outcomes of metastatic and recurrent pancreatic cancer patients. Alkalization therapy, consisting of an alkaline diet and bicarbonate resulted in an increase in urine pH, which may lead to more favorable outcomes in advanced pancreatic cancer patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The Authors declare that they have no conflicts of interest associated with this study.
Authors’ Contributions
Reo Hamaguchi performed the literature review, analyzed the data, and wrote the article. Takashi Ito, Ryoko Narui, Hiromasa Morikawa and Shinji Uemoto performed the acquisition of data. Hiromi Wada supervised the study. All Authors conceived and designed the study and gave final approval for publication.
Acknowledgements
The Authors thank Dr. Helena Akiko Popiel of Tokyo Medical University for her editing of this manuscript.
References
- 1.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/s0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neri D, Supuran CT. Interfering with ph regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 3.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin−one single nature. Biochim Biophys Acta. 2005;1756(1):1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate increases tumor ph and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim-Hashim A, Estrella V. Acidosis and cancer: From mechanism to neutralization. Cancer Metastasis Rev. 2019;38(1-2):149–155. doi: 10.1007/s10555-019-09787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim-Hashim A, Abrahams D, Enriquez-Navas PM, Luddy K, Gatenby RA, Gillies RJ. Tris-base buffer: A promising new inhibitor for cancer progression and metastasis. Cancer Med. 2017;6(7):1720–1729. doi: 10.1002/cam4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis. Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 8.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 9.Hamaguchi R, Narui R, Wada H. Effects of alkalization therapy on chemotherapy outcomes in metastatic or recurrent pancreatic cancer. Anticancer Res. 2020;40(2):873–880. doi: 10.21873/anticanres.14020. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4(10) doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda Y. Investigation of the freely available easy-to-use software 'ezr' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Unicancer GTDo and Intergroup P Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, Isayama H, Boku N. Phase ii study of folfirinox for chemotherapy-naive japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105(10):1321–1326. doi: 10.1111/cas.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, Nakajima TE, Furuse J. Phase i/ii study of nab-paclitaxel plus gemcitabine for chemotherapy-naive japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77(3):595–603. doi: 10.1007/s00280-016-2972-3. [DOI] [PubMed] [Google Scholar]
- 16.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic ph microenvironment. Mol Pharm. 2011;8(6):2032–2038. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerweck LE, Vijayappa S, Kozin S. Tumor ph controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5(5):1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 18.Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: New strategies in prevention and therapy. Cancer Metastasis Rev. 2014;33(4):1095–1108. doi: 10.1007/s10555-014-9531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. Biochem Pharmacol. 2003;66(7):1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 20.Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. Biochem Pharmacol. 2003;66(7):1219–1229. doi: 10.1016/s0006-2952(03)00468-4. [DOI] [PubMed] [Google Scholar]
- 21.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental ph is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D'Ilio S, Lugini L, Violante N, Azzarito T, Majorani C, Brambilla D, Fais S. Exosome release and low ph belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 2014;9(2):e88193. doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of p-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006;8(2):143–152. doi: 10.1593/neo.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007;17(1):239–244. doi: 10.3892/or.17.1.239. [DOI] [PubMed] [Google Scholar]
- 25.Hamaguchi R, Okamoto T, Sato M, Hasegawa M, Wada H. Effects of an alkaline diet on egfr-tki therapy in egfr mutation-positive nsclc. Anticancer Res. 2017;37(9):5141–5145. doi: 10.21873/anticanres.11934. [DOI] [PubMed] [Google Scholar]
- 26.Lopez AM, Robey IF. Safety and tolerability of long-term sodium bicarbonate consumption in cancer care. J Integr Oncol. 2014;4(1):1000128. doi: 10.4172/2329-6771.1000128. [DOI] [Google Scholar]
- 27.Remer T, Manz F. Potential renal acid load of foods and its influence on urine ph. J Am Diet Assoc. 1995;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 28.Martin NK, Robey IF, Gaffney EA, Gillies RJ, Gatenby RA, Maini PK. Predicting the safety and efficacy of buffer therapy to raise tumour phe: An integrative modelling study. Br J Cancer. 2012;106(7):1280–1287. doi: 10.1038/bjc.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The potential role of systemic buffers in reducing intratumoral extracellular ph and acid-mediated invasion. Cancer Res. 2009;69(6):2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigelsen S. Evidence-based complementary treatment of pancreatic cancer: A review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin c, statins, metformin, curcumin, and aspirin. Cancer Manag Res. 2018;10:2003–2018. doi: 10.2147/cmar.S161824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50(11):1610–1619. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC. Vitamin c selectively kills kras and braf mutant colorectal cancer cells by targeting gapdh. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh JL, Wagner BA, van't Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ 2nd, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (pacman): Results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71(3):765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1):e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]