Abstract
Background/Aim: Amino acids are among the most important nutrients for supplying energy and building protein blocks in cancers. L-type amino acid transporter (LAT) 1 is known to play a critical role in cancer growth. We have completed the first-in-human phase I study using the LAT1-specific inhibitor JPH203. Patients and Methods: We evaluated plasma free amino acids (PFAAs), body mass index (BMI), and efficacy of JPH203 in patients enrolled in the phase I study. Results: LAT1-substrate PFAAs and branched chain amino acids (BCAAs) were higher in patients with biliary tract cancer (BTC) than in those with other cancers. High inhibition of uptake of LAT1-substrate PFAAs was associated with survival. BMI of more than the median was associated with disease control and survival. BCAAs tended to be associated with BMI. Conclusion: BCAAs and BMI are useful predictors of the efficacy of JPH203, which shows promising activity against BTC.
Keywords: L-type amino acid transporter, JPH203, amino acids, body mass index, biliary tract cancer
Glucose and amino acids (AA) are major nutrients in animals and humans. In oncology, increase of glucose metabolism within cancer cells is one of the most prominent characteristics. In 1956, Warburg reported that irreversible switching of glucose metabolism from a high efficiency pathway for producing ATP through mitochondrial oxidative phosphorylation to a less efficient glycolytic pathway-initiated transformation of normal cells to cancer cells, which is referred to as the ‘Warburg effect’ (1). Although numerous efforts have been made for several decades, precise mechanism of the ‘Warburg effect’ in cancer development have not been established.
In addition to high glucose metabolism in cancer, we became interested in cancer-specific amino acid metabolism and aimed to explain its metabolic relationship with physiological states and pathophysiological cancerous states. Christensen classified amino acid transporters into three major groups, System-A, -ASC, and -L according to their physiological properties in the pre-genome era (2). In 1998, a novel amino acid transporter belonging to System-L was identified in rat C6 glioma-derived cell line and referred to as the L-type amino acid transporter (LAT) 1 (3). Human LAT1, identified by homology cloning, was highly expressed in cancerous and fetal tissues, and a very weak expression was found in limited normal adult tissues like brain, bone marrow, and reproductive organs (4). LAT1 was expressed in cancer cell membranes together with its chaperon protein, 4F2hc (=CD98). LAT1/CD98 can transport eight AAs (LAT1-substrates) such as histidine, isoleucine, leucine, methionine, phenylalanine, tryptophan, tyrosine, and valine, among which all 7 AAs except tyrosine are essential amino acids (EAA)s in humans. In humans, EAAs consist of 9 AAs, including the above 7 AAs, lysine, and threonine. Moreover, isoleucine, leucine, and valine are known as branched-chain amino acid (BCAA)s. Following identification of LAT1, a second member of System-L transporters, which is expressed ubiquitously in normal cells but seldom in cancer cells, was identified. As this new transporter resembles LAT1 in its molecular structure, it was named LAT2 (5). LAT2 transports 15 AAs, including the 8 LAT1-substrate AAs and alanine, asparagine, cysteine, glutamine, glycine, threonine, and serine.
As predicted, the LAT1 gene and protein are overexpressed in human cancer-derived cell lines (4) and cancer tissues such as prostate (6), stomach (7), colorectum (8), pancreas (9), biliary tract (10), lung (11), and uterus (12). Importantly, immunohistochemical staining in cancer tissues has been shown that LAT1 expression is associated with malignancy in patients with the respective cancer (6-12). Thus, LAT1 could be used as a diagnostic and prognostic marker in patients with cancer. This characteristic of LAT1 could also be exploited to cancer-specific positron emission tomography (PET) imaging analysis using an AA PET probe, [18F]-fluorinated alpha-methyl tyrosine ([18F]-FAMT) (13), because FAMT is taken up by cancer cells via LAT1 (14). In a study using this LAT1 PET probe, [18F]-FAMT showed no false-positive results in lung cancer, indicating that normal parts of the tissues were LAT1-negative in LAT1 PET analysis (15).
Since LAT1 is a cancer type and LAT2 is a normal type, LAT1-specific inhibitors have been investigated as potential new drugs in cancer treatment. Various small molecules inhibitors that differentiate LAT1 and LAT2, have been synthesized. JPH203 was selected as the most potent inhibitor against LAT1, that did not inhibit LAT2 (16,17). Following completion of a series of translationally necessary non-clinical studies, we have completed the first-in-human phase I study using the JPH203 formulation. The study consisted of 17 patients with advanced solid tumors (18). As a result, partial response (PR) was achieved in a patient with biliary tract cancer (BTC) and disease control was observed in 3 of 5 patients with BTC. Therefore, a phase II clinical study of JPH203 with advanced BTC patients is currently under ongoing (UMIN000034080).
The difference in the biology of AAs metabolism between BTC and other solid tumors remains still unclear. The purpose of this investigation was to identify predictive biomarkers for the efficacy of the LAT1 inhibitor JPH203.
Patients and Methods
Study design and treatment of phase I study. Details of this study, including inclusion and exclusion criteria, trial design, and dose-escalation methods have been published previously (18). Briefly, this study was a single-center, open-label, single-arm, first-in-human dose-escalation (3+3 design) phase I study of patients with advanced solid tumors who were refractory to standard chemotherapy. Patients received JPH203 at doses of 12 mg/m2 (n=4), 25 mg/m2 (n=3), 40 mg/m2 (n=3), 60 mg/m2 (n=6), and 85 mg/m2 (n=1), intravenously once per day over 90 minutes for 7 days, followed by 21-day rest periods (one cycle) until tumor progression or unacceptable toxicities. Before starting this schedule, we first confirmed the drug’s safety over an observation period of 7 days after a single-dose administration of JPH203 while the patients were resting.
This study was approved by the institutional review board at Kyorin University and conducted in accordance with the Declaration of Helsinki and good clinical practice. All patients provided written informed consent.
Efficacy analysis. Computed tomography was performed after the first cycle and then every six weeks. Tumor responses were evaluated in accordance with the Response Evaluation Criteria in Solid Tumors 1.1. The response rate was defined as the proportion of patients who experienced a complete response (CR) or PR. The disease control rate (DCR) was defined as the proportion of patients with CR, PR, or stable disease (SD). Progression-free survival (PFS) was defined as period from the date of enrollment in this study to the date of progressive disease (PD) or death for any reason. Overall survival (OS) was defined as the period from the date of enrollment in this study to the date of death from any cause. If PD or death was not observed, the patients were censored for PFS and OS on the last date of confirming no events.
Plasma free amino acid analyses. Blood samples were collected before the single-dose treatment and at 25.5 h following this dose treatment, before the dose administration on day 1, and at 25.5 h after the dose administration for plasma free amino acids (PFAAs) analysis. Thirty-nine PFAAs including 20 AAs and AA-related molecules were measured using Liquid Chromatography/Mass Spectrometry methods. The precise analytical methods used were as described elsewhere (19). We investigated the difference in PFAAs due to the site of the cancer before the dose administration of JPH203, and the association between changes in the LAT1-substrate PFAA levels and survival. Changes in the LAT1-substrate PFAA levels were calculated as the difference in the levels measured before day 1 and after day 7 in cycle 1. If treatment in cycle 1 needed to be discontinued, the level at discontinuation was used instead of that after day 7. The degree of inhibition of uptake of the LAT1-substrate PFAA was divided into the high inhibition group and low inhibition group by the median.
Body mass index and LAT1 immunohistochemical staining analyses. The relationship between the body mass index (BMI) of the patients and the tumor response and survival was investigated. BMI was divided into a High-BMI group and a Low-BMI group by the median. We also analyzed the association between the LAT1 immunohistochemical score and the tumor response. We performed LAT1 immunohistochemical staining by using previously published methods (6). Briefly, the sections were treated with 0.3% hydrogen peroxide for 30 minutes and incubated with a mouse monoclonal antibody against human LAT1 (custom-made by J-Pharma, Yokohama, Japan) for 1 hour at room temperature. The reaction was visualized using the Histofine Simple Stain MAX-PO (Multi) Kit (Nichirei, Tokyo, Japan) according to the manufacturer’s instructions. Formalin/PFA-fixed paraffin-embedded tissues were used for immunohistochemistry. We assigned score 0, 1, 2 and 3 to no staining, weak membrane, moderate complete membrane, and strong complete membrane staining intensity, respectively.
Statistical analysis. The AA data were expressed as means±standard deviation and the statistical significance of differences were determined by a Student’s t-test. OS and PFS were calculated using the Kaplan-Meier method and differences were evaluated using the log-rank test. Comparison of the DCR depending on the BMI (High-BMI and Low-BMI groups) was performed using Fisher’s exact test. The association between LAT1 expression and the tumor response was analyzed using a Chi-squared test. Correlations between BMI and PFAA, and between BMI and BCAA were analyzed using Spearman’s rank test. A p<0.05 was considered as denoting statistical significance in all tests. All analyses were performed using IBM SPSS software (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics and anti-tumor effect. Seventeen patients were enrolled in this study. Most of the patients enrolled had gastrointestinal cancers. The number of patients with colorectal cancer was the highest (6 patients, 35%), followed in frequency by patients with BTC (5 patients, 29%) and pancreatic cancer (PC) (4 patients, 24%). All patients had been heavily treated prior to this study, with a median number of 3 prior chemotherapy regimens (range=2-6). The median follow-up time was 3.7 months (range=1.5-26.8 months).
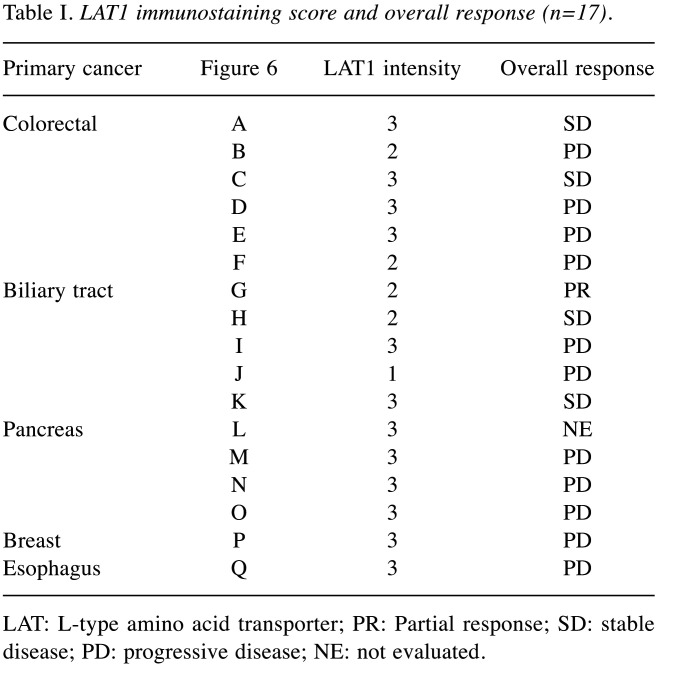
Sixteen of 17 patients were evaluated for survival and tumor responses of JPH203 because one patient with pancreatic cancer was discontinued due to tumor progression after administration of a single dose at 12 mg/m2. The results of overall responses are summarized in Table I. PR was observed in one patient with BTC at the 12 mg/m2 dose, and the DCR for BTC was 60%. In other cancers, only two patients with colorectal cancer had SD (18). All three PC patients together with one breast and esophagus cancer patients had PD.
Table I. LAT1 immunostaining score and overall response (n=17).
LAT: L-type amino acid transporter; PR: Partial response; SD: stable disease; PD: progressive disease; NE: not evaluated.
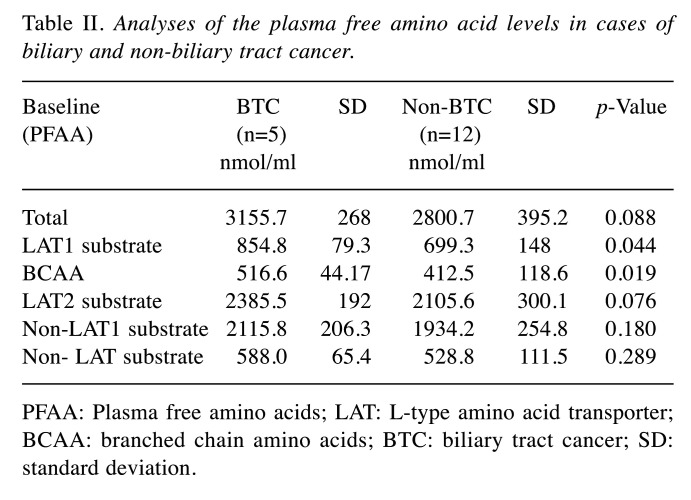
Plasma free amino acids in BTC and non-BTC. To understand the cancer-specific patterns of PFAA, we classified PFAAs to 6 groups, and cancers to two groups. As summarized in Table II, we compared BTC with non-BTC, and found that both LAT1-substrates and BCAA levels in the plasma before treatment were higher in BTC than in non-BTC patients (p<0.05). The difference in BCAA denotes a higher statistical significance than that in LAT1-substrates. There were no significant differences in total PFAAs, LAT2-substrates, non-LAT1 substrates, or non-LAT substrates.
Table II. Analyses of the plasma free amino acid levels in cases of biliary and non-biliary tract cancer.
PFAA: Plasma free amino acids; LAT: L-type amino acid transporter; BCAA: branched chain amino acids; BTC: biliary tract cancer; SD: standard deviation.
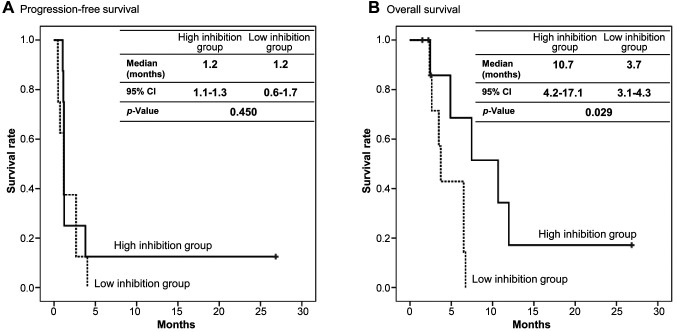
Relationship between LAT1 inhibition and survival in advanced solid tumor patients. There was not statistically significant difference in PFS between the higher degree of uptake inhibition of LAT1-substrate PFAAs (high inhibition group, >–78.0; range=–75.7- +159.6 nmol/ml) and the lower degree of uptake of LAT1-substarte PFAAs (low inhibition group, <–78.0; range=–354.9- –80.2) nmol/ml). The median PFS was 1.2 months [95% confidence interval (CI)=1.1-1.3] in the high inhibition group and 1.2 months (95%CI=0.6-1.7) in the low inhibition group (p=0.450) (Figure 1A).
Figure 1. Efficacy of JPH203 due to L-type amino acid transporter 1 (LAT1)-substrate inhibition. (A) Progression-free survival. (B) Overall survival. The degree of inhibition of uptake of the LAT1-substrate plasma free amino acids (PFAAs) was divided into high and low inhibition groups by the median. Black line, high inhibition group. Dotted line, low inhibition group. CI: Confidence interval.
In contrast, the OS of 10.7 months (95%CI=4.2-17.1) in the high inhibition group was significantly longer than the 3.7 months in the low inhibition group (95%CI=3.1-4.3) (p=0.029) (Figure 1B).
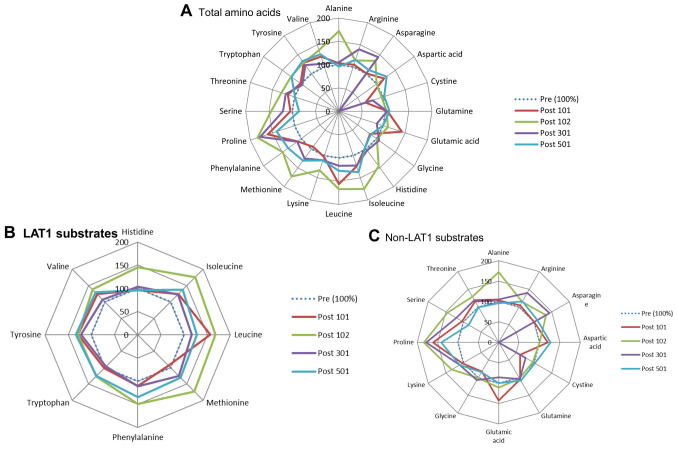
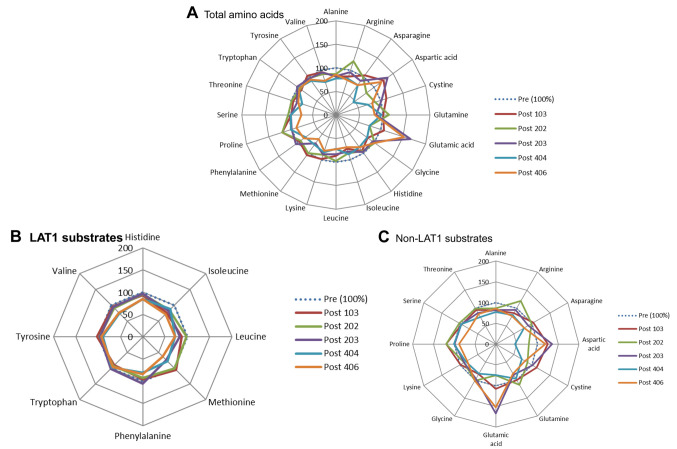
Role of LAT1 substrates in PC and BTC. The results of all 20 PFAAs from the PC and BTC patient groups are shown in Figure 2A and Figure 3A, respectively. We divided these AAs into LAT1- and non-LAT1-substrates and depicted them using redder graphs in Figures 2B, 3B and 2C, 3C. In PC patients, a single intravenous administration of JPH203 increased LAT1-substrates in all patients at 25.5 h after the single-dose administration (Figure 2B), whereas the non-LAT1-substrates changed non-specifically (Figure 2C). Conversely, the results from five patients with BTC were quite different in that JPH203 administration was found to have a decreasing, not increasing, effect on LAT1-substrates (Figure 3B), and non-LAT1 substrates changed non-specifically (Figure 3C).
Figure 2. Effect of JPH203 on plasma free amino acids (PFAAs) in pancreatic cancer. (A) Total amino acids (AAs). (B) L-type amino acid transporter 1 (LAT1) substrate AAs. (C) non-LAT1 substrate AAs.
Figure 3. Effect of JPH203 on plasma free amino acids (PFAAs) in biliary tract cancer. (A) Total amino acids (AAs). (B) L-type amino acid transporter 1 (LAT1) substrate AAs. (C) non-LAT1 substrate AAs.
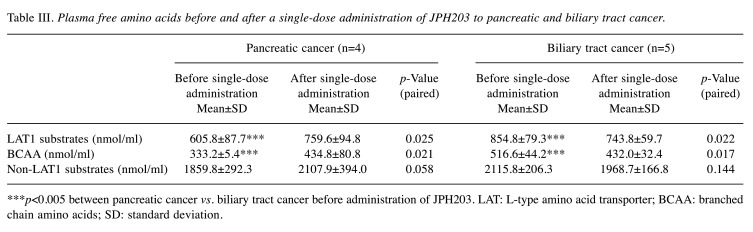
Table III summarizes the comparison of three kinds of PFAA (LAT1-substrates, BCAAs, and non-LAT1 substrates) in PC and BTC. There were clear significant differences between LAT1-substrates and BCAA (p=0.003 and p=0.002, respectively). Tables II and III show that BTC differs from PC and non-BTC, including colorectal cancer (data not shown), not only before JPH203 treatment but also after JPH203 administration.
Table III. Plasma free amino acids before and after a single-dose administration of JPH203 to pancreatic and biliary tract cancer.
***p<0.005 between pancreatic cancer vs. biliary tract cancer before administration of JPH203. LAT: L-type amino acid transporter; BCAA: branched chain amino acids; SD: standard deviation.
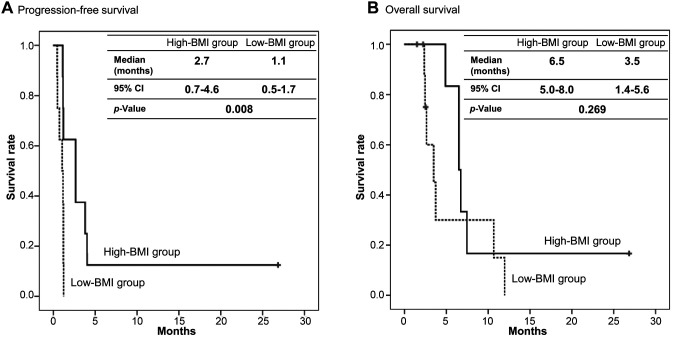
Body mass index analysis. There were eight patients whose BMI was more than 20.5 kg/m2 (High-BMI group), four of whom had BTC and four colorectal cancer. Five patients out of the eight achieved disease control, and the DCR was 62.5%. Conversely, disease control was not achieved in any of the eight patients whose BMIs were less than 20.5 kg/m2 (Low-BMI group). This exploratory analysis between BMI and tumor responses was statistically significant (p=0.026). Furthermore, the median PFS was 2.7 months (95%CI=0.7-4.6) in the High-BMI group and 1.1 months (95%CI=0.5-1.7) in the Low-BMI group (p=0.008), thereby indicating a highly significant difference (Figure 4A). In contrast, there was no difference in OS between the High-BMI group and the Low-BMI group. The median OS was 6.5 months (95%CI=5.0-8.0) in the High-BMI group and 3.5 months (95%CI=1.4-5.6) in the Low-BMI group (p=0.269) (Figure 4B).
Figure 4. Efficacy of JPH203 in High- and Low-body mass index (BMI) groups. (A) Progression-free survival. (B) Overall survival. BMI was divided into a High-BMI group and a Low-BMI group by the median. Black line, High-BMI group. Dotted line, Low-BMI group. CI: Confidence interval.
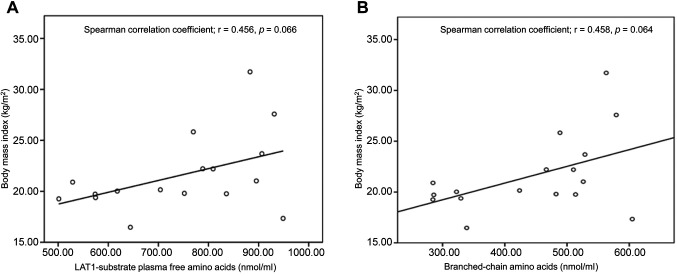
Correlations between body mass index and plasma free amino acids PFAAs indicate a tendency to be associated with BMI according to Spearman correlation coefficient (r=0.458, p=0.064) (Figure 5A). Moreover, the BCAAs of the LAT1-substrate PFAAs also showed a tendency to be associated with BMIs based on the Spearman correlation coefficient (r=0.456, p=0.066) (Figure 5B). However, total PFAAs were not associated with BMI based on the Spearman correlation coefficient (r=0.292, p=0.256) (data not shown).
Figure 5. Correlation between amino acids and body mass index (BMI). (A) Correlation between the L-type amino acid transporter 1 (LAT1)-substrate plasma free amino acid (PFAA) levels and BMI. (B) Correlation between the plasma levels of branched chain amino acids (BCAAs) and BMI.
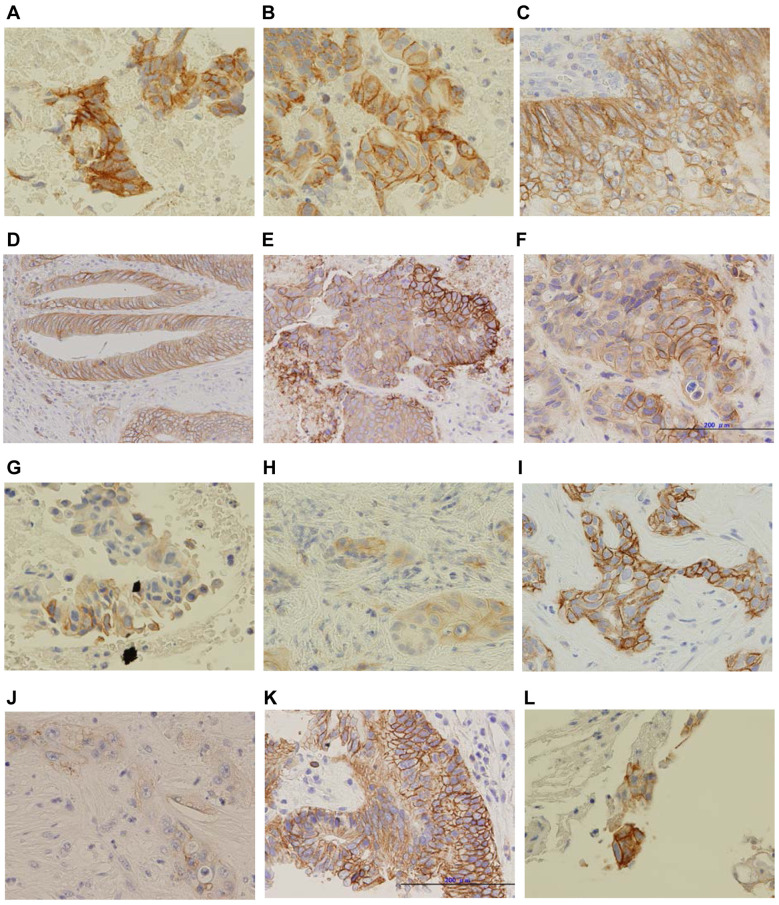
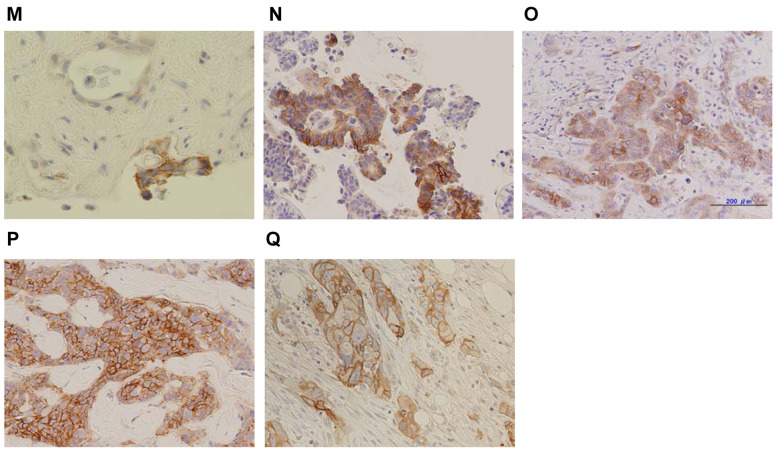
LAT1- immunohistochemical staining analysis. LAT1 immunostaining and tumor response in each patient is shown in Figure 6 and Table I. The LAT1 immunohistochemical intensity for patients with BTC with PR was 2. There were no patients with an intensity of zero. Two of five (40%) patients with an intensity of 1 or 2 were PR+SD, three of eleven (27%) with an intensity of 3 were SD. There was no statistically significant difference in disease control between intensities of 1 or 2 and 3 (p=0.552).
Figure 6. Cancer type and L-type amino acid transporter 1 (LAT1) immunohistochemical staining. (A-F). Colorectal cancer; (G-K) Biliary tract cancer; (L-O) Pancreatic cancer; (P) Breast cancer; (Q) Esophageal cancer. Panel D, E, N, O, and P show magnifications of 200×. The others show magnifications of 400×.
Discussion
This is the first study in humans that evaluated the association between the inhibition of LAT1 by JPH203 and PFAA levels. We found that LAT1-substrate PFAA levels, including those of BCAAs, were higher in patients with BTC than in those with other cancers enrolled in this study (Table II). Miyagi et al. have reported that PFAA profiling has great potential for improving cancer screening and diagnosis of several cancers, though no mechanism was discussed (19). Considering the mechanism of action of JPH203, it would be reasonable to expect a higher antitumor effect in BTC patients, who showed high LAT1-substrate PFAA levels, including BCAA levels before JPH203 treatment, because there might be sufficient gradient of LAT1-substrates between the extracellular and intracellular environment. Patients other than those with BTC, having smaller gradients of LAT1-substartes might reveal no efficacy of JPH203.
Although changes of PFAA monitored under cancerous states have been investigated (19,20), intracellular amino acid handling has scarcely been examined systematically. In relation to cell growth regulation, intracellular leucine taken up by LAT1 stimulates mammalian target of rapamycin complex 1 (mTORC1) signaling pathway followed by cell growth (21). Conversely, in our study, the mTORC1 pathway was inhibited due to attenuation of leucine uptake by JPH203 followed by cell growth inhibition. This could be the mechanism through which JPH203 significantly improves OS (Figure 1B). Although it is hard to explain the clinical evidence from the available data, experimental findings may support this result due to the long-lasting effect of JPH203 few days or longer periods after treatment termination (22-24). In addition to our study on PFAAs, clinical studies with PFAAs, which pertain to individual AAs, have been reported (25,26). Furthermore, LAT1 inhibition may prevent cancer-related cachexia. In a preclinical study, LAT1 inhibition has been shown to decrease cytokine production (27). Although this preclinical study was performed on T cells isolated from healthy subjects, LAT1 inhibition might have a similar effect in cancer patients (27). We postulate that JPH203 exhibits its long-term effect by improving OS, not PFS, in patients with high inhibition of LAT1-substrate uptake.
Hence, our study highlights the important role of LAT1 as a suitable target for diagnosing cancer malignancy and developing novel anti-cancer agents. Indeed, several review articles have been published that enhance our understanding of amino acid transporters in health and diseases (28,29). Recently, in a comprehensive review article, 62 members of amino acid transporters have been classified and characterized, all of which are used for transport of any AAs under physiological and pathophysiological conditions (30). In our current study, we focus on a cancer-specific AA transporter, LAT1 (registered by nomenclature committee as solute carrier 7A5; SLC7A5) consisting of twelve transmembrane spanning domains and having no glycosylation sites (3). Although most membrane transporters are generally glycosylated proteins, LAT1 needs a glycosylated chaperon protein having a single transmembrane spanning domain, traditionally named heavy chain of 4F2 (4F2hc, SLC3A2 same as CD98). With the aid of CD98, LAT1 can be trafficked to the cell membrane. To the best of our knowledge, LAT1/CD98 is the first heterodimer member showing L-type amino acid transporter functions. After the discovery of LAT1, five additional transporters which need CD98 as a chaperon were identified. These are: y+LAT2 (SLC7A6), y+LAT1 (SLC7A7), LAT2 (SLC7A8), Asc-1 (SLC7A10), and System xCT (SLC7A11). Since CD98 can bind six proteins, the selection mechanisms should be of interest and importance for the individually functional expressions of the six partners. Interestingly, LAT1 occupies most of the CD98 and other transporters share minor percentages (30).
In 2019, the LAT1/CD98 complex was directly visualized under cryo-electron micrography. The LAT1 molecule is capped by CD98, and these two molecules bind with a single S-S bonding (31). Moreover, LAT1 protein is covered by CD98 by their positive-negative charges (32). Thus, large molecules like antibodies seem to be difficult to bind possible functional domains because of the overlapped configuration, whereas small molecules like JPH203 have access. None of the above studies (31,32), however, visualized the JPH203 molecule inside LAT1, though JPH203 was shown as one of the most potent inhibitors against the LAT1/CD98 complex (31).
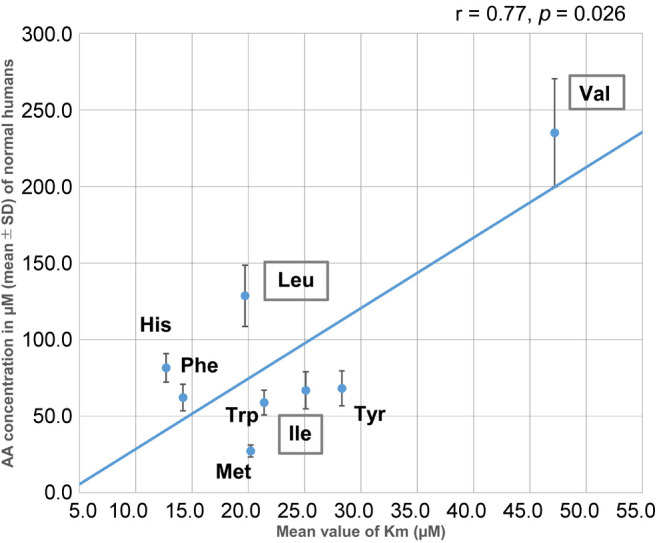
In addition, our study provides important insights into how efficient and sustainable supply of EAAs is associated with aggressive growth of individual cancer cells and tumors by demonstrating the crucial role of LAT1. The evidence may lead us to easily understand why cancer cells overcome normal cells. Considering the biological economy of AA transport, as shown in Figures 2, and 3, we need to consider the affinity of the binding indicated by the Km value of each LAT1-substrate (4) in comparison with its respective PFAA concentration (19). As can be seen in Figure 7, a linear relationship exists between LAT1-substrate PFAA of healthy humans and Km values of respective AAs. It is conceivable that 8 LAT1-substrates behave in human cancers as one group when considering both their import and export through plasma cell membranes. This balanced transport of 8 AAs might have been established through evolutionary processes. The differences in the effects resulting of JPH203 administration to patients with PC and BTC (Table III) can be explained by LAT1 acting as an exchanger to transport 8 AAs inwards or outwards depending on the levels of PFAA concentrations (Figures 2 and 3), and JPH203 inhibiting the influx in PC but not in BTC, thereby resulting in no increase. From the viewpoint of a ‘biologically rational principle’, the regulation of one system would be more practical than the regulation of the 8 substrate AAs individually. Similar to the above rational, one gene knockout may provide a clear understanding for evaluating whether the 8 LAT1-substrates behave as unit in human cancers. This notion was demonstrated in studies by Pouyssegur’s group, which investigated the main role of LAT1 in maintenance of cancer state by LAT1 gene knockout, though several proteins like CD98, ASCT2, xCT and others play additional roles (21,33). However, further preclinical and clinical studies with large sample sizes are needed to confirm this.
Figure 7. Relationship between Km values of L-type amino acid transporter 1 (LAT1) and LAT1-substrates plasma free amino acids (PFAAs). Amino acids (AAs) surrounded by squares denote branched chain amino acids (BCAAs).
This study suggests that BMI may be a useful predictor of the efficacy of a LAT1 inhibitor. Patients with a high BMI showed a higher DCR (62.5%). A tendency towards an association of BMI with the plasma free BCAA levels was also noted. Therefore, BMI and the plasma BCAAs might be predictors of the efficacy of JPH203 against advanced solid tumors. Although a higher DCR and longer PFS were observed in the high BMI group compared with the low BMI group, these differences in efficacy might be reflective of the differences in the tumor biology between the two groups because a low BMI has been reported as a poor prognostic factor. In brief, tumors may progress more rapidly in patients with a low BMI (34,35).
Indeed, BMI may be a predictive factor of the efficacy of immune checkponit inhibitors (36). As mentioned above, because the LAT1 inhibitor may exhibit activated T cell inhibition (27), it is essential to investigate the association between baseline BMI and the efficacy of JPH203. We also recommend that the cut-off values of BMI (20.5 kg/m2) in this study should be validated with larger numbers of patients.
JPH203 is a cytostatic agent that differs from cytotoxic anti-cancer agents. As LAT1 is also a novel drug target, combined therapy of JPH203 with other inhibitors could be considered. In fact, several combined therapies indicate that additive (37,38) or synergistic effects could be observed (39). These results clearly suggest that LAT1 inhibition with JPH203 may become a new anti-cancer agent in the future.
This study has some limitations. First, the sample size was small and multivariate analysis could be not performed. Second, all analyses were performed post-hoc and were not pre-planned. Third, PFAA analyses could not remove the influence of nutritional conditions and the amount of food intake. Finally, this study enrolled patients with different cancer types and with different rounds of previous chemotherapy. Therefore, the association between changes in PFAA levels, BMI, and the anti-tumor effects should be considered as preliminary data.
In conclusion, the LAT1 inhibitor JPH203 showed promising activity against BTC and high BMI patients because of having sufficient gradients of LAT1-substarte PFAAs, especially BCAAs. Further studies in larger samples are needed to clarify these biomarkers.
Conflicts of Interest
Okano N. received personal fee from J-Pharma. Hana K. received salary from J-Pharma. Endou H. received salary from J-Pharma, and has pending patents for LAT1, JPH203, and application of JPH203 in Phase I study of solid tumors. Furuse J. received grant and personal fee from J-Pharma. The other Authors declare that they have no conflicts of interest.
Author’s Contributions
Conception and design: N.O., H.E., and J.F.; Administrative support: H.E.; Provision of study materials or patients: N.O., H.K., D.N., K.K., T.K., F.N., and J.F.; Collection and assembly of data: N.O., D.N., K.K., T.K., F.N., and J.F.; Data analysis and interpretation: N.O., H.K., D.N., F.N., H.E., and J.F.; Manuscript writing: All Authors; Final approval of manuscript: All Authors.
Acknowledgements
This study was sponsored by J-Pharma Co., Ltd. J-Pharma Co., Ltd. supported LAT1 immunohistochemical staining and its interpretation, but had no role in other data acquisition, interpretation, and publications.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273(37):23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 4.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima JI, Fukasawa Y, Tani Y. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514(2):291–302. doi: 10.1016/S0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 5.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274(28):19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 6.Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59(1):7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 7.Ichinoe M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H, Okayasu I. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61(5):281–289. doi: 10.1111/j.1440-1827.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 8.Sakata T, Hana K, Mikami T, Yoshida T, Endou H, Okayasu I. Positive correlation of expression of L-type amino-acid transporter 1 with colorectal tumor progression and prognosis: Higher expression in sporadic colorectal tumors compared with ulcerative colitis-associated neoplasia. Pathol Res Pract. 2020 doi: 10.1016/j.prp.2020.152972. [DOI] [PubMed] [Google Scholar]
- 9.Yanagisawa N, Ichinoe M, Mikami T, Nakada N, Hana K, Koizumi W, Endou H, Okayasu I, Murakumo Y. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol. 2012;65(11):1019–1023. doi: 10.1136/jclinpath-2012-200826. [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa N, Hana K, Nakada N, Ichinoe M, Koizumi W, Endou H, Okayasu I, Murakumo Y. High expression of L-type amino acid transporter 1 as a prognostic marker in bile duct adenocarcinomas. Cancer Med. 2014;3(5):1246–1255. doi: 10.1136/jclinpath-2012-200826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T, Kanai Y. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp Ther Med. 2010;1(5):799–808. doi: 10.3892/etm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe J, Yokoyama Y, Futagami M, Mizunuma H, Yoshioka H, Washiya K, Hana K, Endou H, Okayasu I. L-type amino acid transporter 1 expression increases in well-differentiated but decreases in poorly differentiated endometrial endometrioid adenocarcinoma and shows an inverse correlation with p53 expression. Int J Gynecol Cancer. 2014;24(4):659–663. doi: 10.1097/IGC.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 13.Tomiyoshi K, Amed K, Muhammad S, Higuchi T, Inoue T, Endo K, Yang D. Synthesis of Isomers of 18F-labelled amino acid radiopharmaceutical: Position 2- And 3-L-18F-alpha-methyltyrosine using a separation and purification system. Nucl Med Commun. 1997;18(2):169–175. doi: 10.1097/00006231-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Wiriyasermkul P, Nagamori S, Tominaga H, Oriuchi N, Kaira K, Nakao H, Kitashoji T, Ohgaki R, Tanaka H, Endou H, Endo K, Sakurai H, Kanai Y. Transport of 3-fluoro-L-α-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53(8):1253–1261. doi: 10.2967/jnumed.112.103069. [DOI] [PubMed] [Google Scholar]
- 15.Kaira K, Oriuchi N, Otani Y, Shimizu K, Tanaka S, Imai H, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Dobashi K, Kanai Y, Endou H, Nakajima T, Endo K, Mori M. Fluorine-18-alpha-methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res. 2007;13(21):6369–6378. doi: 10.1158/1078-0432.CCR-07-1294. [DOI] [PubMed] [Google Scholar]
- 16.Endou H, Kanai Y, Tsujihara K, Saito K. Aromatic Amino Acid Derivatives and Medicinal Compositions. US Patent 7,345,068 B2. 2008 [Google Scholar]
- 17.Wempe MF, Jutabha P, Kumar V, Fisher JA, Waers K, Holt MD, Dodson AM, Bautista J, Gehr DT, Backos DS, Kumar A, Rice PJ, Anzai N, Saito K, Oda K, Kanai Y, Endou H. Developingselective L-Amino Acid Transport 1 (LAT1) inhibitors: A structure-activity relationship overview. Med Res Arch. 2019;7(12) doi: 10.18103/mra.v7i12.2014. [DOI] [Google Scholar]
- 18.Okano N, Naruge D, Kawai K, Kobayashi T, Nagashima F, Endou H, Furuse J. First-in-human phase I study of JPH203, an L-type amino acid transporter 1 inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2020 doi: 10.1007/s10637-020-00924-3. [DOI] [PubMed] [Google Scholar]
- 19.Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, Saruki N, Bando E, Kimura H, Imamura F, Moriyama M. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. 2011;6(9):e24143. doi: 10.1371/journal.pone.0024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai HS, Lee JC, Lee PH, Wo ST, Chen WT. Plasma free amino acid profile in cancer patients. Seminars Cancer Biol. 2005;15(4):267–276. doi: 10.1016/j.semcancer.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Cormerais Y, Giuliano S, LeFloch R, Front B, Durivault J, Tambutte E, Massard PA, de la Ballina LR, Endou H, Wempe M, Palacin M, Parks SK, Pouyssegur J. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016;76(15):4481–4492. doi: 10.1158/0008-5472.CAN-15-3376. [DOI] [PubMed] [Google Scholar]
- 22.Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y, Endou H. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101(1):173–179. doi: 10.1111/j.1349-7006.2009.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yothaisong S, Dokduang H, Anzai N, Hayashi K, Namwat N, Yongvanit P, Sangkhamanon S, Jutabha P, Endou H, Loilome W. Inhibition of l-type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment. Tumour Biol. 2017;39(3):1010428317694545. doi: 10.1177/1010428317694545. [DOI] [PubMed] [Google Scholar]
- 24.Saito Y, Li L, Coyaud E, Luna A, Sander C, Raught B, Asara JM, Brown M, Muthuswamy SK. LLGL2 rescues nutrient stress by promoting leucine uptake in ER+ breast cancer. Nature. 2019;569(7755):275–279. doi: 10.1038/s41586-019-1126-2. [DOI] [PubMed] [Google Scholar]
- 25.Katayama K, Higuchi A, Yamamoto H, Ikeda A, Kikuchi S, Shiozawa M. Perioperative dynamics and significance of plasma-free amino acid profiles in colorectal cancer. BMC Surgery. 2018;18(1):11. doi: 10.1186/s12893-018-0344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Y, Chen T, Fu S, Sun X, Wang L, Wang J, Lu Y, Ding S, Ruan G, Teng L, Wang M. Perioperative dynamics and significance of amino acid profiles in patients with cancer. J Transl Med. 2015;13:35. doi: 10.1186/s12967-015-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi K, Jutabha P, Endou H, Sagara H, Anzai N. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Imminol. 2013;191(8):4080–4085. doi: 10.4049/jimmunol.1300923. [DOI] [PubMed] [Google Scholar]
- 28.Broer S, Palacin M. The role of amino acid transporters in inherited and acquired diseases. Biochem J. 2011;436(2):193–211. doi: 10.1042/BJ20101912. [DOI] [PubMed] [Google Scholar]
- 29.Makrides V, Camargo SM, Verrey F. Transport of amino acids in the kidney. Compr Physiol. 2014;4(1):367–403. doi: 10.1002/cphy.c130028. [DOI] [PubMed] [Google Scholar]
- 30.Kandasamy P, Gyimesi G, Kanai Y, Hediger MA. Amino acid transporters revisited: New views in health and disease. Trends Biochem Sci. 2018;43(10):752–789. doi: 10.1016/j.tibs.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Yan R, Zhao X, Lei J, Zhou Q. Structure of the human LAT1–4F2hc heteromeric amino acid transporter complex. Nature. 2019;568(7750):127–130. doi: 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y, Wiriyasermkul P, Jin C, Quan L, Ohgaki R, Okuda S, Kusakizako T, Nishizawa T, Oda K, Ishitani R, Yokoyama T, Nakane T, Shirouzu M, Endou H, Nagamori S, Kanai Y, Nureki O. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol. 2019;26(6):510–517. doi: 10.1038/s41594-019-0237-7. [DOI] [PubMed] [Google Scholar]
- 33.Cormerais Y, Massard PA, Vucetic M, Giuliano S, Tambutté E, Durivault J, Vial V, Endou H, Wempe MF, Parks SK, Pouyssegur J. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5) J Biol Chem. 2018;293(8):2877–2887. doi: 10.1074/jbc.RA117.001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babic A, Rosenthal MH, Bamlet WR, Takahashi N, Sugimoto M, Danai LV, Morales-Oyarvide V, Khalaf N, Dunne RF, Brais LK, Welch MW. Postdiagnosis loss of skeletal muscle, but not adipose tissue, is associated with shorter survival of patients with advanced pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(12):2062–2069. doi: 10.1158/1055-9965.EPI-19-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 36.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non–small cell lung cancer. JAMA Oncol. 2020;6(4):512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueno S, Kimura T, Yamaga T, Kawada A, Ochiai T, Endou H, Sakurai H. Metformin enhances anti-tumor effect of L-type amino acid transporter 1 (LAT1) inhibitor. J Pharmacol Sci. 2016;131(2):110–117. doi: 10.1016/j.jphs.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Imbert V, Nebout M, Mary D, Endou H, Wempe MF, Supuran CT, Winum JY, Peyron JF. Co-targeting intracellular pH and essential amino acid uptake cooperates to induce cell death of T-ALL/LL cells. Leuk Lymphoma. 2018;59(2):460–468. doi: 10.1080/10428194.2017.1339875. [DOI] [PubMed] [Google Scholar]
- 39.Rosilio C, Nebout M, Imbert V, Griessinger E, Neffati Z, Benadiba J, Hagenbeek T, Spits H, Reverso J, Ambrosetti D, Michiels JF, Bailly-Maitre B, Endou H, Wempe MF, Peyron JF. L-type amino acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of t-cell lymphoblastic lymphoma/t-cell acute lymphoblastic leukemia. Leukemia. 2015;29(6):1253–1266. doi: 10.1038/leu.2014.338. [DOI] [PubMed] [Google Scholar]