Abstract
Background/Aim: Immune-related adverse events (irAEs) are associated with the efficacy of immune-checkpoint inhibitors in patients with melanoma and non-small cell lung cancer. We therefore evaluated the relationship between irAEs and nivolumab efficacy against metastatic renal cell carcinoma. Patients and Methods: The medical records of 53 consecutive patients were reviewed and analyzed. Results: Median overall survival was significantly better in patients who showed irAEs at any time compared to patients without irAEs (p=0.013). We identified irAEs in 24 of 53 patients (45.3%), including four patients (7.5%) with grade 3 events. Multivariate analysis also revealed that risk factors for the onset of irAEs were positively associated with a platelet-to-lymphocyte ratio <156 before nivolumab treatment (p=0.006). Conclusion: Development of irAEs was associated with survival outcomes of nivolumab treated patients with metastatic renal cell carcinoma.
Keywords: Immune-related adverse events, nivolumab, overall survival, progression-free survival, metastatic renal cell carcinoma
Therapeutic options for metastatic renal cell carcinoma (mRCC) have changed dramatically over the last decade with the introduction of targeted agents, including vascular endothelial growth factor (VEGF)-targeted therapy and immune checkpoint inhibitors. Nivolumab, a monoclonal antibody targeting programmed cell death 1 (PD1), is the first immune checkpoint inhibitor approved for the treatment of mRCC refractory to antiangiogenic therapy following the results of a phase III clinical trial. Nivolumab conferred a 5.4-month improvement in median overall survival (OS) a more favorable safety profile compared to everolimus (1). However, immune-checkpoint inhibitors can induce immune-related adverse events (irAEs) including rash, colitis, hepatitis, endocrinopathies, and pneumonitis (2,3). IrAEs have been associated with patient characteristics, efficacy and total number of doses of PD-1 inhibitors (4-6). In patients with various malignancies treated with immune-checkpoint inhibitors, irAEs are associated with better survival (7-13). Thus, early development of irAEs may predict more favorable outcomes following immune-checkpoint inhibitor therapy, and optimal management irAEs may be necessary to obtain the best outcome. However, in mRCC, the relationship between irAEs and outcomes following immune-checkpoint inhibitor treatment is unclear. Thus, we assessed irAEs and their association with outcomes of mRCC patients following nivolumab treatment.
Patients and Methods
Study design. We retrospectively reviewed the medical records of all patients with mRCC who were previously treated with VEGF-targeted therapy and who started treatment with nivolumab at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research between September 2016 and September 2018. This review identified 53 eligible patients who received nivolumab monotherapy. Patients with known active or suspected autoimmune disease and patients requiring immunosuppressive medications were excluded from participation in this study.
Treatment regimen and study drugs. Based on the results from a previous clinical trial (1), patients were given nivolumab every 2 weeks until the end of treatment or follow-up. In Japan, the recommended dose of nivolumab was 3 mg/kg until September 2018, and thereafter changed to 240 mg. Blood cell counts obtained within 7 days before beginning nivolumab treatment were considered baseline counts. We obtained all blood specimens, laboratory tests, and information regarding their Eastern Cooperative Oncology Group performance status (ECOG-PS), alcohol habits, smoking habits, and medication history at the same hospital. Follow-up ended on October 31, 2018. Progression-free survival (PFS) was defined as the time from study enrollment to disease progression or death. Each patient underwent computed tomography during follow-up to determine the response to nivolumab, which was assessed with Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (14). The time from the beginning of treatment to death from any cause was defined as OS. Patients were censored if they did not have documented clinical or radiographic progression or were still alive at the last follow-up. irAEs were considered immunologically associated adverse events that required careful monitoring and the possible need for treatment such as immune suppression or endocrine therapy. Pyrexia as well as cutaneous, endocrine, gastrointestinal, hepatic, lymph node, neurological, optic, pancreatic, pulmonary, and renal irAEs were recorded as adverse events (11). Laboratory results, such as an increase in thyroid-stimulating hormone, aspartate aminotransferase, or alanine aminotransferase levels that resolved spontaneously or that were asymptomatic or transient, were not regarded as irAEs. In addition, adverse events that began after initiation of treatment subsequent to nivolumab therapy were not considered. The Common Terminology Criteria for Adverse Events, version 4.0 was used to grade adverse events (15). The patients were categorized into two groups based on the incidence of irAEs: those with irAEs (irAE group) and those without (non-irAE group).
Statistical analyses. Estimates of OS and PFS in the irAE and non-irAE groups were calculated using the Kaplan-Meier method, and statistical significance was analyzed using the log-rank test. A significance level of p<0.05 was employed for statistical analyses. Optimal cut-off values of risk factors for the incidence of irAEs were determined according to receiver operating characteristic (ROC) curves and areas under the ROC curve (AUCs). Next, we performed univariate analysis including baseline clinical characteristics and blood test results as explanatory variables, and the onset of irAEs as the objective variable. The resulting variables showing values of p<0.20 were then entered into multivariate analysis. Statistical analyses were performed using SPSS version 24 software (IBM Corp., Armonk, NY, USA).
Ethical approval. This study was conducted in accordance with the World Medical Association Declaration of Helsinki and independently reviewed and approved by the Clinical Research Ethics Review Committee of the hospital (approval No.: 2018-1154). Patients were not solicited for informed consent, given the retrospective nature of the study. All patient data were processed in anonymity and de-identified prior to analysis.
Results
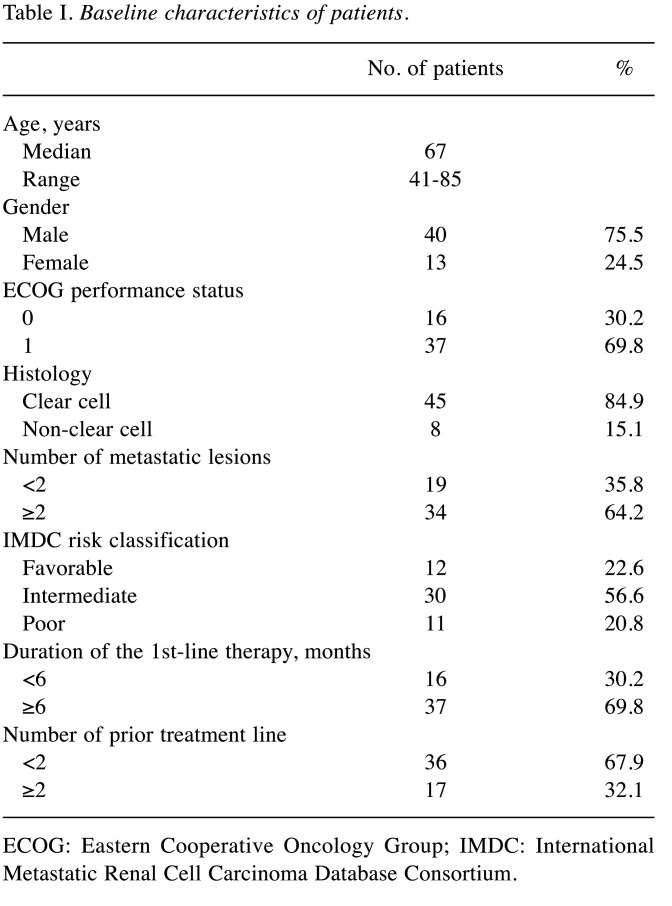
Patient characteristics. A total of 53 patients were included in this study. The median age was 67 years (range=41-85 years). ECOG-PS was 0 in 16 patients (30.2%) and 1 in 37 patients (69.8%) (Table I). The dominant histotype was clear cell RCC in 45 cases (84.9%). According to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk classification (16), the numbers of patients with favorable, intermediate, and poor risk were 12 (22.6%), 30 (56.6%), and 11 (20.8%), respectively. Thirty-six patients received nivolumab as second-line therapy, and 17 patients were administered nivolumab as third-line therapy. The median number of treatment cycles of nivolumab was 10 (range=1-46 cycles). Seven patients (13.2%) died as a result of disease progression. The remaining 46 patients (86.8%) were alive at the last follow-up.
Table I. Baseline characteristics of patients.
ECOG: Eastern Cooperative Oncology Group; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium.
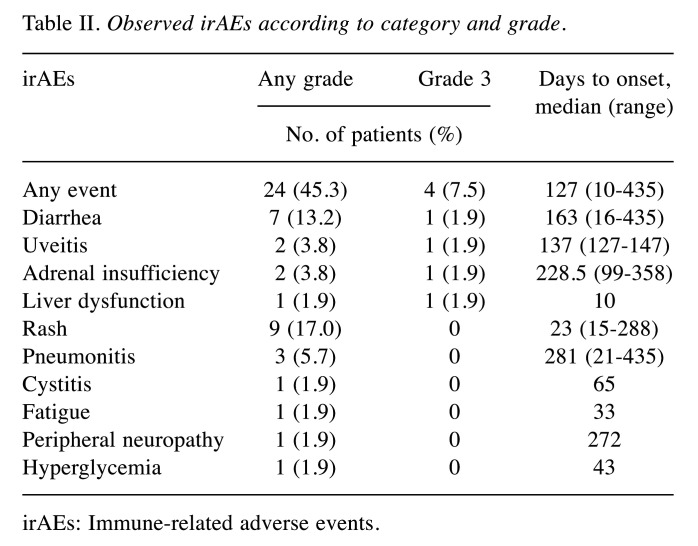
Efficacy and irAEs. Among those patients treated with nivolumab, 45.3% (n=24) showed irAEs of any grade, and 7.5% (n=4) had irAEs of grade 3. Profiles of any-grade irAEs and grade 3 irAEs are shown in Table II. The median time to first onset of irAEs was 127 days (range=10-435 days). The median time to onset of grade 3 irAEs was 159.5 days (range=10-358 days).
Table II. Observed irAEs according to category and grade.
irAEs: Immune-related adverse events.
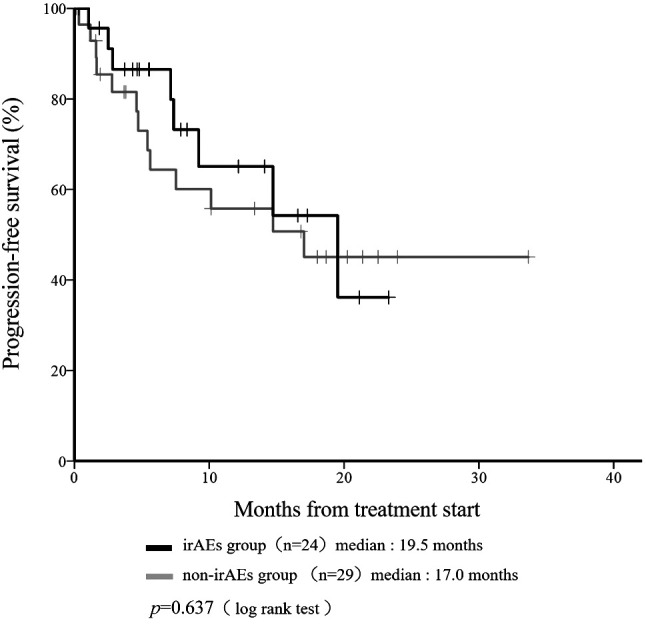
Median OS was not reached in the irAE or non-irAE groups at any time during the study (Figure 1). Median OS in the irAE group was improved compared to the non-irAE group (p=0.013). Median PFS was 19.5 months in the irAE group and 17.0 months in the non-irAE group (Figure 2). Median PFS did not differ significantly among groups (p=0.637).
Figure 1. Kaplan-Meier curve showing overall survival (OS) according to the incidence of any immune-related adverse events (irAEs) at any time in patients administered nivolumab.
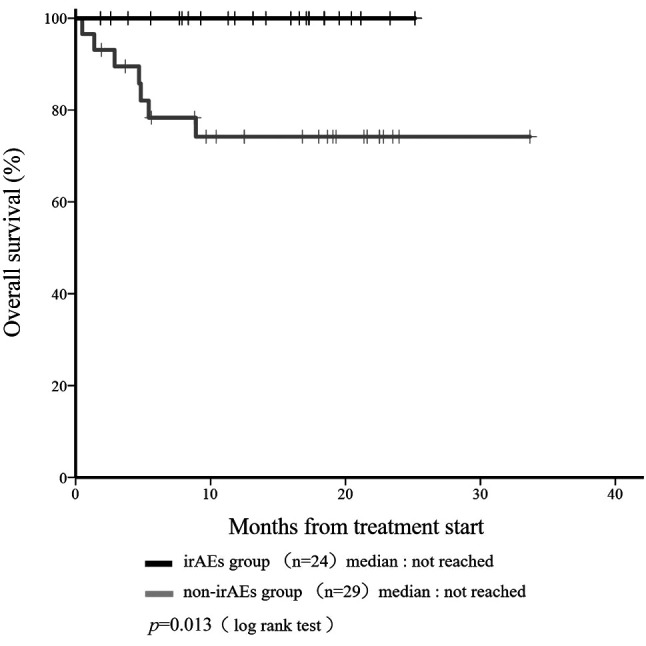
Figure 2. Kaplan-Meier curve showing progression-free survival (PFS) according to the incidence of any immune-related adverse events (irAEs) at any time in patients given nivolumab.
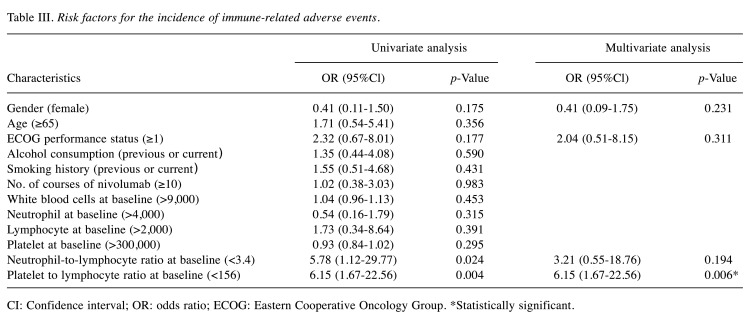
Multivariate analysis of factors associated with the incidence of irAEs. Results of multivariate analysis are shown in Table III. ROC analysis revealed that optimal cut-off values for the neutrophil-to-lymphocyte ratio (NLR) at baseline and platelet-to-lymphocyte ratio (PLR) at baseline were <3.4 and <156, respectively, for predicting the incidence of irAEs (AUC for NLR, 0.688; AUC for PLR, 0.655). Univariate analyses identified baseline NLR <3.4 and baseline PLR <156 as variables associated with the occurrence of irAEs (p=0.024 and p=0.004, respectively). Marginal correlations with irAEs were seen in females and in those with ECOG-PS ≥1 (p<0.20 each). When NLR, PLR, sex, and ECOG-PS were added stepwise to the logistic regression analysis, a significant association was found only for a baseline PLR <156 (odds ratio=6.15; 95% confidence interval=1.67-22.56; p=0.006), which showed the most appropriate regression equation with an error rate of 16.7%.
Table III. Risk factors for the incidence of immune-related adverse events.
CI: Confidence interval; OR: odds ratio; ECOG: Eastern Cooperative Oncology Group. *Statistically significant.
Discussion
We observed that development of irAEs was associated with a good outcome following nivolumab treatment in patients with mRCC. Recently, a Japanese series of 47 mRCC patients treated with nivolumab showed that the median PFS and OS after initiation of nivolumab therapy were significantly longer in patients with irAEs than in patients without irAEs (PFS: 13.1 vs. 4.8 months, p<0.001; OS: 26.0 months vs. not reached, p=0.007) (17), and that the median OS in patients who developed irAEs at any time was better (Figure 1). However, median PFS did not differ significantly among groups in this study (p=0.637). Kobari et al. described a case report about a rapid progressive disease just after nivolumab was administered as third- or fourth-line therapy for mRCC (18). Because our patients seemed to show relatively good baseline characteristics compared to their report, median PFS would not differ significantly.
Regarding the incidence of irAEs, a baseline PLR <156 was identified with multivariate logistic regression analysis as a significant risk factor (p=0.006). Pavan et al. observed that a baseline PLR <180 was significantly associated with occurrence of any irAEs among non-small lung cancer patients (19). They also reported that the occurrence of any irAE was associated with a baseline NLR <3.0 and a baseline PLR <180 by univariate analyses. Multivariate modeling confirmed a baseline PLR <180 as the only independent predictive factor (19). These findings agree with the findings of the present study.
Inflammation is an important feature of the tumor microenvironment and has been associated with poor prognosis for various tumors (20). Hematological inflammatory parameters such as neutrophils, lymphocytes, monocytes, and platelets can reflect the immune status and offer important predictive value for tumor prognosis (21,22). Giorgi et al. identified a PLR <232 as a statistical biomarker for improved OS among mRCC patients treated with nivolumab (23). The incidence of irAEs and PLR may thus be considered as a reliable marker reflecting the therapeutic efficacy of nivolumab in patients with mRCC. For the first time, our current study demonstrated an association between baseline PLR and irAEs in mRCC patients.
The present study has some limitations that warrant consideration. First, the study was retrospective in nature, which may have introduced potential biases and confounding factors. However, this single-center study included all mRCC patients treated with nivolumab, limiting the potential bias of heterogeneity in the patient population in this type of analysis. Second, we were not able to include all potential confounding factors in our multivariate analysis because of the small number of covariates that were identified in the study cohort. Third, the follow-up period was too short to fully assess long-term survival outcomes. The association between irAEs and nivolumab efficacy in mRCC, thus, remains inconclusive and warrants clarification in a larger cohort over a longer period.
In conclusion, our findings indicate that the incidence of irAEs is associated with nivolumab efficacy in patients with mRCC. To the best of our knowledge, this study is the first to reveal an association between PLR at baseline and irAEs among mRCC patients.
Conflicts of Interest
K.S. reports personal fees from Ono, Taiho, Eli Lilly, and Takeda, outside the submitted work. The other Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
K.K., M.H., T.A., T.Y., S.S., K.H., and Y.I. made substantial contributions to study conception and interpretation of data, and were involved in collecting data and drafting the manuscript. K.K. and K.S. made substantial contributions to study conception. K.S., M.H., T.A., T.Y., H.S., E.S., M.T., and T.H. were involved in critically revising the manuscript for important intellectual content. All Authors gave final approval of the version to be published.
Acknowledgements
The Authors thank Dr. Yonese, Dr. Yuasa, Dr. Numao, Dr. Yamamoto, and Dr. Fujiwara from the Department of Urology, Cancer Institute Hospital of the Japanese Foundation for Cancer Research for their invaluable data collection and management, secretarial assistance, and support.
References
- 1.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C. Safety profiles of anti-ctla-4 and anti-pd-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 3.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016;2(10):1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki H, Iwasaki H, Yamashita T, Yoshida T, Suganuma N, Yamanaka T, Masudo K, Nakayama H, Kohagura K, Rino Y, Masuda M. Potential risk factors for nivolumab-induced thyroid dysfunction. In Vivo. 2017;31(6):1225–1228. doi: 10.21873/invivo.11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiala O, Sorejs O, Sustr J, Kucera R, Topolcan O, Finek J. Immune-related adverse effects and outcome of patients with cancer treated with immune checkpoint inhibitors. Anticancer Res. 2020;40(3):1219–1227. doi: 10.21873/anticanres.14063. [DOI] [PubMed] [Google Scholar]
- 6.Sukari A, Nagasaka M, Alhasan R, Patel D, Wozniak A, Ramchandren R, Vaishampayan U, Weise A, Flaherty L, Jang H, Kim S, Gadgeel S. Cancer site and adverse events induced by immune checkpoint inhibitors: A retrospective analysis of real-life experience at a single institution. Anticancer Res. 2019;39(2):781–790. doi: 10.21873/anticanres.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, Tsurumi K, Suzuki K, Shimizu H, Sugisaka J, Ono H, Domeki Y, Terayama K, Nakamura A, Yamanda S, Kimura Y, Honda Y. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358–1365. doi: 10.1634/theoncologist.2017-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y, Goda M, Imanishi M, Chuma M, Hidaka N, Sayama K, Kubo Y, Tanaka A, Ishizawa K. Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: A multicenter retrospective study. Clin Ther. 2018 doi: 10.1016/j.clinthera.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: Long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2018 doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed] [Google Scholar]
- 11.Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, Imai Y, Ishida K, Fukuoka J, Tomii K. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol. 2017;12(12):1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, Aoki M, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Boku N. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer. 2019;19(1):974. doi: 10.1186/s12885-019-6150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi K, Suzuki K, Hiraide M, Aoyama T, Yokokawa T, Shikibu S, Hashimoto K, Iikura Y, Sato H, Sugiyama E, Tajima M, Hama T. Association of immune-related adverse events with pembrolizumab efficacy in the treatment of advanced urothelial carcinoma. Oncology. 2020;98(4):237–242. doi: 10.1159/000505340. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Common terminology criteria for adverse events (CTCAE) version 4.0 published: May 28, 2009 (v4.03: June 14, 2010) U.S. Department of health and human services national institutes of health, national cancer institute. [Google Scholar]
- 16.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/jco.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara H, Takagi T, Kondo T, Homma C, Tachibana H, Fukuda H, Yoshida K, Iizuka J, Kobayashi H, Okumi M, Ishida H, Tanabe K. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. 2019;37(6):355.e321–355.e329. doi: 10.1016/j.urolonc.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Kobari Y, Kondo T, Takagi T, Omae K, Nakazawa H, Tanabe K. Rapid progressive disease after nivolumab therapy in three patients with metastatic renal cell carcinoma. In Vivo. 2017;31(4):769–771. doi: 10.21873/invivo.11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, Guarneri V, Aprile G, Conte P, Bonanno L. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist. 2019;24(8):1128–1136. doi: 10.1634/theoncologist.2018-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 21.Peng F, Hu D, Lin X, Chen G, Liang B, Li C, Chen Y, Cui Z, Zhang H, Lin J, Zheng X, Niu W. The monocyte to red blood cell count ratio is a strong predictor of postoperative survival in colorectal cancer patients: The fujian prospective investigation of cancer (fiesta) study. J Cancer. 2017;8(6):967–975. doi: 10.7150/jca.18000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–2248. doi: 10.3892/ol.2016.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli P, Ferrau F, Crino L, Frassoldati A, Marchetti P, Mini E, Scoppola A, Verusio C, Fornarini G, Carteni G, Caserta C, Sternberg CN. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25(13):3839–3846. doi: 10.1158/1078-0432.Ccr-18-3661. [DOI] [PubMed] [Google Scholar]