Abstract
Background/Aim: Head and neck cancers account for 8% of all cancer cases worldwide. However, identifying the optimal treatment for recurrent or metastatic head and neck cancer (R/MHNSCC) has been challenging. The aim of this study was to evaluate the efficacy, safety, and prognostic factors of the outcome of patients with R/MHNSCC who were treated with weekly cetuximab and paclitaxel (Cmab-PTX). Patients and Methods: The records of R/MHNSCC patients who were treated with Cmab-PTX in our institution between June 2013 and September 2017 were collected. We analyzed Overall survival (OS), progression-free survival (PFS), prognostic factors and adverse events. Results: The records of 59 patients treated with Cmab-PTX were analyzed. The median PFS was 5.7 months, and the median OS was 11.8 months. Patients who had been administered cetuximab previously had shorter PFS and OS than those who had not. Conclusion: Cmab-PTX may be considered as a treatment option in head and neck R/MHNSCC patients.
Keywords: Head and neck squamous cell carcinoma, paclitaxel, cetuximab, recurrent, metastasis
Head and neck cancers account for 8% of all cancer cases worldwide; 1,450,000 new cases are diagnosed every year, and there are 500,000 related deaths annually (1). Identifying the optimal treatment for recurrent or metastatic head and neck squamous cell carcinoma (R/MHNSCC) has been challenging. However, treatment with immune checkpoint inhibitors (ICIs) in cases with high PD-L1 expression and biochemotherapy in other cases has recently gained attention (2). Increased treatment options contribute to a prolonged median overall survival (OS) of R/MHNSCC ranging between 7.5-14.7 months (3). Therefore, not only the efficacy and safety of the treatment but also the choice of second- and later-line treatment plays an important role in oncologic outcomes.
The NCCN (National Comprehensive Cancer Network) guidelines recommend cetuximab (Cmab), cisplatin, and 5-fluorouracil (5-FU) combination therapy (Extreme regimen) or pembrolizumab as first-line treatment (4-6). Recently, the combination of taxanes and Cmab, for example, exchanging 5-FU for taxanes in the Extreme regimen, has drawn attention (7). Cmab and paclitaxel combination therapy (Cmab-PTX), first reported by Hitt et al. (8), is one such combination, and some studies have reported that Cmab-PTX has good efficacy for R/MHNSCC patients (7-16). However, the prognostic factors associated with Cmab-PTX have been inadequately reported (9,10).
In this study, we retrospectively examined the efficacy and safety of Cmab-PTX in R/MHNSCC patients. We also assessed the predictors of outcomes in these patients.
Patients and Methods
Patients. This study was a retrospective analysis at a single institution. We reviewed the clinical records of patients with R/MHNSCC treated with Cmab-PTX between June 2013 and September 2017. The inclusion criteria were 18 years of age or older; at least one measurable lesion evaluated by computed tomography (CT) or magnetic resonance imaging (MRI); ECOG performance status (PS) of 2 or less and adequate hematologic, renal, and hepatic function; at least a 3-month life expectancy; and ability to provide written consent. Prior chemotherapy was allowed if administered as part of a multimodal treatment of head and neck squamous cell carcinoma. Exclusion criteria were pregnancy, drop-out before first evaluation for R/MHNSCC without disease progression or severe adverse events, and treatment with Cmab-PTX as maintenance therapy with the therapeutic effect of the Extreme regimen.
Written informed consent for the publication of this work was obtained from all patients. This study was approved by the institutional review board of the International University of Health and Welfare Mita Hospital (No. 5-19-66).
Treatment. Weekly Cmab was administered at an initial dose of 400 mg/m2 and subsequently at doses of 250 mg/m2. Weekly PTX was administered at a dose of 80 mg/m2. All patients received dexamethasone before Cmab and H2-blocker before PTX. Treatment was continued until disease progression or unacceptable toxicity.
Dose reduction or delay was considered in the event of grade 3 non-hematologic adverse events or at the patient’s request, and delay was considered in the event of grade 4 hematologic toxicity. Administration of PTX was reduced or delayed in the event of grade 3 hematologic toxicity.
Study design. The primary end point was OS. Secondary end points were overall response rate [ORR: complete response (CR)+partial response (PR)], disease control rate [DCR: CR+PR+stable disease (SD)], progression-free survival (PFS), duration of response, and safety. Additional end points were the prognostic factors affecting OS and PFS.
OS was defined as the period from the day of the initiation of treatment until the day of death, regardless of the cause. PFS was defined as the period from the day of the initiation of treatment to the day of disease progression or death. Duration of response was defined as the period from the first response [SD, PR, or progressive disease (PD)] until disease progression. Tumor response was assessed by CT or MRI before treatment and every 4 to 8 weeks until disease progression or treatment discontinuation according to the Response Evaluation Criteria in Solid Tumors, version 1.1. The evaluation of AEs was based on the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis. OS and PFS were estimated by the Kaplan-Meier method. Analysis of the relationship between age, sex, PS, existence of metastatic lesions, treatment line, and previous administration of platinum, Cmab, or docetaxel and OS or PFS were calculated using a univariate Cox regression model. All statistical analysis was carried out using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
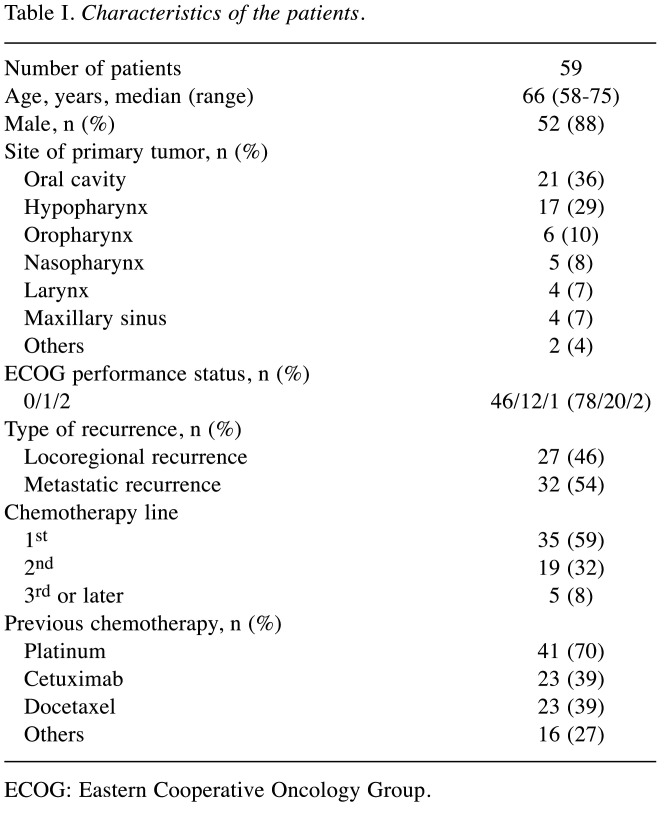
Baseline clinical characteristics. Fifty-nine patients were eligible for inclusion. The median follow-up period was 16.3 months (95%CI=8.5-30.0). Table I shows the baseline clinical characteristics of the patients. The mean age was 66 years old. Fifty-two patients were men (88%). The most frequent primary site of the tumor was the oral cavity, followed by the hypopharynx and oropharynx. Most patients had a PS of 0 or 1. Thirty-two patients had metastatic recurrence with or without locoregional recurrence. Cmab+PTX was the first-line regimen for recurrent or metastatic lesions in 35 patients. Forty-nine patients had previously received cisplatin and 23 patients had previously received Cmab.
Table I. Characteristics of the patients.
ECOG: Eastern Cooperative Oncology Group
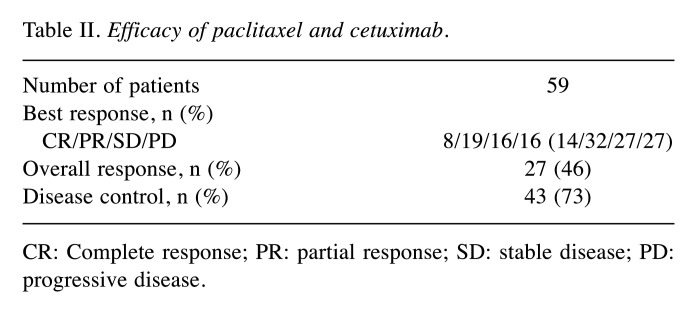
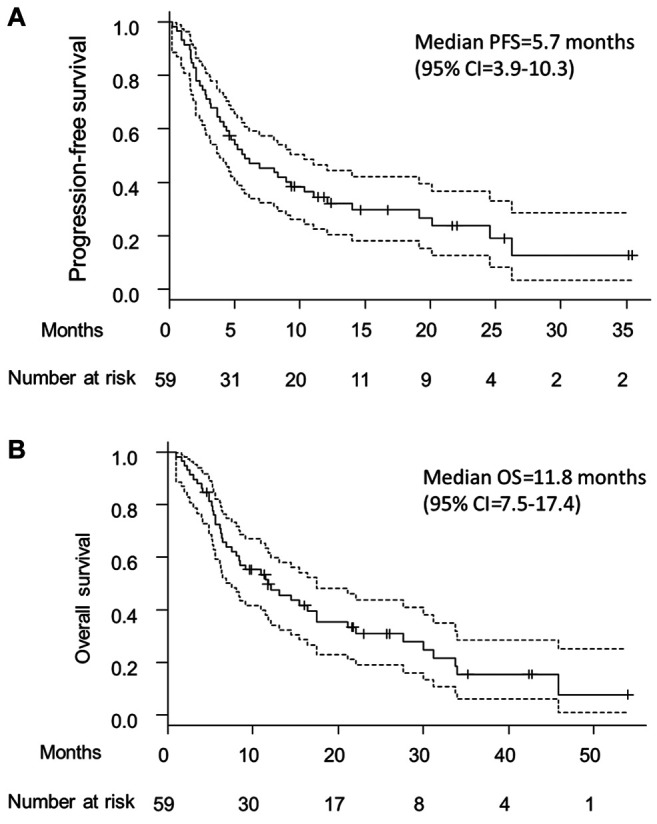
Efficacy. The efficacy of Cmab+PTX in this study is shown in Table II. The ORR was 46% (95%CI=33-60), and the DCR was 73% (95%CI=60-84). Figure 1A and 1B show the Kaplan-Meier plots for the PFS and OS, respectively. The median PFS was 5.7 months (95%CI=3.9-10.3), and the median OS was 11.8 months (95%CI=7.5-17.4).
Table II. Efficacy of paclitaxel and cetuximab.
CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease.
Figure 1. Sample collection and flow-chart of the study. A small number of cases were randomly selected as pilot studies and microarrays were performed. For saliva samples, the OSCC patient group (n=4) was compared with the HV group (n=4), and the expression levels of mRNA were compared. The expression levels of mRNA in tissue samples were compared between the OSCC tissue group (n=5) and the adjacent noncancerous tissues group (n=5). In saliva, there were 207 mRNAs that demonstrated a >2-fold change in the OSCC patient group, compared to the HV group. In tissue, there were 3,041 mRNAs that demonstrated >2- fold-change in the OSCC tissue group. Nine mRNAs that were upregulated more than 2-fold in both saliva and tissues were identified, and among these, NUS1 and RCN1 were selected for further investigation. HV: Healthy volunteers, OSCC: patients with oral squamous cell carcinoma.
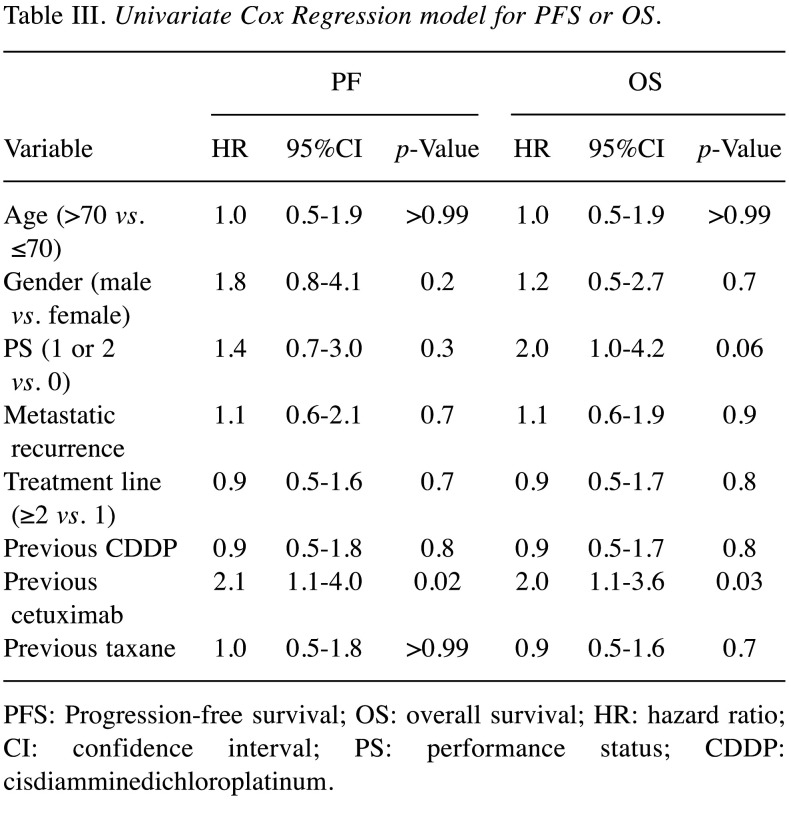
Prognostic factors. Table III shows the results of the Cox regression analysis for prognostic factors for the efficacy of Cmab-PTX. Patients who had previously received Cmab had significantly shorter PFS and OS than those who had not (PFS: HR=2.1, 95%CI=1.1-4.0; OS: HR=2.0, 95%CI=1.1-3.6). There were no differences in PFS or OS between patients receiving Cmab-PTX as second- or later-line chemotherapy and those receiving it as first-line chemotherapy (PFS: HR=0.9, 95%CI=0.5-1.6; OS: HR=0.9, 95%CI=0.5-1.7). There were also no differences in PFS or OS between patients with a PS of 1 or 2 and those with a PS of 0 (PFS: HR=1.4, 95%CI=0.7-3.0; OS: HR=2.0, 95%CI=1.0-4.2).
Table III. Univariate Cox Regression model for PFS or OS.
PFS: Progression-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; PS: performance status; CDDP: cisdiamminedichloroplatinum.
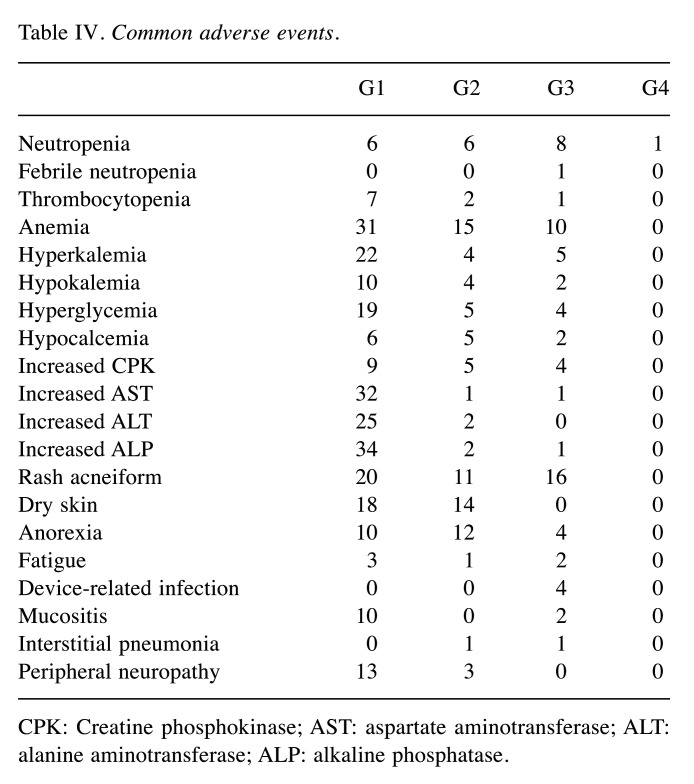
Adverse events. Table IV shows the grade 1 to 4 (G1 to G4) adverse events of this study. The most common G3 or G4 adverse events were rash-acneiform (n=16, 27%), anemia (n=10, 17%), and neutropenia (n=9, 15%). The most common G1 or G2 adverse event was anemia (n=46, 78%). Rash-acneiform was observed in 31 patients. G1 or G2 peripheral neuropathies were observed in 26 patients, and there were no G3 or G4 peripheral neuropathy cases. Interstitial pneumonia was observed in two patients, one G2 and another G3. G1 or G2 infusion-related reactions were observed in two patients; no G3 or G4 infusion-related reactions were observed.
Table IV. Common adverse events.
CPK: Creatine phosphokinase; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase.
Discussion
This retrospective study of weekly Cmab-PTX for R/MHNSCC revealed an ORR of 44%, a median PFS of 5.7 months, and a median OS of 11.8 months. Patients who had previously received Cmab had shorter PFS and OS than those who had not.
Hitt et al. (8) reported the first phase II study of Cmab-PTX in 2007. Other studies have reported ORRs of 30-55%, median PFS of 3.9-7.7 months, and median OS of 7.6-16.8 months (8-16). The results of our study are consistent with those of previous studies. Conversely, Vermorken et al. (5) have reported the results of a phase III Extreme study of platinum-based chemotherapy and cetuximab in 2008, which had an ORR of 36%, an OS of 10.1 months, and a PFS of 5.6 months. Additionally, Ferris et al. (6) have reported that the phase III Checkmate 141 trial of nivolumab in 2016 had an ORR of 13.3%, an OS of 7.5 months, and a PFS of 2.0 months. Comparing the results of our study including second-line and later-line therapy with those of the Extreme study and CheckMate 141 trial, we suggest that weekly Cmab-PTX should be considered as a treatment option for R/MHNSCC, although we should take care when comparing retrospective studies to phase III clinical trials. Cmab-PTX might become one of the treatment options for patients who are refractory to platinum-based chemotherapy, have PD after receiving ICIs, or for whom ICIs are not suitable.
In subgroup analysis, we also examined the prognostic factors for the response to Cmab-PTX. Patients who had previously received Cmab had significantly poorer prognosis than those who had not. To our knowledge, this is the first study to report that previous administration of Cmab is a prognostic factor for survival in this cohort. The prognosis did not differ significantly between patients with a PS≥1 and those with a PS of 0. This result is inconsistent with those of previous studies, which have shown that patients with a PS of 2 had significantly poorer prognosis than those with a PS of 0 or 1 (9). This might be because there were few patients with a PS of 2 in this study.
In our study, the most frequent G3 or G4 toxicities were rash (27%), anemia (17%), and neutropenia (15%). Rash was more frequent in our study than in previous studies (11-13). This may be because there were more patients who had previously received regimens including Cmab in this study than in the previous studies. Thus, the total period of Cmab administration was longer. Anemia was also more frequent in our study than in previous studies (11-13). This may be because there were more patients who had previously received chemotherapy and more patients in poor condition. However, all adverse events were tolerable and manageable.
Our study had several limitations. Because this was not a phase III study, we cannot compare the efficacy of Cmab-PTX with that of other treatments. Further, this study was a single-institution retrospective series and we only examined the medical records to assess adverse events. Because of the small sample size, we could not perform multivariate Cox regression analysis. Therefore, our findings should be interpreted with caution.
Conclusion
In conclusion, our findings suggest that Cmab-PTX should be considered for the treatment of R/MHNSCC patients. Patients who previously received Cmab might have poorer prognosis than those who did not. Further research is needed to confirm whether previous administration of Cmab is a predictor of poor response to Cmab-PTX.
Conflicts of Interest
The Authors declare that they have no competing interests related to this study.
Authors’ Contributions
YT, DB and CF designed the study. DB, YT and CF contributed to the collection and interpretation of the data. CF, DB, TM, MY, YK, TK, JT, KH, NT, KM and YT contributed to data collection and patient management. CF was a major contributor in writing the manuscript. All Authors read and approved the final manuscript.
Acknowledgements
This work was supported by JSPS Grants-in-Aid for Scientific Research (C) to Dr. Yuichiro Tada (No. 18K09386). The Authors thank Editage (www.sditage.jp) for English language editing.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (keynote-048): A randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 3.Argiris A, Harrington KJ, Tahara M, Schulten J, Chomette P, Ferreira Castro A, Licitra L. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. doi: 10.3389/fonc.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network Head and neck cancer 2019. Available at: https://www.nccn.org/ professionals/physician_gls/ [Last accessed 20 May, 2020]
- 5.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guigay J, Tahara M, Licitra L, Keilholz U, Friesland S, Witzler P, Mesía R. The evolving role of taxanes in combination with cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: Evidence, advantages, and future directions. Front Oncol. 2019;9:668. doi: 10.3389/fonc.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hitt R, Irigoyen A, Cortes-Funes H, Grau JJ, García-Sáenz JA, Cruz-Hernandez JJ. Phase ii study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. 2012;23(4):1016–1022. doi: 10.1093/annonc/mdr367. [DOI] [PubMed] [Google Scholar]
- 9.Pellini Ferreira B, Redman M, Baker KK, Martins R, Eaton KD, Chow LQM, Baik CS, Goulart B, Lee SM, Santana-Davila R, Rodriguez CP. Predictors of outcome with cetuximab and paclitaxel for head and neck squamous cell carcinoma. Laryngoscope. 2017;127(7):1583–1588. doi: 10.1002/lary.26422. [DOI] [PubMed] [Google Scholar]
- 10.Uozumi S, Enokida T, Suzuki S, Nishizawa A, Kamata H, Okano T, Fujisawa T, Ueda Y, Okano S, Tahara M, Yamaguchi M. Predictive value of cetuximab-induced skin toxicity in recurrent or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2018;8:616. doi: 10.3389/fonc.2018.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano K, Marshall S, Taira S, Sato Y, Tomomatsu J, Sasaki T, Shimbashi W, Fukushima H, Yonekawa H, Mitani H, Kawabata K, Takahashi S. A comparison of weekly paclitaxel and cetuximab with the extreme regimen in the treatment of recurrent/metastatic squamous cell head and neck carcinoma. Oral Oncol. 2017;73:21–26. doi: 10.1016/j.oraloncology.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Sosa AE, Grau JJ, Feliz L, Pereira V, Alcaraz D, Muñoz-García C, Caballero M. Outcome of patients treated with palliative weekly paclitaxel plus cetuximab in recurrent head and neck cancer after failure of platinum-based therapy. Eur Arch Otorhinolaryngol. 2014;271(2):373–378. doi: 10.1007/s00405-013-2537-6. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez B, Trigo JM, Pajares BI, Sáez MI, Quero C, Navarro V, Llácer C, Medina L, Rueda A, Alba E. Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients. Oral Oncol. 2013;49(2):182–185. doi: 10.1016/j.oraloncology.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Bernad IP, Trufero JM, Urquizu LC, Pazo Cid RA, de Miguel AC, Agustin MJ, Lanzuela M, Antón A. Activity of weekly paclitaxel-cetuximab chemotherapy in unselected patients with recurrent/metastatic head and neck squamous cell carcinoma: Prognostic factors. Clin Transl Oncol. 2017;19(6):769–776. doi: 10.1007/s12094-016-1604-z. [DOI] [PubMed] [Google Scholar]
- 15.Noronha V, Patil VM, Joshi A, Bhattacharjee A, Paul D, Dhumal S, Juvekar S, Arya S, Prabhash K. A tertiary care experience with paclitaxel and cetuximab as palliative chemotherapy in platinum sensitive and nonsensitive in head and neck cancers. South Asian J Cancer. 2017;6(1):11–14. doi: 10.4103/2278-330x.202558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Péron J, Ceruse P, Lavergne E, Buiret G, Pham BN, Chabaud S, Favier B, Girodet D, Zrounba P, Ramade A, Fayette J. Paclitaxel and cetuximab combination efficiency after the failure of a platinum-based chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Anticancer Drugs. 2012;23(9):996–1001. doi: 10.1097/CAD.0b013e32835507e5. [DOI] [PubMed] [Google Scholar]