Abstract
Background/Aim: STK11/LKB1 mutation has been suggested as a poorly responding candidate biomarker of the anti-programmed cell death-1 (PD-1) antibody; however, the association between STK11/LKB1 expression and the effects of anti-PD-1 antibodies is uncertain. The aim of the study was to correlate the efficacy of pembrolizumab monotherapy and STK11/LKB1 expression in untreated patients with non-small-cell lung carcinoma (NSCLC) and high PD-ligand 1 expression. Patients and Methods: From February 2017 to January 2020, we retrospectively analyzed 30 previously untreated patients with NSCLC and a tumor proportion score (TPS) ≥50% treated with pembrolizumab monotherapy. STK11/LKB1 expression in tumor tissue was evaluated by immunohistochemistry. Results: Twenty-three (76.7%) of the 30 patients were classified with low-STK11/LKB1 expression. The median progression-free survival and overall survival of patients with low-STK11/LKB1 expression was shorter than those with high-STK11/LKB1 expression, although the results were not statistically significant. The disease progression rate for the low-STK11/LKB1 group was higher than that of the high-STK11/LKB1 group. Conclusion: STK11/LKB1 expression, as measured by immunohistochemistry, could be a useful biomarker associated with the efficacy of pembrolizumab monotherapy for patients with NSCLC and a TPS ≥50%.
Keywords: Non-small-cell lung carcinoma, PD-1, pembrolizumab, STK11/LKB1
Lung cancer remains the leading cause of cancer-related deaths worldwide (1). On the other hand, the treatment for advanced non-small-cell lung carcinoma (NSCLC) is dramatically changing. Immune checkpoint inhibitors (ICI) such as anti-programmed death 1 (PD-1) or its ligand (PD-L1) antibody are some of most important drugs in the treatment and survival of patients with NSCLC. Nivolumab, pembrolizumab, atezolizumab and durvalumab are currently approved for advanced NSCLC and are available in clinical practice. The phase III studies KEYNOTE-024 (2) and KEYNOTE-042 (3) compared pembrolizumab monotherapy with platinum-based chemotherapy as first-line therapy for patients with untreated NSCLC who had tumor proportion scores (TPS) ≥50% and ≥1%, respectively. Both studies demonstrated that pembrolizumab prolonged overall survival (OS) compared to chemotherapy. Based on the results of these studies, pembrolizumab monotherapy is considered one of the standard therapies for untreated advanced NSCLC with a TPS ≥1%, especially for a TPS ≥50%. However, the survival curves in the KEYNOTE-042 study were crossed, and it was suggested that a number of patients (even patients with a TPS ≥50%) might not benefit from pembrolizumab (3). A number of patients treated with anti-PD-1/L1 antibodies experienced rapid disease progression, which was sometimes reported as hyperprogression during anti-PD-1/L1 therapy (4). Not only good response biomarkers, but also poor response biomarkers are necessary to prevent hyperprogression during anti-PD-1/L1 antibody therapy.
STK11/LKB1 is a tumor suppressor gene that regulates cell metabolism, proliferation, and polarity by activating adenosine monophosphate (AMP)-activated protein kinase (AMPK), which inhibits the mammalian target of rapamycin signaling. STK11/LKB1 encodes a serine/threonine protein kinase and is located on the human chromosome 19p13.3 (5). Skoulidis et al. demonstrated that patients with Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant lung adenocarcinoma with the STK11/LKB1 mutation (which nullifies STK11/LKB1 function) showed poor response to anti-PD-1 antibodies (6). The authors considered that STK11/LKB1 genomic alterations facilitate the establishment of a non-T-cell inflammatory tumor immune microenvironment with poor PD-L1 expression in tumor cells. Several retrospective analyses of clinical trials for NSCLC have suggested that STK11/LKB1 mutations are associated with the poor efficacy of anti-PD-1/L1 therapies for NSCLC (7,8). STK11/LKB1 inactivation could, therefore, be related to primary resistance to anti-PD-1/L1 therapies for NSCLC. However, the association between STK11/LKB1 expression in tumor cells and the effects of ICI has not been fully investigated. We hypothesized that NSCLC with low STK11/LKB1 expression results in the poor efficacy of anti-PD-1/L1 therapies for NSCLC and STK11/LKB1 expression is a biomarker for predicting primary resistance. In this study, we retrospectively examined the correlation between STK11/LKB1 expression in the tumor and the efficacy of pembrolizumab monotherapy in untreated patients with advanced NSCLC and a TPS ≥50%.
Patients and Methods
Patients. The study enrolled patients who matched the following criteria: pathologically confirmed untreated stage III or IV NSCLC or recurrent NSCLC; a TPS ≥50%; and having undergone pembrolizumab monotherapy as first-line therapy at the Cancer Institute Hospital, Japanese Foundation for Cancer Research between February 2017 and January 2020. Patients were excluded if they had sensitizing epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangement/fusion, active autoimmune disease for which they had undergone systemic treatment, or active interstitial lung disease. During the study, the patients were administered pembrolizumab intravenously at a dosage of 200 mg every 3 weeks.
Data collection. We collected data on the patients’ age, sex, Eastern Cooperative Oncology Group performance status, smoking status, histology, stage, progression-free survival (PFS), OS, overall response rate (ORR), and disease progression rate (DPR). The data cutoff date was January 31, 2020. We evaluated tumor response using computed tomography every 6-8 weeks and according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (9). All patients enrolled in the study were evaluable. We measured PFS from treatment initiation to clinical or radiographic progression or death from any cause. OS was measured from treatment initiation to death from any cause.
Pathology and immunohistochemical staining. We employed biopsy specimens or surgical specimens fixed in 10% formalin for the pathology evaluation and immunohistochemical (IHC) staining. The TPS was analyzed at a certified commercial laboratory (SRL Inc., Tokyo, Japan) with a monoclonal antibody targeting PD-L1 (22C3 Pharm Dx, Agilent Technologies, Santa Clara, CA, USA). STK11/LKB1 expression in the tumors was evaluated through IHC in our institute’s laboratory. After deparaffinizing the specimens, we performed antigen activation through heat treatment with 10-mM sodium citrate buffer (pH 6.0) at approximately 100˚C for 10 min. We exposed sections to 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity and then washed them with deionized water for several minutes, after which they were blocked with the blocking solution. The primary antibody employed in the study was the STK11/LKB1 rabbit monoclonal antibody (clone D60C5F10, dilution 1:250, Cell Signaling Technology). Subsequent visualization was performed with the Leica Bond-III automatic immunostainer (Leica, Bannockburn, IL, USA) with the Leica Refine detection kit (DS9800, Leica) for 30 min at room temperature. A pathologist (H.N.) and 2 researchers (T.H and N.Y.) evaluated the STK11/LKB1 expression, grading the staining intensity as follows: IHC score 0, no discernable cytoplasmic staining; IHC score 1+, weak or incomplete cytoplasmic staining; IHC score 2+, weak to moderate continuous cytoplasmic staining; IHC score 3+, strong cytoplasmic staining readily apparent at 400× magnification. Figure 1 shows a representative image of each score. We defined IHC scores of 0 or 1+ as low-STK11/LKB1 group and 2+ or 3+ as high-STK11/LKB1 group.
Figure 1. STK11/LKB1 expression by immunohistochemical analysis. Immunohistochemical analysis of formalin-fixed paraffin-embedded (FFPE) specimens of non–small-cell lung carcinoma tumor tissue prior to pembrolizumab administration using STK11/LKB1 rabbit monoclonal antibody (clone D60C5F10, dilution 1:250, Cell Signaling Technology). (A) 3+, strong cytoplasmic staining (adenocarcinoma, lymph node biopsy specimen), (B) 2+, weak to moderate continuous cytoplasmic staining (squamous cell carcinoma, transbronchial lung biopsy specimen), (C) 1+, weak and/or incomplete cytoplasmic staining (adenocarcinoma, transbronchial lung biopsy specimen), (D) 0, no discernable cytoplasmic staining (large-cell carcinoma, surgical specimen).
Statistical analysis. We employed the Fisher’s exact test or the Mann-Whitney U-test to evaluate the association between baseline variables and STK11/LKB1 expression (low vs. high). We performed a Kaplan-Meier analysis of PFS and OS, with differences between each pair of variables assessed with the log-rank test. We calculated hazard ratios (HR) and 95% confidence intervals (CI) with the univariate Cox proportional hazard model, setting the two-sided significance level at p<0.05. All data were analyzed with EZR on R commander version 1.27 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). The study was conducted according to protocols approved by our hospital’s institutional review board (approval number: 2018-1123).
Results
Patient characteristics. We identified 30 patients who met the inclusion criteria; Table I shows their baseline characteristics. Twenty-three (76.7%) of patients were men, and most patients were PS0 or PS1 (93.3%) and had a history of smoking (96.7%). The median age was 71.0 years (range=51-82 years). Twenty-one (70.0%) of the 30 patients had non-squamous-cell carcinoma. All patients had a TPS ≥50%, and 21 (70.0%) had a TPS ≥75%. We evaluated STK11/LKB1 expression in all patients, classifying 18 patients with an IHC score of 0, 5 with an IHC score of 1+, 6 with an IHC score of 2+, and 1 with an IHC score of 3+. Twenty-three patients (76.7%) had low STK11/LKB1 expression, and 7 patients (23.3%) had high STK11/LKB1 expression. The characteristics of the low- and high-STK11/LKB1 groups are shown in Table I. The low-STK11/LKB1 group included significantly more patients with non-squamous-cell carcinoma (p=0.014), as well as more women, although the difference was not statistically significant.
Table I. Patient characteristics.
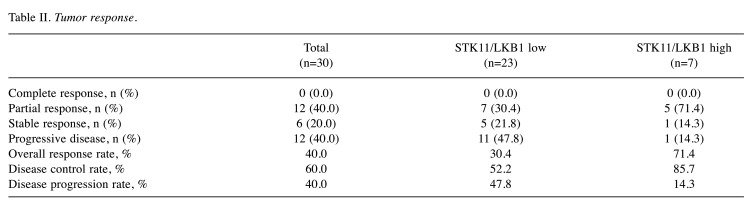
Efficacy. The median follow-up was 11.3 months (range=0.9-30.8). Four patients (13.3%) were still undergoing pembrolizumab therapy by the end of the follow-up, 2 in the low-STK11/LKB1 group and 2 in the high-STK11/LKB1 group. The median PFS for all patients was 5.2 months (95% CI=1.5-11.2). The median PFS for the low- and high-STK11/LKB1 groups was 4.0 months and 11.2 months, respectively; however, the difference was not statistically significant (HR=1.81; 95% CI=0.67-4.87; p=0.24). Figure 2A shows the Kaplan-Meier PFS curves for the low- and high-STK11/LKB1 groups. The median OS for all patients was 11.3 months (95% CI=5.2-not reached). The median OS of the low- and high-STK11/LKB1 groups was 8.6 months and not reached, respectively; however, the difference was not statistically significant (HR=2.97; 95% CI=0.68-13.05; p=0.15). Figure 2B shows the Kaplan-Meier OS curves for the low- and high-STK11/LKB1 groups. While Table II shows the tumor response. The ORR for the low- and high-STK11/LKB1 groups was 30.4% and 71.4%, respectively; however, the difference was not statistically significant (p=0.084). The DPR for the low- and high-STK11/LKB1 groups was 47.8% and 14.3%, respectively; however, the difference was not statistically significant (p=0.19).
Figure 2. Progression-free and overall survival. Kaplan-Meier curves showing the relationship between STK11/LKB11 expression and PFS (A) and OS (B). PFS: Progression-free survival, OS: overall survival, CI: confidence interval, NR: not reached.
Table II. Tumor response.
Discussion
To our knowledge, there have been no studies to have evaluated the association between STK11/LKB1 expression and anti-PD-1 antibody efficacy in patients with high PD-L1-expressing NSCLC. Our study showed a high DPR (47.8%) and short PFS (4.0 months) and OS (8.6 months) for pembrolizumab monotherapy in the low-STK11/LKB1 group, even though the patients had NSCLC with a TPS ≥50%. Compared to previous studies that evaluated the efficacy of pembrolizumab monotherapy for NSCLC with a TPS ≥50%, the results of our study were remarkably inferior. In the KEYNOTE-024 study (2), which evaluated pembrolizumab monotherapy for NSCLC with a TPS ≥50%, the median PFS was 10.3 months. For the TPS ≥50% NSCLC subset of the KEYNOTE-042 study (3), which evaluated pembrolizumab monotherapy for NSCLC with a TPS ≥1%, the median PFS was 7.1 months. In the TPS ≥50% NSCLC subset of the KEYNOTE-001 study (10,11), a phase I/II study of pembrolizumab monotherapy, the median PFS was 12.5 months. The median OS was 30.0 months, 20.0 months and 35.4 months for the KEYNOTE-024 study (2), the TPS ≥50% subset of the KEYNOTE-042 study (3) and the TPS ≥50% subset of the KEYNOTE-001 study (10,11), respectively. The ORR was 44.8%, 39% and 66.7% for the KEYNOTE-024 study (2), the TPS ≥50% subset of the KEYNOTE-042 study (3) and the TPS ≥50% subset of KEYNOTE-001 study (10,11), respectively. In the TPS ≥50% NSCLC subset of the KEYNOTE-042 study (3), the DPR was 18%. Given these previous studies, we assumed the efficacy of pembrolizumab to be poor in our low-STK11/LKB1 group. Several recent studies have reported on the efficacy of ICI monotherapy in patients with NSCLC and the STK11/LKB1 mutation. Gainor et al. reported that the STK11/LKB1 mutation was associated with low ORR compared with the STK11/LKB1 wild-type in patients with NSCLC and PD-L1 TPS ≥50% (22% vs. 41%, p=0.141) (8). Rizvi et al. reported that STK11/LKB1 mutations were significantly enriched in the nondurable benefit group compared with the durable clinical benefit group (19% vs. 27%) in patients with advanced NSCLC treated with PD-1/PD-L1 monotherapy (7). Skoulidis et al. also examined the efficacy of chemoimmunotherapy with pemetrexed, carboplatin and pembrolizumab, the first-line standard of care for patients with metastatic non-squamous-cell carcinoma with STK11/LKB1 mutations (12). Patients with STK11/LKB1 mutations had lower ORR and higher DPR than those with wild-type STK11/LKB1 [ORR=31.3% vs. 60.6% (p=0.07); DPR, 37.5% vs. 6.1% (p=0.01)]. PFS was significantly shorter in the patients with STK11/LKB1 mutations compared with those with wild-type STK11/LKB1 (4.4 vs. 11.0 months, p=0.039). It has been reported that STK11/LKB1 mutation correlates with low STK11/LKB1 expression in KRAS mutation-positive NSCLC (6). Our results showing that low-STK11/LKB1 group had a tendency for shorter PFS and higher DPR are in line with these results.
The evaluation of STK11/LKB1 expression by IHC is a simple, fast and low-cost method that can be easily employed in clinical practice compared with identifying STK11/LKB1 mutations. Sahin et al. reported that STK11/LKB1 expression assessed by IHC could be a valid surrogate for the genetic analysis of STK11/LKB1 mutations in pancreatic and biliary neoplasia (13). The evaluation of STK11/LKB1 expression by IHC is, therefore, one of our study’s strengths. Another possibility is that STK11/LKB1 expression is simply a poor prognostic factor for NSCLC. However, a previous report suggested that STK11/LKB1 expression was not a prognostic factor because STK11/LKB1 expression was not related to the patients’ postoperative survival (14).
The mechanism by which STK11/LKB1 expression influences the effects of pembrolizumab is the following. One of the immune escape mechanisms with STK11/LKB1 inactivation is due to the correlation between STK11/LKB1 expression and the stimulator of interferon genes (STING) (15). In the endoplasmic reticulum, STING senses free double-strand DNA inside the cell (such as from viruses and bacteria) and triggers type I interferon and inflammatory responses to eliminate the foreign substances (16). STING not only plays a critical role in the host’s defense against microbial infection, but also activates the cancer-immunity cycle (17). The activation of the STING intracellular phosphorylation cascade leads to the release of several immune inflammatory cytokines that stimulate PD-L1 expression and anti-tumor immunity. STK11/LKB1 enhances STING expression by activating the AMPK pathway (15). Low STK11/LKB1 expression, such as that due to STK11/LKB1 mutations, therefore leads to STING suppression and triggers cold-immune states with resistance to anti-PD-1 antibodies.
The differences between the low- and high-STK11/LKB1 groups in terms of ORR, DPR, PFS and OS were not statistically significant [ORR=30.4% vs. 71.4% (p=0.084); DPR, 47.8% vs. 14.3%, (p=0.19); PFS, 4.0 vs. 11.2 months (p=0.24); OS, 8.6 months vs. not reached (p=0.15)]. This lack of statistical significance might be due to the small number of patients, especially considering that the high-STK11/LKB1 group had only 7 patients (23.3%). There have been few reports on the rate of STK11/LKB1 expression. STK11/LKB1 mutation rates of approximately 10-20% have been reported for Caucasian patients with lung adenocarcinoma (18,19) and as low as 4% in Chinese patients with lung adenocarcinoma (20). Skoulidis et al. reported that patients with STK11/LKB1 mutations have low STK11/LKB1 expression, while patients with wild-type STK11/LKB1 have a STK11/LKB1 expression rate of 82.4% in KRAS mutation-positive NSCLC treated with anti-PD-1 antibodies (6). Although few studies have examined STK11/LKB1 expression rates in both Caucasian and Asians, Asians with few STK11/LKB1 mutations might have high STK11/LKB1 expression. In this study, STK11/LKB1 mutation was not measured, and the relationship between expression and mutation is unknown.
Our study has several limitations, the first of which is its retrospective single-center nature. Secondly, this study failed to show any statistical significance due to the small sample size, and the results are therefore inconclusive. Finally, the evaluation method and cutoff for STK11/LKB1 expression by IHC were not quantitative, and standardization of the IHC scores is necessary. Further clinical studies are therefore warranted to confirm the results and establish STK11/LKB1 expression as a biomarker.
Conclusion
The evaluation of STK11/LKB1 expression by IHC could be related to the efficacy of pembrolizumab monotherapy for NSCLC with a TPS ≥50% and lead to an easy-to-use and useful biomarker.
Conflicts of Interest
All Authors approved the submission of this manuscript to your journal. Makoto Nishio reports honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer-ingelheim, MSD, and Novartis, research funding from Novartis, Daiichi Sankyo, Taiho Pharmaceutical, BMS, Boehringer-ingelheim, Ono Pharmaceutical, Eli Lilly, Chugai Pharmaceutical, AstraZeneca, Merck Sernon, MSD and Pfizer. Noriko Yanagitani reports employment/ledership position/ advisory role of Chugai Pharmaceutical. Atsushi Horiike reports horaria from Chugai Pharmaceutical, AstraZeneca, Pfizer, Ono Pharmaceutical, Bristol-Myers Squibb, A2 Healthcare and MSD oncology, research funding from Chugai Pharmaceutical, MSD, Abbvie, AstraZeneca and Loxo.
Authors’ Contributions
All Authors contributed to the study conception and design. Tsukasa Hasegawa, Noriko Yanagitani, Hiroaki Sakamoto, Takehiro Tozuka, Hiroshi Yoshida, Yoshiaki Amino, Shinya Uematsu, Takahiro Yoshizawa, Ryo Ariyasu, Ken Uchibori, Satoru Kitazono, Atsushi Horiike, and Makoto Nishio were involved in preparing the material and collecting and analyzing the data. Tomoyo Kakitani and Hironori Ninomiya was performed by the sample processing by immunohistochemistry. Hironori Ninomiya and Kengo Takeuchi provided assistance in evaluating the specimens. Tsukasa Hasegawa wrote the first draft of the manuscript, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The Authors would like to thank all of the study participants who provided clinical data for the analysis.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for pd-l1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G, KEYNOTE-042 Investigators Pembrolizumab versus chemotherapy for previously untreated, pd-l1-expressing, locally advanced or metastatic non-small-cell lung cancer (keynote-042): A randomised, open-label, controlled, phase 3 trial. The Lancet. 2019;393(10183):1819–1830. doi: 10.1016/s0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 4.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferte C. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 5.Zhao N, Wilkerson MD, Shah U, Yin X, Wang A, Hayward MC, Roberts P, Lee CB, Parsons AM, Thorne LB, Haithcock BE, Grilley-Olson JE, Stinchcombe TE, Funkhouser WK, Wong KK, Sharpless NE, Hayes DN. Alterations of lkb1 and kras and risk of brain metastasis: Comprehensive characterization by mutation analysis, copy number, and gene expression in non-small-cell lung carcinoma. Lung Cancer. 2014;86(2):255–261. doi: 10.1016/j.lungcan.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, Ali SM, Elvin JA, Singal G, Ross JS, Fabrizio D, Szabo PM, Chang H, Sasson A, Srinivasan S, Kirov S, Szustakowski J, Vitazka P, Edwards R, Bufill JA, Sharma N, Ou SI, Peled N, Spigel DR, Rizvi H, Aguilar EJ, Carter BW, Erasmus J, Halpenny DF, Plodkowski AJ, Long NM, Nishino M, Denning WL, Galan-Cobo A, Hamdi H, Hirz T, Tong P, Wang J, Rodriguez-Canales J, Villalobos PA, Parra ER, Kalhor N, Sholl LM, Sauter JL, Jungbluth AA, Mino-Kenudson M, Azimi R, Elamin YY, Zhang J, Leonardi GC, Jiang F, Wong KK, Lee JJ, Papadimitrakopoulou VA, Wistuba II, Miller VA, Frampton GM, Wolchok JD, Shaw AT, Janne PA, Stephens PJ, Rudin CM, Geese WJ, Albacker LA, Heymach JV. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, Hollmann T, Schalper KA, Gainor JF, Shen R, Ni A, Arbour KC, Merghoub T, Wolchok J, Snyder A, Chaft JE, Kris MG, Rudin CM, Socci ND, Berger MF, Taylor BS, Zehir A, Solit DB, Arcila ME, Ladanyi M, Riely GJ, Schultz N, Hellmann MD. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/jco.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor JF, Rizvi H, Aguilar Jimenez E, Skoulidis F, Yeap BY, Naidoo J, Khosrowjerdi S, Mooradian M, Lydon C, Illei P, Zhang J, Peterson R, Ricciuti B, Nishino M, Zhang J, Roth JA, Grishman J, Anderson D, Little BP, Carter BW, Arbour K, Sauter JL, Mino-Kenudson M, Heymach JV, Digumarthy S, Shaw AT, Awad MM, Hellmann MD. Clinical activity of programmed cell death 1 (PD-1) blockade in never, light, and heavy smokers with non-small-cell lung cancer and PD-L1 expression ≥50. Ann Oncol. 2020;31(3):404–411. doi: 10.1016/j.annonc.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, KEYNOTE-010 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 11.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, Patnaik A, Gubens M, Ramalingam SS, Felip E, Goldman JW, Scalzo C, Jensen E, Kush DA, Hui R. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi: 10.1200/jco.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoulidis F, Arbour KC, Hellmann MD, Patil PD, Marmarelis ME, Awad MM, Murray JC, Hellyer J, Gainor JF, Dimou A, Bestvina CM, Shu CA, Riess JW, Blakely CM, Pecot CV, Mezquita L, Tabbò F, Scheffler M, Papadimitrakopoulou V, Heymach J. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J Clin Oncol. 2019;37(15_suppl):102–102. [Google Scholar]
- 13.Sahin F, Maitra A, Argani P, Sato N, Maehara N, Montgomery E, Goggins M, Hruban RH, Su GH. Loss of STK11/LKB1 expression in pancreatic and biliary neoplasms. Mod Pathol. 2003;16(7):686–691. doi: 10.1097/01.MP.0000075645.97329.86. [DOI] [PubMed] [Google Scholar]
- 14.Onozato R, Kosaka T, Achiwa H, Kuwano H, Takahashi T, Yatabe Y, Mitsudomi T. LKB1 gene mutations in japanese lung cancer patients. Cancer Sci. 2007;98(11):1747–1751. doi: 10.1111/j.1349-7006.2007.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitajima S, Ivanova E, Guo S, Yoshida R, Campisi M, Sundararaman SK, Tange S, Mitsuishi Y, Thai TC, Masuda S, Piel BP, Sholl LM, Kirschmeier PT, Paweletz CP, Watanabe H, Yajima M, Barbie DA. Suppression of sting associated with LKB1 loss in kras-driven lung cancer. Cancer Discov. 2019;9(1):34–45. doi: 10.1158/2159-8290.CD-18-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber GN. Sting: Infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. Sting: A master regulator in the cancer-immunity cycle. Mol Cancer. 2019;18(1):152. doi: 10.1186/s12943-019-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015;16(7):e342–e351. doi: 10.1016/s1470-2045(15)00077-7. [DOI] [PubMed] [Google Scholar]
- 20.Shen H, Zhu M, Wang C. Precision oncology of lung cancer: Genetic and genomic differences in chinese population. NPJ Precis Oncol. 2019;3:14. doi: 10.1038/s41698-019-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]