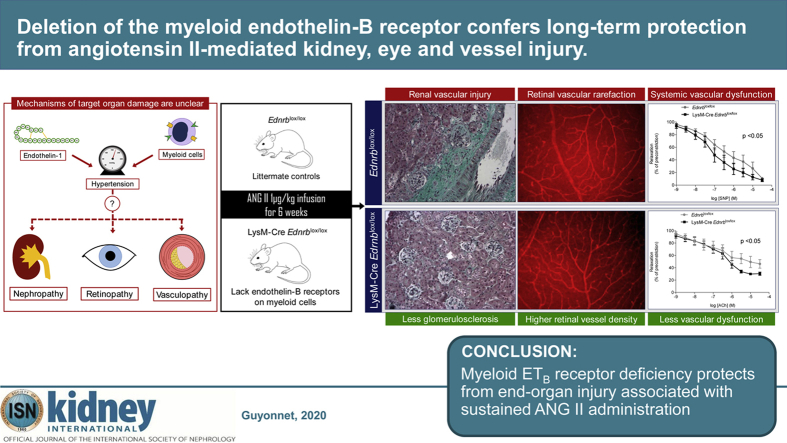
Abstract
The endothelin system may be an important player in hypertensive end-organ injury as endothelin-1 increases blood pressure and is pro-inflammatory. The immune system is emerging as an important regulator of blood pressure and we have shown that the early hypertensive response to angiotensin-II infusion was amplified in mice deficient of myeloid endothelin-B (ETB) receptors (LysM-CreEdnrblox/lox). Hypothesizing that these mice would display enhanced organ injury, we gave angiotensin-II to LysM-CreEdnrblox/lox and littermate controls (Ednrblox/lox) for six weeks. Unexpectedly, LysM-CreEdnrblox/lox mice were significantly protected from organ injury, with less proteinuria, glomerulosclerosis and inflammation of the kidney compared to controls. In the eye, LysM-CreEdnrblox/lox mice had fewer retinal hemorrhages, less microglial activation and less vessel rarefaction. Cardiac remodeling and dysfunction were similar in both groups at week six but LysM-CreEdnrblox/lox mice had better endothelial function. Although blood pressure was initially higher in LysM-CreEdnrblox/lox mice, this was not sustained. A natriuretic switch at about two weeks, due to enhanced ETB signaling in the kidney, induced a hypertensive reversal. By week six, blood pressure was lower in LysM-CreEdnrblox/lox mice than in controls. At six weeks, macrophages from LysM-CreEdnrblox/lox mice were more anti-inflammatory and had greater phagocytic ability compared to the macrophages of Ednrblox/lox mice. Thus, myeloid cell ETB receptor signaling drives this injury both through amplifying hypertension and by inflammatory polarization of macrophages.
Keywords: endothelin, hypertension, macrophages, organ injury
Graphical abstract
Translational Statement.
Hypertension is the single largest contributor to the global burden of disease and to global mortality. It is a major risk factor for chronic kidney disease, atherosclerosis and retinopathy, and end-organ effects with devastating consequences. Preventing this injury is the principal therapeutic goal in patients with hypertension. Our data provide novel insights into the roles of the immune and endothelin systems in the hypertensive end-organ injury seen in the kidney, eye, and vasculature, providing a rational basis for further investigation into the modulation of these pathways for therapeutic gain. These and other successful studies may encourage the industry to take a lead in this relatively orphan area, potentially resulting in a more rational prescribing of endothelin receptor antagonists for hypertension.
Hypertension is the biggest single contributor to the global burden of disease and to global mortality.1 Currently, it is estimated that a quarter of the world’s adult population is hypertensive, projected to reach approximately 30% by 2025.2 Arterial hypertension is a major risk factor for atherosclerosis, coronary artery disease, stroke, and chronic kidney disease, which underpin its devastating effects.3 Thus, preventing end-organ damage is the principal therapeutic goal in patients with hypertension. The last few decades have seen few advances in the treatment of hypertension and its complications. An improved understanding of the mechanisms underlying hypertensive end-organ damage might provide new therapeutic opportunities, particularly for those resistant to conventional blood pressure (BP)-lowering strategies.
In this context, animal models provide an important discovery platform.4 Chronic angiotensin II (ANG II) infusion is widely used to capture the cardinal features of clinical disease,4,5 and studies extending the model beyond 4 weeks demonstrate stable hypertension and significant cardiac, renal, and vascular injury.4 Earlier work shows that these are blunted by endothelin-1 (ET-1) receptor antagonism.6,7 Indeed, ET receptor antagonism is an emerging therapeutic option in a number of clinical conditions including chronic kidney disease8 and resistant hypertension.9 This partly reflects a hemodynamic benefit because ET-1 exerts powerful, sustained vasoconstriction10 via activation of endothelin-A (ETA) receptors present on vascular smooth muscle. In addition, ETA receptor activation is directly proinflammatory and profibrotic, so BP-independent benefits of ET-1 antagonism may also be therapeutically important.
There has been less focus given to the role of the endothelin-B (ETB) receptor, which exerts a countervailing influence on BP. Endothelial ETB activation causes vasodilation and activation of ETB in the distal renal tubule promotes natriuresis.11 In addition, the ETB receptor plays a key role in clearing ET-1 from the circulation and curtailing its bioactivity.12 Indeed, we recently showed that ETB receptor–mediated clearance of ET-1 by myeloid cells protected against the early development of experimental hypertension.13 Other studies also point to the innate immune system, particularly macrophages, as an important modulator of the development and progression of hypertension.14, 15, 16, 17, 18 Whether this also modulates target organ injury is less clearly defined. We therefore hypothesized that targeted deletion of the mouse myeloid ETB receptor would exaggerate hypertensive end-organ injury. In the current study, we infused ANG II for 6 weeks and examined injury in the kidney, eye, heart, and vasculature. Surprisingly, myeloid ETB receptor deficiency protected from end-organ injury. These effects partly reflected a reduction in BP but also involved a tuning of macrophages to an anti-inflammatory phenotype. Thus, targeting the myeloid ET system may provide an attractive and novel therapeutic strategy in the context of hypertensive end-organ damage.
Results
Myeloid ETB receptor deficiency protects from ANG II-mediated renal injury
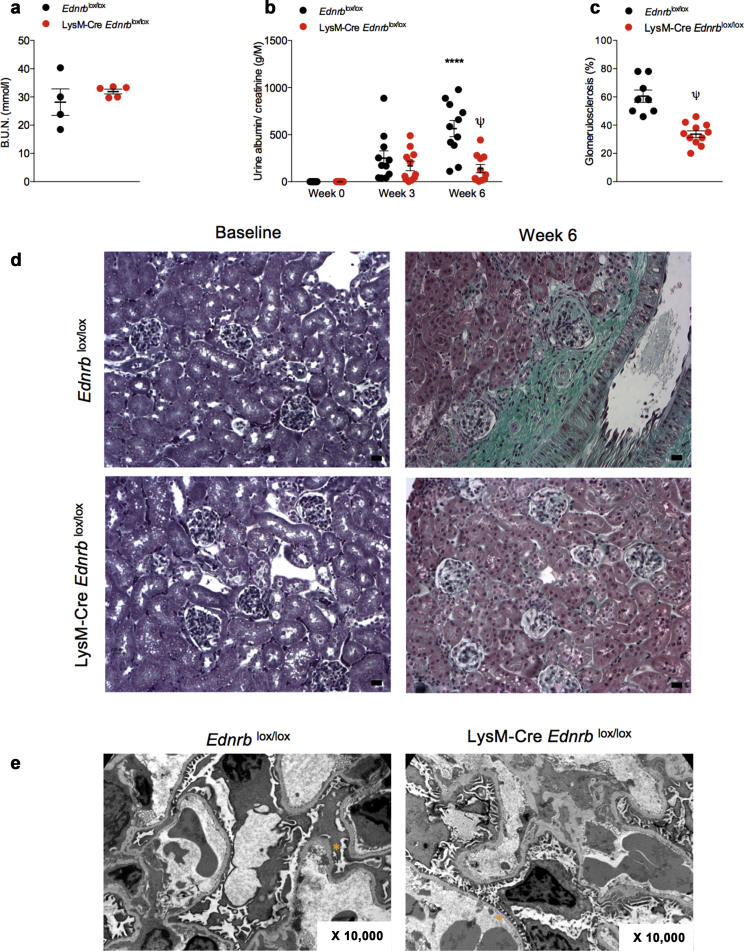
After 6 weeks of ANG II infusion, renal excretory function was no different between mice with a myeloid-specific disruption of the Ednrb gene (LysM-Cre Ednrblox/lox) and littermate controls (mice with no expression of Cre recombinase Ednrblox/lox) (Figure 1a). Ednrblox/lox mice developed progressive, heavy albuminuria over this period. By contrast, LysM-Cre Ednrblox/lox mice displayed only a modest rise in urinary albumin excretion that did not progress between week 3 and week 6 (at 6 weeks: 564 ± 86 vs. 139 ± 44 g/M, P < 0.001, Figure 1b). Consistently, there was less glomerulosclerosis at 6 weeks in LysM-Cre Ednrblox/lox mice compared with controls (61 ± 4 vs. 34 ± 2%, P < 0.0001, Figure 1c and d). In addition, there was a greater preservation of glomerular ultrastructure—less glomerular basement membrane thickening and podocyte foot process broadening and effacement—in LysM-Cre Ednrblox/lox mice compared with control mice (Figure 1e).
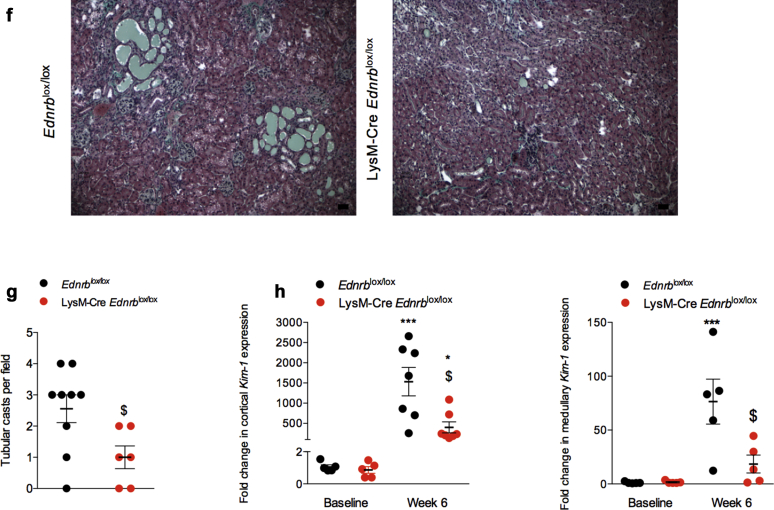
Figure 1.
Specific deletion of Ednrb from myeloid cells protects from angiotensin II (ANG II)–mediated renal injury. (a) Blood urea nitrogen concentration at 6 weeks, (b) urinary albumin excretion, and (c) glomerulosclerosis at 6 weeks in Ednrblox/lox and LysM-Cre Ednrblox/lox mice after ANG II. (d) Representative images of Masson’s trichrome–stained kidney cortex from Ednrblox/lox and LysM-Cre Ednrblox/lox mice after 6 weeks of ANG II. (e) Representative transmission electron micrographs of glomeruli from Ednrblox/lox and LysM-Cre Ednrblox/lox at 6 weeks. ∗An area of podocyte foot process effacement and fusion in Ednrblox/lox mice and an area of normal podocyte ultrastructure in LysM-Cre Ednrblox/lox mice. (f) Representative images of Masson’s trichrome–stained kidney medulla from Ednrblox/lox and LysM-Cre Ednrblox/lox mice after 6 weeks of ANG II with (g) associated quantification of tubular casts. (h) Quantitative reverse transcription polymerase chain reaction analysis of cortical and medullary Kim-1 message at baseline and after 6 weeks. Data represent mean ± SEM of n = 10 mice/group. ∗P < 0.05, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 for baseline compared with week 6 within the same genotype; $P < 0.05 and ψP < 0.001 for one genotype compared with the other at the same time point. Between-group comparisons for normally distributed data were by a t test (c,g) or 2-way analysis of variance with Sidak post test of plan comparisons (b,h). For between-group comparisons of non-normally distributed variables, a Mann-Whitney test (c) or Kruskal-Wallis test was employed. Bars = 20 μm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
The protective effects of the myeloid ETB receptor extended to ANG II-mediated tubular injury with LysM-Cre Ednrblox/lox mice demonstrating fewer tubular casts after 6 weeks of ANG II compared with Ednrblox/lox (Figure 1f and g). This was supported by lower cortical and medullary Kim-1 expression in knockout mice compared with controls at 6 weeks (Figure 1h).
Myeloid ETB receptor deficiency reduces ANG II-mediated renal inflammation
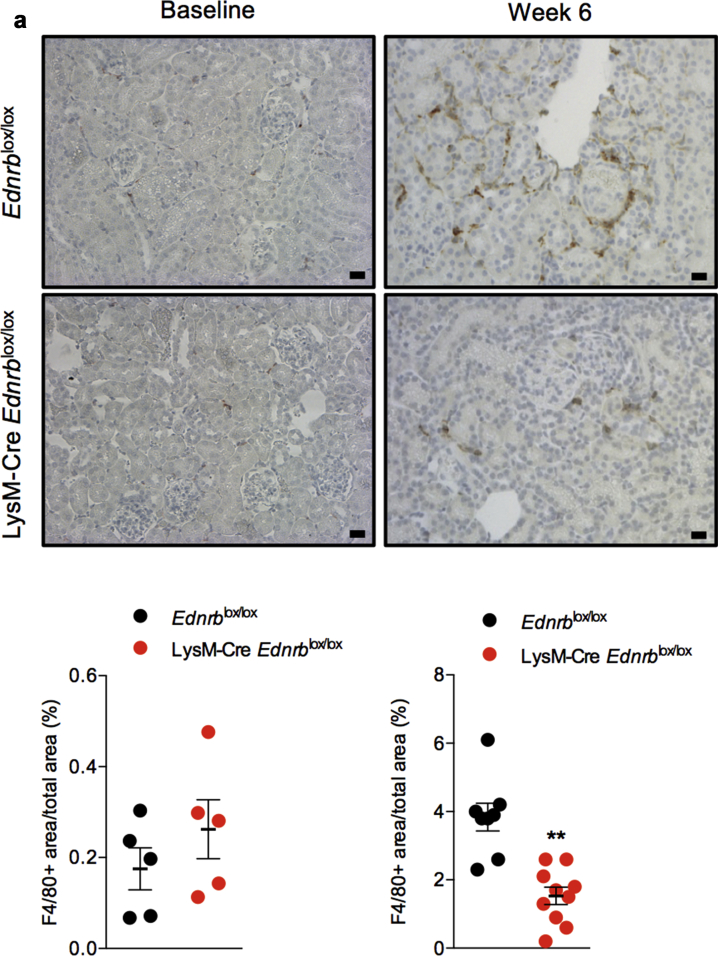
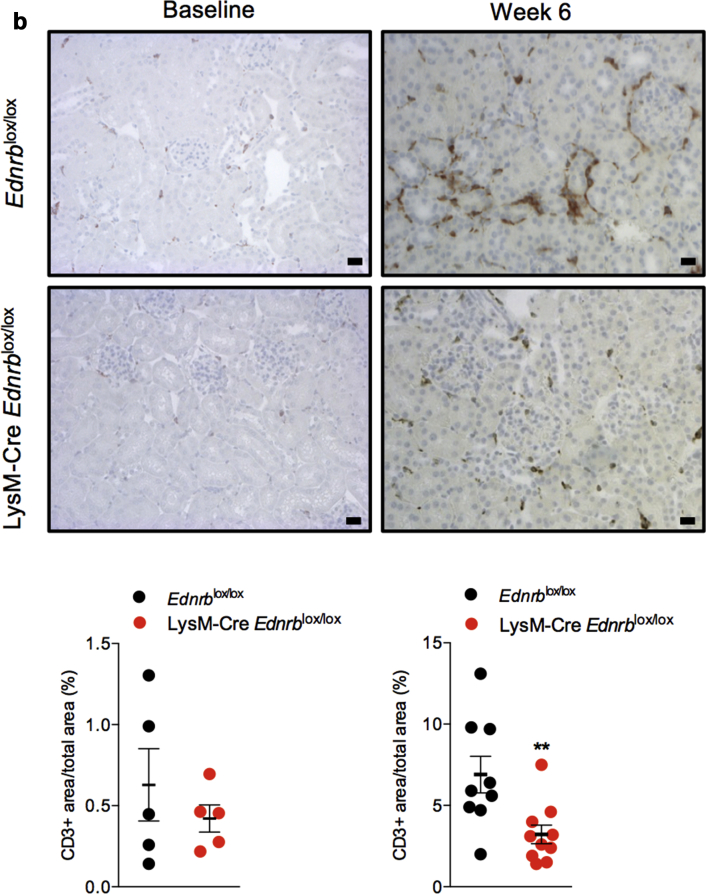
At baseline, there was no difference in the number of renal resident macrophages between LysM-Cre Edrnblox/lox and Ednrblox/lox mice. Six weeks of ANG II increased the number of renal macrophages to a greater extent in Ednrblox/lox mice than in those deficient in myeloid ETB receptors (at 6 weeks, F4/80+ area/total area: 1.5 ± 0.3 vs. 3.8 ± 0.4%, P < 0.001, Figure 2a). Similarly, the number of renal T lymphocytes was no different between the 2 groups at baseline and increased to a greater extent in Edrnblox/lox mice after 6 weeks of ANG II than in Lys M-Cre Edrnblox/lox mice (at 6 weeks, CD3+ area/total area: 3.2 ± 0.6 vs. 6.9 ± 1.1%, P < 0.01, Figure 2b).
Figure 2.
Specific deletion of Ednrb from myeloid cells reduces angiotensin II (ANG II)–mediated renal inflammation. Representative images of renal (a) F4/80 and (b) CD3 staining on kidney sections from Ednrblox/lox and LysM-Cre Ednrblox/lox mice at baseline and after 6 weeks of ANG II infusion. Data represent mean ± SEM of n = 8 mice/group. ∗∗P < 0.01 for one genotype compared with the other. Between-group comparisons were made by an unpaired t test. Bars = 20 μm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Myeloid ETB receptor deficiency promotes an anti-inflammatory renal environment in response to ANG II
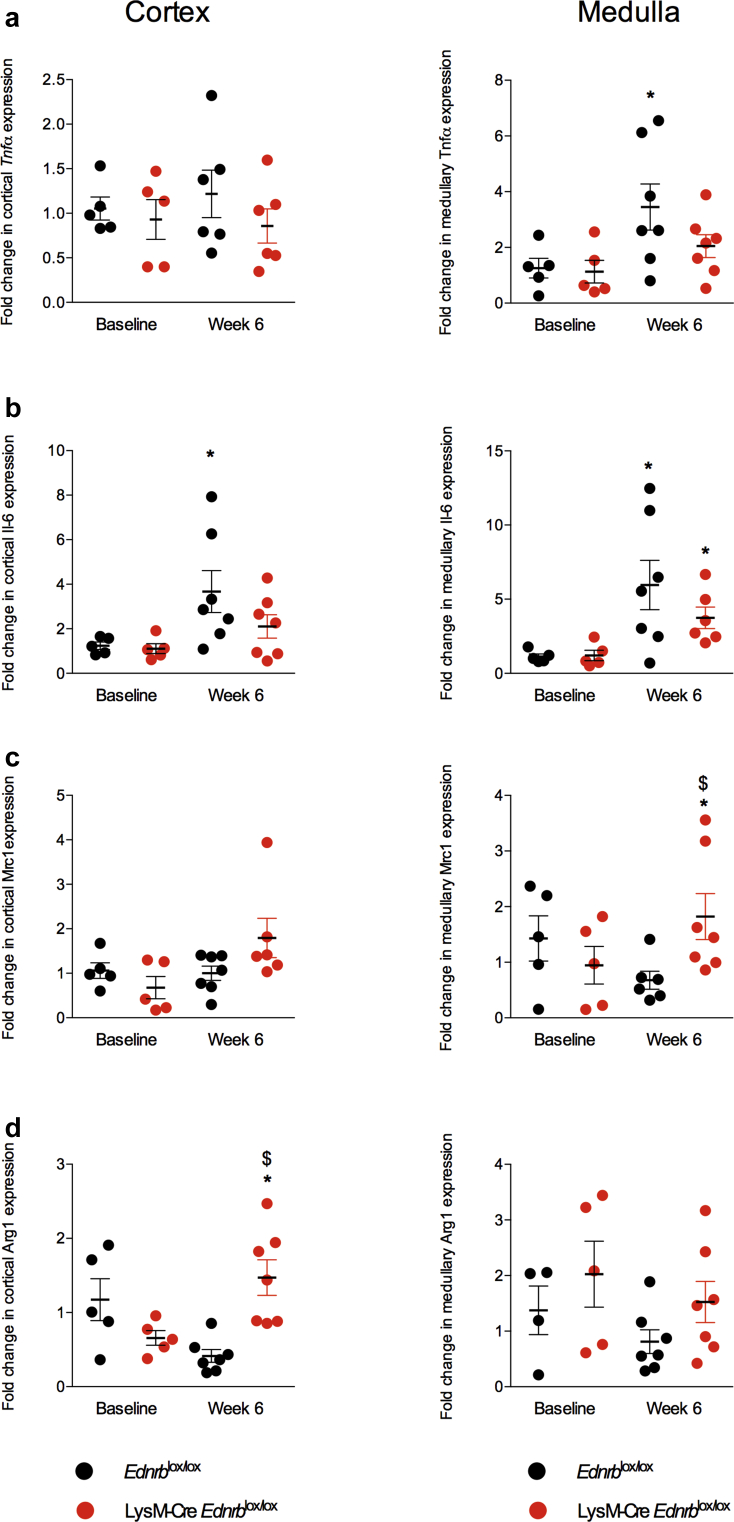
We went on to further examine the effects of ANG II on the balance between a proinflammatory (Tnfα and Il-6) and anti-inflammatory (Mrc1 and Arg1) environment within the kidney. At baseline, and in both cortex and medulla, Tnfα, Il-6, Mrc1, and Arg1 expression did not differ between LysM-Cre Edrnblox/lox and Edrnblox/lox mice (Figure 3). After 6 weeks of ANG II, cortical and medullary Il-6 expression increased approximately 4- to 6-fold and medullary Tnfα approximately 3-fold in control mice, whereas LysM-Cre Ednrblox/lox mice displayed a modest increase in medullary Il-6 but no change in Tnfα in either compartment (Figure 3a and b). Conversely, whereas Edrnblox/lox mice demonstrated no changes in cortical or medullary Mrc1 and Arg1 expression after ANG II, knockout mice upregulated cortical Arg1 and medullary Mrc1 (Figure 3c and d).
Figure 3.
Specific deletion of Ednrb from myeloid cells promotes an anti-inflammatory renal environment in response to angiotensin II (ANG II). Quantitative reverse transcription polymerase chain reaction analysis of renal cortex and medulla of Ednrblox/lox and LysM-Cre Ednrblox/lox mice before and after 6 weeks of ANG II. Data represent mean ± SEM fold change from baseline; n = 7 mice/group. ∗P < 0.05 for baseline compared with week 6 within the same genotype; $P < 0.05 for one genotype compared with the other at the same time point. Comparisons were made by 2-way analysis of variance with Sidak post-test for planned comparisons.
Myeloid ETB receptor deficiency protects from ANG II-mediated retinal injury
Prolonged uncontrolled hypertension leads to the development of retinal hemorrhages and immune cell infiltration. Deletion of the ETB receptor on myeloid cells alone significantly reduced the number of retinal hemorrhages seen after ANG II infusion by approximately 90% (Figure 4a). Six weeks of ANG II led to vessel rarefaction in the inner retina plexus in Edrnblox/lox mice, whereas the vessel density of LysM-Cre Ednrblox/lox mice was preserved (Figure 4b). In addition, control mice demonstrated a greater number of activated microglia in the inner retina in response to 6 weeks of ANG II compared with knockout mice (Figure 4c). Activated microglia exhibited a typical round shape with larger cell body and short neurites.
Figure 4.
Specific deletion of Ednrb from myeloid cells protects from angiotensin II (ANG II)–mediated retinal injury. All data are after 6 weeks of ANG II. (a) Quantification of retinal hemorrhagic spots in Ednrblox/lox and LysM-Cre Ednrblox/lox mice. (b) Representative images of the inner retina vascular network stained with lectin from Ednrblox/lox and LysM-Cre Ednrblox/lox mice. Vascular network density was calculated from an average of 4 images/retina using the NeuronJ plugin on ImageJ software (National Institutes of Health, Bethesda, MD). (c) Representative images of microglia within the inner retina stained with iba-1 antibody from Ednrblox/lox and LysM-Cre Ednrblox/lox mice. Activated microglia, which have an amoeboid morphology with a large cell body and short neurites (∗), were counted from 4 images/retina. Data represent mean ± SEM; n = 5 mice per group. ∗P < 0.05 and ∗∗P < 0.01 for one genotype compared with the other. Between-group comparisons were performed with a Mann-Whitney test. Bars = 50 μm To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Myeloid ETB receptor deficiency does not affect ANG II-mediated cardiac hypertrophy and dysfunction
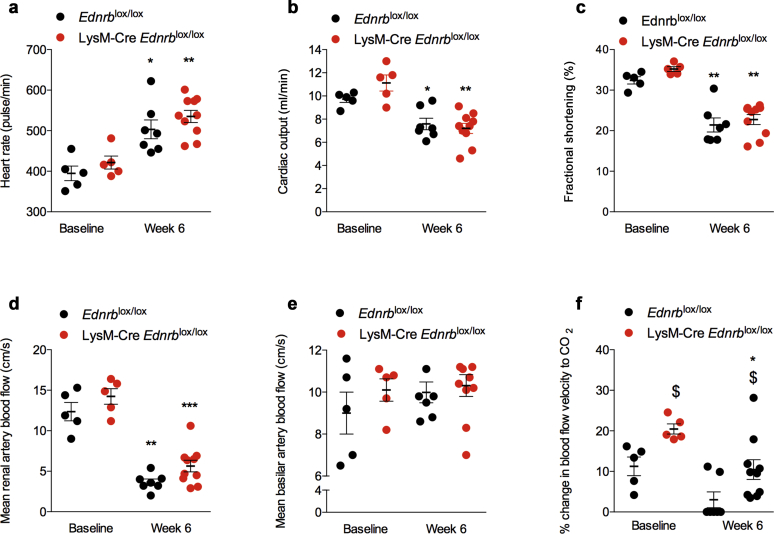
Six weeks of ANG II led to changes in cardiac structure that were in keeping with the development of left ventricular hypertrophy; these did not differ between LysM-Cre Edrnblox/lox and Edrnblox/lox mice (data not shown). ANG II infusion led to a similar increase in heart rate (Figure 5a) and reduction in cardiac output (Figure 5b) in both groups. LysM-Cre Ednrblox/lox and littermates also showed a similar reduction in left ventricular shortening fraction (Figure 5c).
Figure 5.
Specific deletion of Ednrb from myeloid cells does not affect angiotensin II (ANG II)–mediated cardiac injury. Effects on (a) heart rate, (b) cardiac output, (c) fractional shortening, (d) renal artery blood flow, (e) basilar artery blood flow, and (f) basilar artery vascular function at baseline and after 6 weeks of ANG II in Ednrblox/lox and LysM-Cre Ednrblox/lox mice. Data represent mean ± SEM of n = 8 mice/group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for baseline compared with week 6 within the same genotype; $P < 0.05 for one genotype compared with the other at the same time point. Comparisons were made by 2-way analysis of variance with Sidak post-test for planned comparisons.
Myeloid ETB receptor deficiency protects from ANG II-mediated endothelial dysfunction
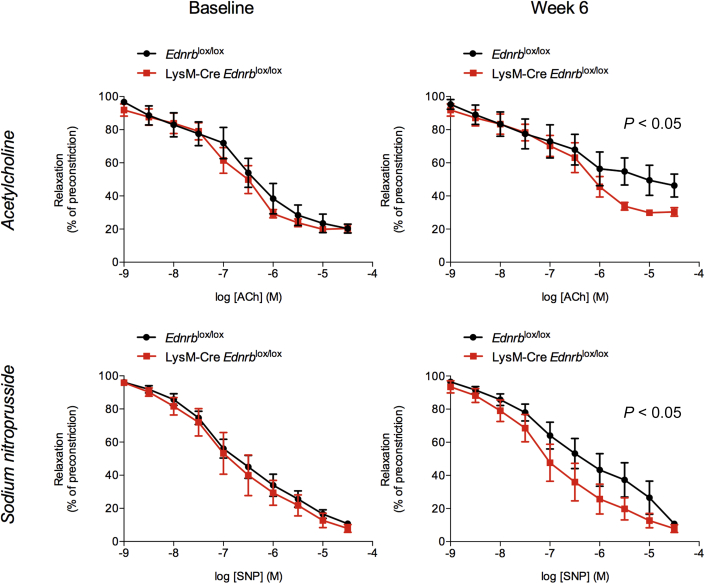
As expected, 6 weeks of ANG II infusion reduced renal artery blood flow by approximately 50%, and this fall was no different between LysM-Cre Edrnblox/lox and control mice (Figure 5d). ANG II did not affect basilar artery blood flow (Figure 5e) in either group. Interestingly, LysM-Cre Edrnblox/lox displayed greater basilar artery vascular reactivity at baseline compared with controls (20 ± 1 vs. 11 ± 2%, P < 0.01). After 6 weeks of ANG II, and in keeping with the development of significant vascular dysfunction, this response was abolished in Edrnblox/lox mice, whereas LysM-Cre Edrnblox/lox mice retained a significant, albeit reduced, basilar artery vasodilatory capacity that remained within the physiological range (10 ± 2 vs. 0 ± 3%, P < 0.05, Figure 5f). In keeping with these data, whereas there were no differences in the responses of isolated mesenteric vessels from knockout and control mice to acetylcholine (endothelium-dependent) and sodium nitroprusside (endothelium-independent) vasodilation at baseline, 6 weeks of ANG II led to impairment of both but with less marked effects in myeloid ETB receptor–deficient mice compared with controls (Figure 6).
Figure 6.
Specific deletion of Ednrb from myeloid cells protects from angiotensin II (ANG II)–mediated endothelial dysfunction. Endothelium-dependent (acetylcholine) and endothelium-independent (sodium nitroprusside) vascular function in Ednrblox/lox and LysM-Cre Ednrblox/lox mice at baseline and after 6 weeks of ANG II. Data represent mean ± SEM of n = 8 mice/group. Between-group comparisons were performed with a 2-way analysis of variance.
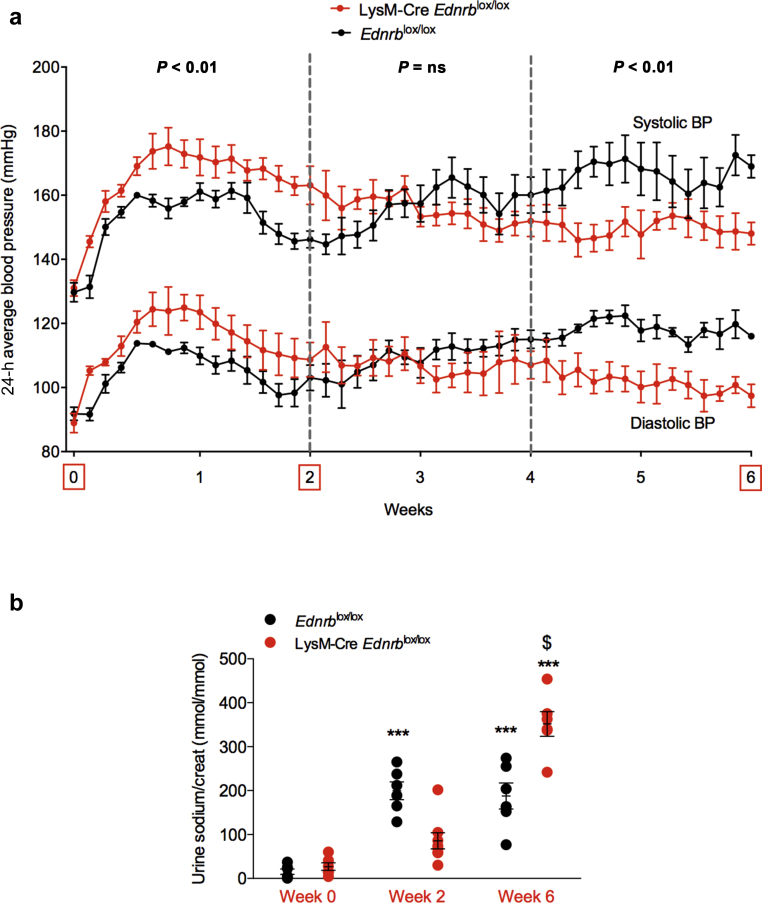
Myeloid ETB receptor deficiency has a biphasic effect on long-term ANG II-mediated hypertension and promotes natriuresis
LysM-Cre Edrnblox/lox mice demonstrated an exaggerated BP response to ANG II during the first 2 weeks of infusion, confirming our previous work.13 Extending the protocol, we demonstrated that this exaggerated BP response was not sustained. Indeed, there was a reversal during weeks 3–4 such that in weeks 5 and 6, knockout mice now had a significantly lower BP compared with controls (Figure 7a).
Figure 7.
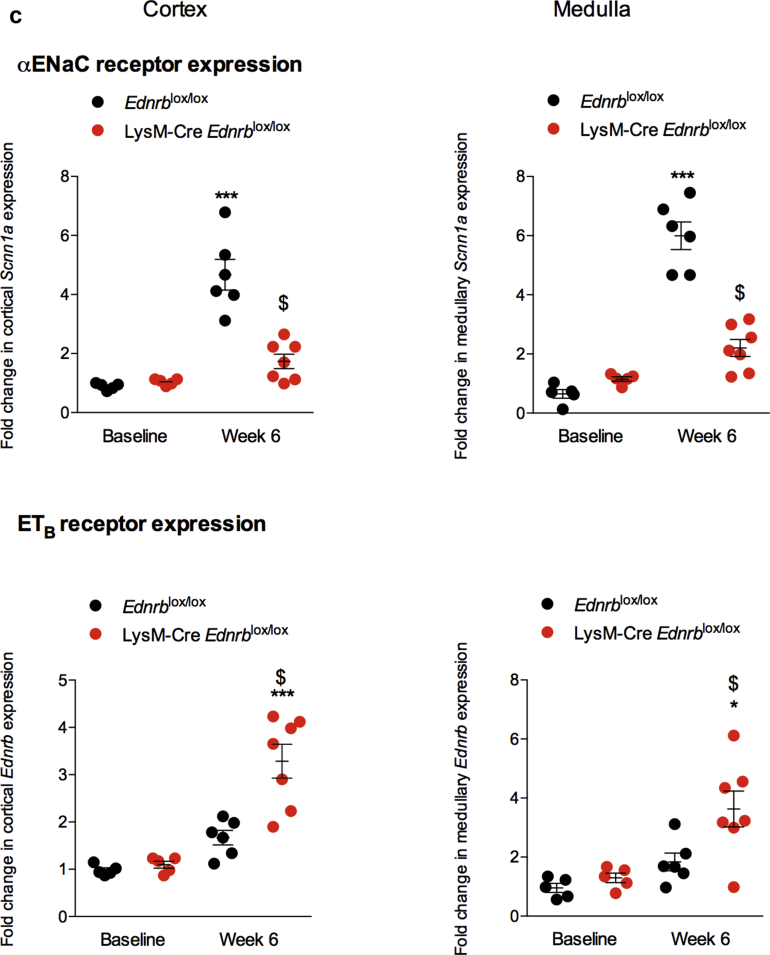
Specific deletion of Ednrb from myeloid cells has a biphasic effect on the blood pressure (BP) response to angiotensin II (ANG II) and promotes long-term natriuresis. (a) Systolic and diastolic BP of Ednrblox/lox and LysM-Cre Ednrblox/lox mice during 6 weeks of ANG II infusion. (b) Urinary sodium excretion over this 6-week period. (c) Expression of cortical and medullary α epithelial sodium channel (ENaC) and endothelin-B (ETB) receptor in Ednrblox/lox and LysM-Cre Ednrblox/lox mice at baseline and after 6 weeks of ANG II. Data represent mean ± SEM of n = 5–7 mice/group. ∗P < 0.05 and ∗∗∗P < 0.001 for baseline compared with week 6 within the same genotype; $P < 0.05 for one genotype compared with the other at the same time point. Between-group comparisons were performed with 2-way analysis of variance.
Next, we examined renal salt excretion at key time points over the course of the 6-week study. Before ANG II infusion, sodium excretion was no different between genotypes. Infusion of ANG II induced an increase in natriuresis in control mice, and this was sustained through the 6-week course of the study. The response was different in myeloid ETB receptor–deficient mice: the early natriuresis was blunted (Figure 7b), concomitant with the exaggerated hypertensive response to ANG II; by week 6, the natriuresis was significantly greater than in controls, coincident with the reversal of BP. ETB receptors are expressed in the principal cell of the renal tubule. Activation of ETB here inhibits the epithelial sodium channel (ENaC) to promote natriuresis. We therefore examined renal expression of the ETB receptor and αENaC subunit (the rate-limiting step for functional ENaC formation). Before ANG II, there was no difference between genotypes in the expression of either ETB or αENaC mRNA. At 6 weeks, control mice showed a significantly increased abundance of αENaC mRNA in both the cortex and medulla, which was not evident in LysM-Cre Edrnblox/lox mice. Notably, only LysM-Cre Edrnblox/lox mice showed a significant increase in ETB expression after 6 weeks of ANG II infusion (Figure 7c).
Myeloid ETB receptor deficiency promotes an anti-inflammatory macrophage and neutrophil phenotype in response to ANG II
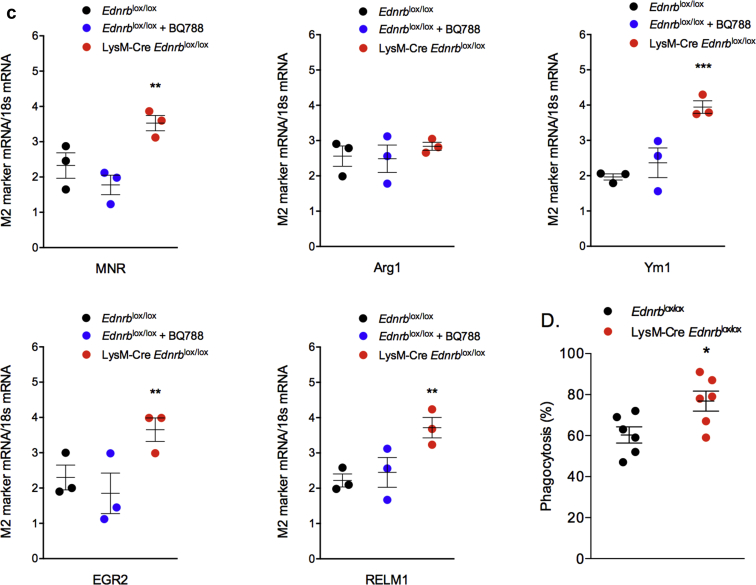
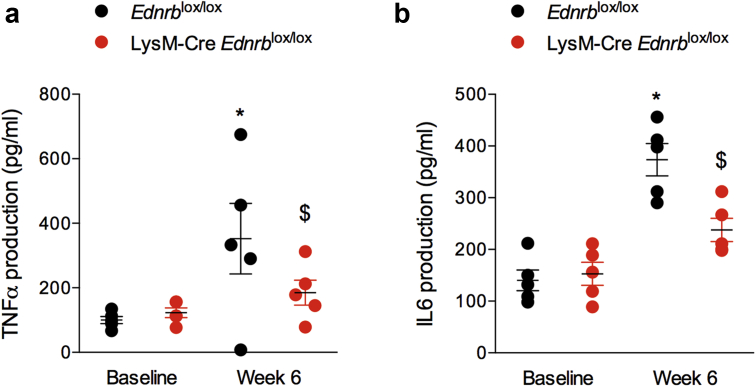
Next, we explored the effects of ANG II and myeloid ETB receptor deficiency on macrophage and neutrophil phenotype. At baseline, there were no differences seen between macrophages from Edrnblox/lox or LysM-Cre Edrnblox/lox mice in terms of their ability to polarize to an inflammatory (M1) or anti-inflammatory (M2) phenotype, or their phagocytic ability (Supplementary Table S1). Interestingly, after 6 weeks of exposure to ANG II, macrophages harvested from LysM-Cre Edrnblox/lox mice demonstrated less of an M1 phenotype and more of an M2 phenotype than macrophages from littermate controls (Figure 8a–c). Knockout macrophages also displayed a greater phagocytic capacity than controls at this timepoint (Figure 8d). Neutrophils from Edrnblox/lox or LysM-Cre Edrnblox/lox mice did not differ at baseline in terms of cytokine production. However, after exposure to 6 weeks of ANG II, neutrophils from knockout mice were less inflammatory compared with control cells (Figure 9).
Figure 8.
Specific deletion of Ednrb from myeloid cells promotes an anti-inflammatory macrophage phenotype in response to angiotensin II (ANG II). Peritoneal macrophages were harvested from Ednrblox/lox and LysM-Cre Ednrblox/lox mice after 6 weeks of ANG II. (a) A range of M1 macrophage markers and (b) cytokines were assessed after stimulation with lipopolysaccharide/interferon-γ; (c) M2 markers were assessed after stimulation with interleukin (IL) 4/IL13; (d) phagocytosis. Data are mean ± SEM; n = 6/group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for one genotype compared with the other. Between-group comparisons were performed with a 1-way analysis of variance with Sidak post-test for planned comparisons.
Figure 9.
Specific deletion of Ednrb from myeloid cells promotes a less inflammatory neutrophil phenotype in response to angiotensin II (ANG II). Bone marrow–derived neutrophils from Ednrblox/lox and LysM-Cre Ednrblox/lox mice were cultured in vitro after 6 weeks of ANG II. (a) Tumor necrosis factor-α (TNFα) and (b) interleukin (IL) 6 production after stimulation with lipopolysaccharide. Data are mean ± SEM; n = 5/group. ∗P < 0.05 for baseline compared with week 6 within the same genotype; $P < 0.05 for one genotype compared with the other at the same time point. Comparisons were made by 2-way analysis of variance with Sidak post-test for planned comparisons.
Discussion
Damage to the kidney, heart, eye, and arteries underpins the devastating consequences of sustained hypertension. In this study, we purposefully used an extended-duration ANG II infusion to model such end-organ injury with stable hypertension. In the kidney, injury to both the glomerular and interstitial compartments was apparent and the gradual development of proteinuria and glomerulosclerosis is analogous to that observed in human progressive chronic kidney disease. We examined how ET-1/ETB signaling in myeloid cells contributed to the development of organ injury. Our study had 3 main findings. First, we have demonstrated, for the first time, that activation of the myeloid ETB receptor makes a significant contribution to renal, retinal, and vascular injury induced by ANG II. Second, the injurious role of myeloid cell ET-1/ETB signaling may reflect polarization of macrophages to a proinflammatory phenotype. Third, myeloid cell ETB receptors modulate the BP response to ANG II infusion in a biphasic way, a Janus-faced role of macrophages that is relevant to progressive human disease.
Current research shows that macrophages play diverse roles in hypertension. Crowley et al.16 showed that mice lacking ANG type 1 receptors on bone marrow–derived cells develop exaggerated hypertension and proteinuria during ANG II infusion, whereas Rickard et al.17 studied mice with a macrophage-specific knockout of the mineralocorticoid receptor and found that they were protected from deoxycorticosterone acetate/salt-induced hypertension. As opposed to altering macrophage phenotype, Wenzel et al.15 elegantly depleted macrophages and found that this markedly blunted virtually all consequences of chronic ANG II infusion, including BP elevation, superoxide production, vascular dysfunction, and fibrosis (some of these effects may have been due to modulation of the ET system). We also found that macrophages provide a buffering mechanism in the development of hypertension. The presence of a functional ET-1/ETB signaling cascade in macrophages slowed the initial rise in BP induced by a 2-week infusion of either ET-1 or ANG II.13 The hypertension induced by ANG II is partly mediated by ET-1, and we found in both models that the initial BP-lowering effect of macrophages reflected clearance of ET-1, dependent on an unblocked ETB receptor and intact endocytosis.13 We now show that this “protective” role of ET-1/ETB in macrophages is not sustained. The BP differential between mice with and without macrophage ETB receptors is lost beyond 2 weeks and indeed reverses after 4 weeks of ANG II treatment. An “injurious” role for macrophage ETB ultimately emerges such that specific deletion of this receptor substantially reduces organ injury in the kidney, eye, and vasculature, at least in the setting of ANG II hypertension. This remains to be confirmed in an ET-1 infusion model. Similarly, we have not yet explored whether the myeloid ETA receptor, expressed at much lower levels than the ETB receptor,13 contributes to injury progression.
By extending our examination of ANG II hypertension beyond 2 weeks, we highlight diverse and changing roles for macrophages in BP regulation. The hypertensive reversal after 3–4 weeks was associated with a marked improvement in the natriuretic response. Our data point to a mechanism involving increased tubular ET-1/ETB signaling and downregulation of ENaC-mediated sodium reabsorption in the distal nephron. Moreover, the magnitude of the natriuresis is large, and it is likely that transporters in other segments of the nephron are downregulated. Defining how the nephron functionally remodels sodium transport could be assessed by examining the natriuretic response to a panel of diuretics19 and is a future direction of our research. Similarly, how deletion of myeloid cell ETB receptor leads to upregulation of the same system in the principal cell is currently unclear. Recent studies show that renal epithelial cells produce chemokines to orchestrate intrarenal distribution of monocyte-derived cells.20,21 Whether our data uncover direct “myeleo-tubular” crosstalk within the normal operation of the ET-1 pathway requires further investigation.
Chronic ANG II infusion also promotes renal T-cell and macrophage infiltration, shown here and by others.22,23 This infiltration was substantially reduced by myeloid ETB deficiency, which also shifted the renal corticomedullary environment from an inflammatory to a more anti-inflammatory one. Together these suggest that the macrophage ET-1/ETB signaling system operates to promote inflammation and injury in the long term. Certainly, sustained ANG II treatment upregulates the vascular and renal ET system.6,7 We have previously shown that macrophages display ETB-dependent chemokinesis toward ET-1. It is plausible that the increase in renal macrophages seen after 6 weeks of ANG II infusion reflects recruitment of monocytes to the kidney in chemokinetic response to a high systemic-intrarenal ET gradient. Moreover, effective ETB blockade disrupts the myeloid ETB sensing mechanism, leading to fewer circulating monocytes transitioning to the kidneys. Others have explored renal inflammation and the contribution that the ET system makes to this. Boesen et al.24 showed that during a 2-week infusion of ANG II in mice, ETA receptor activation mediated a BP-independent increase in renal cortical T cells, whereas medullary T-cell and corticomedullary macrophage infiltration was BP-dependent. Similarly, in a model of diabetic nephropathy, treatment with an ETA antagonist reduced renal macrophage infiltration and fibrosis.25 In both studies, ET receptor blockade was given systemically and so the specific cell mediating the beneficial effects was unclear.
Although no different at baseline, macrophages from myeloid ETB knockout mice taken after 6 weeks of exposure to ANG II displayed a more M2/anti-inflammatory and reparative phenotype than those from littermate control mice that showed a more M1/inflammatory one. The ability of ANG II to promote an inflammatory macrophage phenotype is well recognized.26, 27, 28 However, its exact mechanism remains unclear. Our data suggest an interaction between macrophage ANG II receptors and ETB to promote this phenotypic switch. ANG II-mediated hypertension and end-organ injury are to a large extent mediated by an upregulation of the ET system. Thus, ET receptor antagonism is able to abrogate these effects.6,7 However, previous studies have not explored the type and location of the ET receptor involved. Our novel findings support a key role for macrophage ETB receptors in mediating these effects.
Few recent studies have explored the effects of ANG II-mediated hypertension on the eye. Retinal hemorrhages and vascular loss are characteristic of hypertensive eye injury and both were seen here. They were reduced with myeloid ETB deficiency as was retinal inflammation assessed by microglial activation. ANG II contributes to a number of eye disorders including diabetic retinopathy, glaucoma, and retinopathy of prematurity.29 In retinopathy of prematurity, blockade of ANG type 1 receptors promotes the re-establishment of normal vessel growth and reduces microglia recruitment.30 These effects are similar to those seen here, and so targeting the myeloid ET system may provide a novel direction for future studies in these areas. Indeed, one study in diabetic mice showed an improvement in the retinal vascular network and reduced pericyte loss with the ETA antagonist, atrasentan.31
As expected, chronic ANG II infusion resulted in both endothelium-dependent and endothelium-independent dysfunction. This injury is a result of damage to both the vascular endothelial cell and the underlying smooth muscle cell. This was ameliorated by myeloid ETB receptor deficiency. The mechanisms for this protection probably relate in part to the shift in the macrophage phenotype we observed in response to ANG II between the two groups of mice. It is well recognized that inflammatory macrophages contribute to endothelial dysfunction.15,32 Phagocytosis also contributes to inflammation resolution through removal of cell debris.33 Here, phagocytic capacity of knockout macrophages was greater than in controls, and so this may also explain the differential effects seen in the kidneys, eye, and vasculature.
In the current study, the magnitude of the antiproteinuric and antifibrotic effects of effective myeloid ETB receptor blockade (approximately 40%–60%) is similar to that seen with ET receptor antagonists. As both selective ETA and mixed ETA/B receptor antagonists are available in the clinic, the addition of our own data to these earlier studies suggests that both approaches may be beneficial in reducing ANG II-mediated renal damage, which is one of the main mechanisms in the development and progression of chronic kidney disease. Consistent with this, the recent SONAR study demonstrated the efficacy of atrasentan, an endothelin-A antagonist, in slowing diabetic chronic kidney disease progression when added to renin-angiotensin system blockade.8
In summary, our study has identified a novel role for the myeloid ET system in mediating ANG II-associated injury in the eye, kidney, and vasculature. The long-term protective effects of deleting the myeloid ETB receptor were only resolved by extending the duration of our experiment, highlighting the importance of “time” as a factor when modeling chronic, progressive human disorders. The protection ultimately conferred by myeloid ETB receptor depletion may be partly hemodynamic, reflecting the lowering of BP after natriuresis. Our data also indicate that reprogramming of macrophage and/or neutrophil phenotype and function is likely to be important. We cannot currently discriminate the relative contributions of macrophages and neutrophils to this protective phenotype, a limitation of the study requiring further work. Moreover, we cannot yet clearly map across to humans the biphasic BP response to myeloid ETB receptor deletion. We previously reported that cyclophosphamide therapy, depleting circulating monocytes and tissue macrophages (as well as T and B cells), increased BP in patients with vasculitis,13 at least initially and a longer study is now needed. This is relevant, because targeting the immune system is recognized as an effective treatment for hypertension in man as seen in studies using interleukin (IL)6 antagonism34 and immunization against ANG II.35 Similarly, macrophage cell therapy is currently being explored as an anti-inflammatory approach in a number of disease states.36, 37, 38 Combining these with newer monoclonal antibodies that specifically target ETA and ETB39 might allow the current data to be explored in the clinical setting.
Methods (Please also see Supplementary Material)
ANG II-mediated end-organ damage
Mice were infused ANG II (1 μg/kg/min, Sigma Aldrich) subcutaneously using osmotic mini pumps (Alzet, model 2006) for a period of 6 weeks. Animals were fed a high-salt diet (NaCl 3%) and had free access to food and water. BP was measured in conscious, unrestrained mice using a radio-telemetry system (PA-C10 and Dataquest software, Data Sciences International). Recordings were obtained every 15 minutes for 60 seconds; daytime was defined as 0700–1900 and nighttime as 1900–0700.
Assessment of renal function and albuminuria
Blood urea nitrogen and urinary creatinine concentrations were quantified spectrophotometrically by colorimetric methods. Urinary albumin was measured with a specific enzyme-linked immunosorbent assay (Cusabio, CSB-E13878m).
Renal histopathology and immunohistochemistry
Kidneys were immersed in 10% formalin and embedded in paraffin. Four-millimeter sections were processed for histopathology or immunohistochemistry. Glomerulosclerosis was defined by focal and segmental consolidation of the tuft by increased extracellular matrix, obliterating the glomerular capillary lumen. For the assessment of glomerulosclerosis, an examiner blinded to the experimental conditions assessed the percentage of glomeruli showing glomerulosclerosis with at least 50 glomeruli examined per field. For immunohistochemistry, paraffin embedded sections were stained with the following primary antibodies: rabbit anti-CD3 (DAKO, A 0452, 1:200), rat anti-F4/80 (AbDSerotec, MCA497, 1:500). CD3 and F4/80 staining were exposed with Histofine reagent (Nichirei Biosciences, 414141F) and color formation stopped by washing in phosphate-buffered saline. Slides were then counterstained with hematoxylin. Photomicrographs were taken with an Axiophot Zeiss photomicroscope (Jena, Germany). Quantification of CD3 and F480 staining was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Electron microscopy
Sections of renal cortex were fixed in Trump’s solution (EMS, 11750) and embedded in Araldite M (Sigma Aldrich, 10951). Ultrathin sections were counter-stained with uranyl acetate and lead citrate and examined with a transmission electron microscope (JEM-1011, JEOL, Peabody, MA).
Assessment of retinal injury
After enucleation, eyes were fixed in 4% paraformaldehyde for 30 minutes at room temperature. After washing in phosphate-buffered saline, and removal of the cornea and lens, the retina was dissected from the retinal pigment epithelium/choroid/sclera. Retinas were incubated overnight with primary antibodies (lectin Alexa conjugated, 1:100, Invitrogen; rabbit anti-Iba1, 1:400, Wako) in phosphate-buffered saline supplemented with 0.5% Triton X-100, followed by incubation with appropriate Alexa-coupled secondary antibodies (Life Technologies). Retinas were flat-mounted and viewed with a fluorescence microscope (DM5500B, Leica, France). Vascular network and activated Iba1+ cell density were calculated from an average of 8 images/animal (4/retina). Vascular network density was obtained using NeuronJ plugin on ImageJ software.
Noninvasive ultrasound assessment of cardiac, renal, and cerebral hemodynamics
Ultrasound examination was carried out under isoflurane anesthesia using an echocardiograph (Acuson S3000; Siemens, Erlangen, Germany) equipped with a 14 MHz linear transducer (14L5 SP). Mice were placed on a heating blanket (38 °C) to avoid hypothermia.
Cardiac parameters and cardiac output
A parasternal long-axis B-mode image was used to measure cardiac output using the mean of 3 successive measurements. Heart dimensions measured, in both systole and diastole, included left ventricular posterior wall thickness, left ventricular internal diameter, and interventricular septum thickness. Heart rates were obtained with an electrocardiogram.
Renal artery blood flow velocity
A pulsed Doppler sample gate was placed on the longitudinal axis of the right renal artery and the pulsed Doppler spectrum was recorded. Time-averaged mean blood flow velocity was calculated with correction of the angle between the long axis of each vessel and the Doppler beam.
Cerebral vasoreactivity
A horizontal B-mode image at the skull base allowed imaging of the basilar trunk and both internal carotid arteries. Color Doppler mode was activated and the basilar trunk with the circle of Willis was drawn and localized on the screen by their color-coded blood flow. A pulsed Doppler sample gate was placed on the longitudinal axis of the basilar trunk, and the pulsed Doppler spectrum was recorded by a masked operator. Vasoreactivity was quantified as the percentage increase in mean blood flow velocity after 5-minute inhalation of 16% O2/5% CO2/79% N2 compared with mean blood flow velocity on ambient air.40
Ex vivo myography
This is described in detail elsewhere.41 In brief, 2-mm segments of aorta and second-order mesenteric arteries were mounted in a multi-myography system (610 M; Danish Myo Technology, Denmark) containing a physiological salt solution aerated at 37 °C with 95% O2/5% CO2. In all studies, vessel viability was first confirmed by a contractile response on addition of 80 mM KCl, repeated 3 times. Vessel contractility was then assessed by cumulative concentration-response curves to phenylephrine (1 nM to 3 μM), noradrenalin (1 nM to 3 μM), and ET-1 (1 pM to 30 nM), and we selected a concentration that produced 80% maximum contraction for each individual vascular ring. To assess vasodilator capacity, vessels were preconstricted with phenylephrine and cumulative concentration curves to acetylcholine (1 nM to 3 μM) and sodium nitroprusside (1 nM to 3 μM) were obtained. At least 30-minute washout was allowed between drugs. Results are presented as percentage of a maximal response.
In vitro culture of peritoneal macrophages and bone marrow–derived neutrophils
Freshly isolated peritoneal macrophages were plated in 6-well plates at a density of 5 × 105 cells/3.8 cm2 in the Roswell Park Memorial Institute 1640 culture medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin (Life Technologies, 15140-122) and left to adhere for 24 hours. Cells were then incubated with lipopolysaccharide/interferon-γ (100 ng/ml/10 ng/ml, Sigma Aldrich) or IL4/IL13 (10 ng/ml for both, Invitrogen) for 24 hours. Bone marrow–derived neutrophils were isolated and cultured in vitro as previously described.42 Cells were then incubated with lipopolysaccharide (100 ng/ml, Sigma Aldrich) for 24 hours.
Cell culture supernatants were removed and frozen immediately at −80 °C until analysis. IL1β, IL6, and tumor necrosis factor-α were determined using an enzyme-linked immunosorbent assay (R&D Systems) according to the manufacturer’s instructions. Samples were analyzed in duplicate at 450 nm with wavelength correction at 570 nm (Synergy HT BioTEK). Concentrations were calculated from a standard curve using a second-order (quadratic) regression analysis. To assess phagocytic ability, macrophages were incubated with fluorescent beads (10:1 ratio) for 60 minutes. Cells were vigorously washed and detached from the plates before quantification of phagocytosis by flow cytometry.
Disclosure
All the authors declared no competing interests.
Acknowledgments
TEF is supported by a Clinical Research Training Fellowship from the Medical Research Council (MR/R017840/1). ND was supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/13/30/29994). P-LT was supported by the Institut National de Santé et de la Recherche Médicale and a research grant from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 107037. LG was supported by the Société Française d'Hypertension Artérielle (SFHTA) and Fondation de Recherche sur l'Hypertension Artérielle (FRHTA).
Author Contributions
P-LT and ND designed the study and provided funding; LG, AC, TEF, VB, PB, AC OL, FS, and CR performed the experiments and carried out analyses; DJW, DCK, MAB, and ND wrote the manuscript; and all authors provided approval for the final manuscript.
Footnotes
Supplemental Table S1. Differences between baseline and week 6 for macrophages from Edrnblox/lox and LysM-Cre Edrnblox/lox mice in terms of their ability to polarize to an inflammatory (M1) or anti-inflammatory (M2) phenotype, or their phagocytic ability.
Supplemental Methods.
Contributor Information
Pierre-Louis Tharaux, Email: pierre-louis.tharaux@inserm.fr.
Neeraj Dhaun, Email: bean.dhaun@ed.ac.uk.
Supplementary Material
References
- 1.Lim S.S., Vos T., Flaxman A.D. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney P.M., Whelton M., Reynolds K. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Poulter N.R., Prabhakaran D., Caulfield M. Hypertension. Lancet. 2015;386:801–812. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 4.Lerman L.O., Kurtz T.W., Touyz R.M. Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension. 2019;73:e87–e120. doi: 10.1161/HYP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galis Z.S., Thrasher T., Reid D.M. Investing in high blood pressure research: a national institutes of health perspective. Hypertension. 2013;61:757–761. doi: 10.1161/HYPERTENSIONAHA.111.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopalan S., Laursen J.B., Borthayre A. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Moreau P., d'Uscio L.V., Shaw S. Angiotensin II increases tissue endothelin and induces vascular hypertrophy. Reversal by ETA-receptor antagonist. Circulation. 1997;96:1593–1597. doi: 10.1161/01.cir.96.5.1593. [DOI] [PubMed] [Google Scholar]
- 8.Heerspink H.J.L., Parving H.H., Andress D.L. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02603809?term=act-132577&rank=6 Available at: Accessed September 30, 2020.
- 10.Dhaun N., Webb D.J. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol. 2019;16:491–502. doi: 10.1038/s41569-019-0176-3. [DOI] [PubMed] [Google Scholar]
- 11.Dhaun N., Goddard J., Webb D.J. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17:943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- 12.Dhaun N., Pollock D.M., Goddard J., Webb D.J. Selective and mixed endothelin receptor antagonism in cardiovascular disease. Trends Pharmacol Sci. 2007;28:573–579. doi: 10.1016/j.tips.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Czopek A., Moorhouse R., Guyonnet L. A novel role for myeloid endothelin-B receptors in hypertension. Eur Heart J. 2019;40:768–784. doi: 10.1093/eurheartj/ehy881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machnik A., Neuhofer W., Jantsch J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nature Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel P., Knorr M., Kossmann S. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 16.Crowley S.D., Song Y.S., Sprung G. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickard A.J., Morgan J., Tesch G. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel P. Monocytes as immune targets in arterial hypertension. Br J Pharmacol. 2018;176:1966–1977. doi: 10.1111/bph.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter R.W., Craigie E., Homer N.Z. Acute inhibition of NCC does not activate distal electrogenic Na+ reabsorption or kaliuresis. Am J Physiol. 2014;306:F457–F467. doi: 10.1152/ajprenal.00339.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry M.R., Mathews R.J., Ferdinand J.R. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell. 2017;170:860–874.e19. doi: 10.1016/j.cell.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Stewart B.J., Ferdinand J.R., Young M.D. Spatiotemporal immune zonation of the human kidney. Science. 2019;365:1461–1466. doi: 10.1126/science.aat5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barhoumi T., Kasal D.A., Li M.W. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J.D., Patel M.B., Song Y.S. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boesen E.I., Krishnan K.R., Pollock J.S., Pollock D.M. ETA activation mediates angiotensin II-induced infiltration of renal cortical T cells. J Am Soc Nephrol. 2011;22:2187–2192. doi: 10.1681/ASN.2010020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasser J.M., Sullivan J.C., Hobbs J.L. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18:143–154. doi: 10.1681/ASN.2006030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn A.W., Jonas U., Buhler F.R., Resink T.J. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347:178–180. doi: 10.1016/0014-5793(94)00531-1. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S., Yancey P.G., Zuo Y. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2856–2864. doi: 10.1161/ATVBAHA.111.237198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L.J., Corsa B.A., Zhou J. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am J Physiol. 2011;300:F1203–F1213. doi: 10.1152/ajprenal.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher E.L., Phipps J.A., Ward M.M. The renin-angiotensin system in retinal health and disease: its influence on neurons, glia and the vasculature. Progr Retin Eye Res. 2010;29:284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Deliyanti D., Miller A.G., Tan G. Neovascularization is attenuated with aldosterone synthase inhibition in rats with retinopathy. Hypertension. 2012;59:607–613. doi: 10.1161/HYPERTENSIONAHA.111.188136. [DOI] [PubMed] [Google Scholar]
- 31.Chou J.C., Rollins S.D., Ye M. Endothelin receptor-A antagonist attenuates retinal vascular and neuroretinal pathology in diabetic mice. Invest Ophthalmol Vis Sci. 2014;55:2516–2525. doi: 10.1167/iovs.13-13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenzel P., Rossmann H., Muller C. Heme oxygenase-1 suppresses a pro-inflammatory phenotype in monocytes and determines endothelial function and arterial hypertension in mice and humans. Eur Heart J. 2015;36:3437–3446. doi: 10.1093/eurheartj/ehv544. [DOI] [PubMed] [Google Scholar]
- 33.Gordon P., Okai B., Hoare J.I. SOCS3 is a modulator of human macrophage phagocytosis. J Leuk Biol. 2016;100:771–780. doi: 10.1189/jlb.3A1215-554RR. [DOI] [PubMed] [Google Scholar]
- 34.Kapoor S. Interleukin-6 antagonists for the management of hypertension. Hypertension. 2007;49:e18. doi: 10.1161/01.HYP.0000257805.64783.d9. author reply e9. [DOI] [PubMed] [Google Scholar]
- 35.Tissot A.C., Maurer P., Nussberger J. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 36.Chernykh E.R., Shevela E.Y., Starostina N.M. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transplant. 2016;25:1461–1471. doi: 10.3727/096368915X690279. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03337165?term=macrophage+cell+therapy&draw=10&rank=52 Available at: Accessed September 30, 2020.
- 38.Chen T., Cao Q., Wang Y., Harris D.C.H. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int. 2019;95:760–773. doi: 10.1016/j.kint.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Davenport P., Hyndman K., Dhaun N. Endothelin. Pharmacol Rev. 2016;68:357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poittevin M., Bonnin P., Pimpie C. Diabetic microangiopathy: impact of impaired cerebral vasoreactivity and delayed angiogenesis after permanent middle cerebral artery occlusion on stroke damage and cerebral repair in mice. Diabetes. 2015;64:999–1010. doi: 10.2337/db14-0759. [DOI] [PubMed] [Google Scholar]
- 41.Miller M.R., Borthwick S.J., Shaw C.A. Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ Health Perspec. 2009;117:611–616. doi: 10.1289/ehp.0800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozman S., Yousefi S., Oberson K. The generation of neutrophils in the bone marrow is controlled by autophagy. Cell Death Differ. 2015;22:445–456. doi: 10.1038/cdd.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.