Abstract
Islet autotransplantation (IAT) is used to preserve as much insulin-secretory capacity as possible in patients undergoing total pancreatectomy for painful chronic pancreatitis. The enzyme used to dissociate the pancreas is a critical determinant of islet yield, which is correlated with posttransplant function. Here, we present our experience with IAT procedures to compare islet product data using the new enzyme SERVA/Nordmark (SN group; n = 46) with the standard enzyme Liberase-HI (LH group; n = 40). Total islet yields (mean ± standard deviation; 216 417 ± 79 278 islet equivalent [IEQ] in the LH group; 227 958 ± 58 544 IEQ in the SN group; p = 0.67) were similar. However, the percentage of embedded islets is higher in the SN group compared to the LH group. Significant differences were found in pancreas digestion time, dilution time, and digested pancreas weight between the two groups. Multivariate linear regression analysis showed the two groups differed in portal venous pressure changes. The incidence of graft function and insulin independence was not different between the two groups. The SN and LH enzymes are associated with similar outcomes for IAT. Further optimization of the collagenase/neutral protease ratio is necessary to reduce the number of embedded islets obtained when using the SN enzyme.
Keywords: Collagenase, islet autotransplantation, islet isolation, islet transplantation
Introduction
Chronic pancreatitis is an inflammatory disease characterized by progressive damage to the pancreas and persistent abdominal pain. Pancreatic duct drainage procedures, currently usually done endoscopically, or partial resection, can relieve the pain in some patients (1), but when not able to do so, total pancreatectomy (TP) is an option to remove the root cause. The main drawback of TP is the occurrence of surgical diabetes. Intraportal islet autotransplantation (IAT) at the time of TP first done in 1977 (2), was developed to preserve as much beta cell mass and insulin-secretory capacity as possible to prevent or minimize the otherwise inevitable postpancreatectomy endocrine deficiency (3). TP-IAT is increasingly being used to treat patients with chronic pancreatitis, but because of the variability of islet yield from case to case they must still accept becoming diabetic for the relief of pain (4).
The probability of achieving insulin independence after IAT correlates significantly with the islet cell mass that is transplanted (5–8). In our series of TP-IAT cases from 1977 to 2007, if >5000 islet equivalent/kilogram (IEQ/kg) were transplanted, the probability of insulin independence at 1 year was 63%; if 2500–5000 IEQ/kg were transplanted, it was 23% and if <2500 IEQ/kg, it was only 7% (8). Thus, the key to increasing the success rate with IAT is improving islet yield.
Although human islet isolation techniques have been constantly improving, it is still a challenge to produce consistently high islet yields (9–11). The source of isolation enzyme appears to be a critical factor affecting islet yield and therefore may significantly affect the clinical outcome of IAT (12,13). The introduction of Liberase-HI (LH), a highly purified enzyme blend with low endotoxin burden, has improved the consistency of islet yield and quality of islets (14). In spite of significant interlot variability (15) and deleterious effects after islet isolation (16), LH was the choice of enzyme and has been widely used for human clinical islet isolation.
In April 2007, it was revealed that the LH manufacturing process utilizes bovine brain-derived raw material. This caused serious concern because bovine brain-derived materials have a potential to transmit prions causing transmissible spongiform encephalopathies (TSEs). In response to this situation, the SERVA/Nordmark (SN) Collagenase NB1 and Neutral protease NB blend was chosen as an alternate source of isolation enzyme to eliminate the risk of TSEs transmission. The SERVA/Nordmark enzyme has several other advantages compared to LH (17). It is available as a GMP-grade reagent, has lower endotoxin levels and has separated vials of collagenase and neutral protease. However, the effectiveness of the SN enzyme for human islet isolation from chronic pancreatitis specimens for subsequent IAT (i.e. autologous islet isolation) has not been established.
In this study, we have successfully utilized SN Collagenase NB1 plus Neutral protease NB blend for autologous islet isolation and compared the results with LH. Islet isolation results, portal venous pressure changes and graft survival were compared between LH and SN groups.
Materials and Methods
Patients
Between January 2006 and April 2008, 87 patients with chronic pancreatitis underwent a pancreas resection and subsequent IAT at the University of Minnesota Medical Center. All patients gave written informed consent before pancreas resection. The diagnosis of chronic pancreatitis was based on the results of the patient’s medical history and imaging studies of the pancreas. For all patients, the indication for pancreas resection was intractable pain, regardless of whether the gross morphologic changes detected in the pancreas were minimal or severe.
The autologous islet isolation data of 87 consecutive cases were retrospectively reviewed. One case was excluded from the analysis because of the use of SERVA premium-grade enzyme. Graft function data based on insulin requirements with sufficient follow-up for analysis were available in 68 consecutive cases between January 2006 and December 2007. Nine patients were excluded from graft function analysis because the pancreatectomy was partial (n = 6) or because laboratory test reports were not available during follow-up (n = 3). Patients were divided into two groups based on the type of enzyme used to dissociate the pancreas: LH group, from January 2006 to March 2007, n = 40 for islet isolation analysis, n = 33 graft function analysis; SN group, from April 2007 to April 2008, n = 46 for islet isolation analysis, n = 26 for graft function analysis.
Operative procedures (pancreatectomy)
The technique of TP involved removal of the pancreas with a segment of the duodenum while preserving the pylorus. Reconstruction after resection was by duodenoduodenostomy or duodenojejunostomy and choledochoenterostomy just proximal or distal to the enteroenterostomy. Blood supply to the pancreas was preserved as long as possible to minimize the warm ischemia time for islets; thus, ligation and division of the splenic artery at its origin and the splenic vein at its termination were the last steps in the pancreatectomy. Thus, warm ischemia as usually less than a minute. After resection, the pancreas was immediately immersed in the cold modified University of Wisconsin (UW) preservation solution (gluconic acid instead of lactonic acid and high sodium/low potassium rather than the reverse) without arterial flush, and the duodenum, excess fat and connective tissue were dissected off of the gland. The pancreas was divided at the surgical neck and for each segment (proximal, head and uncinate process; distal, body and tail) the pancreatic duct was cannulated for subsequent collagenase infusion. The pancreas was then transported to the islet-processing laboratory in modified UW solution. Cold ischemia time prior to beginning the islet isolation process was typically <1 h.
Enzyme preparation
In the LH group, one vial of Liberase-HI or one vial of Liberase Collagenase Blend supplemented with one vial of Liberase Thermolysin (Roche Molecular Biochemicals, Indianapolis, IN), which were stored at −20°C prior use, was used for each pancreas digestion.
In the SN group, we used SERVA/Nordmark enzyme (GMP grade, SERVA Electrophoresis GmbH, Heidelberg, Germany) which was stored at 4°C prior use and examined three different preparations to achieve high islet yield. One vial of Collagenase-NB1 supplemented with one vial of Neutral Protease-NB was used in the first 26 isolations. In two isolations, 1600 units of Collagenase NB1 supplemented with 150 units (dimethylcasein [DMC] assay, unrelated to caseinase assay for Liberase Thermolysin) of Neutral protease NB were used. In the most recent isolations (n = 18), 1600 units of Collagenase NB1 supplemented with 100 units of Neutral protease NB were used. Enzyme profiles provided by the manufacturer are shown in Table 1 for each lot used in both groups.
Table 1:
Enzyme profiles
| Collagenase lot no. | Neutral protease lot no. | # Isolation | Wunsch (LH) or PZ1 (SN) units | Caseinase (LH) or DMC2(SN) units | |
|---|---|---|---|---|---|
| LH group | |||||
| A | 93 201 820 | – | 4 | 2440 | 71 314 |
| B | 93 246 220 | – | 2 | 2195 | 64 336 |
| C | 93 431 220 | – | 2 | 2159 | 75 497 |
| D | 93 471 520 | – | 29 | 2345 | 58 673 |
| E | 98 404 0203 | 93 403 9204 | 3 | 2203 | 71 652 |
| SN group | |||||
| F | 20 080 005 | 20 030 105 | 4 | 2348 | 250 |
| G | 20 120 005 | 20 030 205 | 3 | 2332 | 250 |
| H | 20 130 005 | 20 030 205 | 1 | 2443 | 250 |
| I | 20 130 005 | 20 130 005 | 1 | 2443 | 184 |
| J | 20 150 005 | 20 030 205 | 1 | 2709 | 250 |
| K | 20 150 005 | 20 130 005 | 4 | 2709 | 184 |
| L | 20 170 005 | 20 130 105 | 12 | 2597 | 184 |
| M | 20 150 005 | 20 130 105 | 1 | 1600 | 150 |
| N | 20 150 005 | 20 180 005 | 1 | 1600 | 150 |
| O | 20 080 005 | 20 130 105 | 1 | 1600 | 100 |
| P | 20 130 005 | 20 130 105 | 1 | 1600 | 100 |
| Q | 20 150 005 | 20 180 005 | 2 | 1600 | 100 |
| R | 20 170 005 | 20 130 105 | 13 | 1600 | 100 |
| S | 20 170 005 | 20 180 005 | 1 | 1600 | 100 |
LH = Liberase-HI; SN = SERVA/Nordmark.
PZ = 4-phenylazobenzyloxycarbonyl-l-prolyl- l-leucylglycyl-l-prolyl-l-arginine. PZ units, identical to Wunsch units.
DMC = dimethylcasein. DMC units, unrelated to caseinase units.
Liberase collagenase blend.
Liberase thermolysin.
Islet isolation
Islet isolations were done in the University of Minnesota Molecular and Cellular Therapeutics GMP Facility in compliance with federal regulations. The islet isolation method has been reported elsewhere (18). Briefly, the pancreas was distended with the cold enzyme solution through the pancreatic duct using a pressure-controlled pump system(19). The distended pancreas was digested using the semiautomated method (20). The switch from digestion phase to dilution phase occurred when most of the islets were free from exocrine tissue. The digested tissue was purified by continuous iodixanol (OptiPrep, Axis-Shield, Oslo, Norway) density gradient (18) on a COBE 2991 cell separator (21) when the pellet volume exceeded 20 mL. In some cases, when the recovered pellet volume measured 15–20 mL, purification was still done after consultation with the surgeon. The islet yield and purity were assessed by counting duplicate aliquots stained with diphenylthiocarbazone (Sigma, St. Louis, MO) (22). In some instances, many islets were found in the COBE bag fraction after purification. During such purification failure we also collected tissue from this fraction and transplanted this highly impure preparation as long as portal pressures remained low. The liberated islets were suspended in the Connaught Medical Research Laboratories-1066 medium (Mediatech, Inc, Manassas, VA) supplemented with 25 mM HEPES, antibiotics and 2.5% human serum albumin (American Red Cross, Washington, DC). The islets were collected in a transplant bag for delivery to the operative room.
Islet viability assay
Islet viability after isolation was assessed by simultaneous use of two fluorescent dyes, fluorescein diacetate (Sigma, St. Louis, MO) and propidium iodide (Sigma), as described previously (23). Briefly, 100–200 islets were stained with 0.67 μM fluorescein diacetate and 4.0 μM propidium iodide in phosphate-buffered saline for 30 min. Quadruplicate sets of 25–50 islets in 100 μL phosphate-buffered saline were taken into four wells of a black 96-well plate (Dynatech Microfluor B, Naperville, IL). The intensity of green fluorescence from live cells and of red fluorescence from dead cells was determined by a FL 600 fluorescence microplate reader (BioTek Instruments, Inc, Winooski,VT,USA), using the 485/530 and 530/645 excitation/emission dual-filter setting. Islet viability was expressed as the percentage of live cells in islets.
Islet autotransplantation procedure and postoperative care
Patients who received intraportal transplants received 70 U/kg of heparin intravenously to prevent propagation of clots around the islet tissue. Portal venous pressure was measured before transplant by direct saline manometry. The islet suspension was infused into the portal vein circulation over 15–60 min. Portal venous pressure was measured periodically during the infusion. If portal pressure elevated by more than 25–30 cm H2O, intraportal infusion of islets was stopped. In three cases in the SN group, the remaining islets were transplanted into the intraperitoneal cavity due to elevated portal pressure, and two were included in graft function analysis.
After infusion of the islet suspension, the patient was placed on an intravenous exogenous insulin drip to maintain blood glucose levels between 80 and 140 mg/dL. Total parenteral nutrition or enteral nutrition via feeding jejunostomy was administered until the patient tolerated oral nutrition. Insulin infusion was continued during immediate postoperative period to minimize glucose-stimulated insulin secretion from the transplanted islets. Once the patient began eating, a transition was made to subcutaneous insulin with the adjusted dose or insulin free.
Assessment of short-term graft function after transplantation
Follow-up information was obtained by retrospective review of the patient’s medical record from the University of Minnesota Medical Center. For data analysis, short-term (within 1 year after transplant) islet graft function was stratified as full for patients who were insulin independent, partial for those requiring insulin only intermittently or requiring long-acting insulin once daily, or graft failure for those on a basal-bolus (typical diabetic) insulin regimen. The time of pancreatic islet graft loss was determined by return to a basal-bolus insulin regimen after full or partial graft function.
Statistical analyses
Data are expressed as means ± standard deviation (SD). Statistical comparisons between the groups were performed with the two-sample t-test or Mann-Whitney U-test, as appropriate, for comparison of the two groups. Comparison of categorical variables was performed with a chi-square test or the Fisher exact test, as appropriate. Correlation was expressed as the Pearson correlation coefficient. Multivariate linear regression analysis using stepwise elimination procedure was used to identify significant independent predictors of the change in portal venous pressure during IAT Insulin independence and graft survival were calculated with the Kaplan-Meier method, and the statistical significance of differences was determined by the Mantel-Cox log-rank test. All statistical calculations were performed using Statistical Product and Services Solutions, version 11.0 (SPSS Inc, Chicago, IL). Values for p less than 0.05 were considered statistically significant.
Results
Table 2 summarizes the patient (donor) characteristics. Patients in the LH group were significantly older than patients in the SN group (p = 0.04). There were marked variabilities in pancreas weight and degree of fibrosis in both enzyme groups; however, when we compared the variability of pancreas characteristics between groups, they were not significant. All other characteristics between groups were similar.
Table 2:
Patient characteristics
| LH | SN | p-Value | |
|---|---|---|---|
| No. of patients | 40 | 46 | |
| Age (years) | 39.6 ± 11.8 | 33.5 ± 14.4 | 0.04 |
| Sex (male/female) | 14/26 | 10/36 | 0.17 |
| Body weight (kg) | 69.8 ± 18.2 | 66.4 ± 23.0 | 0.46 |
| BMI (kg/m2) | 24.7 ± 6.2 | 24.5 ± 5.7 | 0.83 |
| Cause of pancreatitis | 0.35 | ||
| Idiopathic | 11 | 19 | |
| Alcoholic | 1 | 2 | |
| Divisum | 1 | 3 | |
| Others | 27 | 22 | |
| Previous operations | 0.66 | ||
| Whipple | 6 | 4 | |
| Distal pancreatectomy | 2 | 4 | |
| Surgical drainage | 0 | 2 | |
| Others | 1 | 1 | |
| None | 31 | 35 | |
| Pancreas weight (g) | 63.8 ± 28.6 | 64.7 ± 40.1 | 0.67 |
| Severity of fibrosis | 0.64 | ||
| Severe | 19 | 19 | |
| Moderate | 14 | 21 | |
| Normal | 7 | 6 | |
| Section | 0.21 | ||
| Total | 31 | 31 | |
| Head | 0 | 4 | |
| Body and tail | 9 | 11 |
LH = Liberase-HI; SN = SERVA/Nordmark; BMI = body mass index. Values are means ± SD.
Comparison of islet isolation-related variables
Table 3 summarizes the islet isolation data. Pancreatic digestion time was longer in the SN group than it was in the LH group, whereas dilution time was shorter with SN than with LH. The degree of pancreas digestion by weight was significantly higher in the SN group compared with the LH group. Despite these differences, postdigest tissue volumes were similar in both groups. The number of purifications performed and postdigest islet yields were also similar in both groups. Interestingly, fewer islets were embedded in the LH group than in the SN group. In the SN group, we found significant differences in patient age, between cases of more than 50% islets embedded and cases of less than 50% islets embedded (age 27.3 ± 15.1 vs. 38.2 ± 12.2; p < 0.05). There were no statistically significant differences in islet size distribution (Table 4) and fluorescein diacetate/propidium iodide viability staining between groups. Figure 1 shows postdigest islet yield and embedded islet percentage in five different lots of LH and 14 different combinations of SN enzyme. In the SN group, there were no significant differences among different ratios of neutral protease to collagenase activity in terms of islet yields and embedded islet percentage (data not shown). Likewise, there was no strong correlation between this ratio and islet yield in cases of fibrotic pancreata (data not shown). All autologous islet products examined in this review were released for IAT
Table 3:
Human autograft islet isolation results
| LH | SN | p-Value | |
|---|---|---|---|
| Digestion time (min) | 18.4 ± 4.3 | 22.5 ± 4.3 | 0.0001 |
| Dilution time (min) | 42.5 ± 8.5 | 30.6 ± 7.2 | 0.0001 |
| Percent of digest pancreas weight (%) | 75.6 ± 14.2 | 81.5 ± 11.3 | 0.05 |
| Postdigest IEQ (103) | 222.1 ± 139.1 | 230.4 ± 122.5 | 0.77 |
| Postdigest IEQ/g pancreas | 3642±2330 | 3980± 1924 | 0.46 |
| Postdigest IEQ/islet number | 1.020 ± 0.320 | 0.915 ± 0.291 | 0.07 |
| Postdigested tissue volume (mL) | 13.2 ± 9.9 | 16.6 ± 13.3 | 0.31 |
| No. of purification | 11 (27.5%) | 15 (32.6%) | 0.64 |
| Postpurification IEQ (103) | 216.4 ± 79.3 | 228.0 ± 58.5 | 0.67 |
| Postpurification IEQ/g pancreas (103) | 2566 ± 919 | 2414 ± 1021 | 0.70 |
| Percent recovery (%) | 72.8 ± 25.0 | 72.9 ± 18.7 | 0.99 |
| Postpurification purity (%) | 33.8 ± 20.2 | 34.0 ± 21.5 | 0.87 |
| Postpurification IEQ/islet number | 1.216 ± 0.271 | 1.034 ± 0.219 | 0.10 |
| Embedded islet (%) | 26.8 ± 25.6 | 45.8 ± 26.6 | 0.001 |
| Islet viability (FDA/PI; %) | 91.4 ± 9.8 | 94.1 ± 4.8 | 0.07 |
| Final product volume (mL)/g pancreas | |||
| Digest | 0.20 ± 0.11 | 0.23 ± 0.12 | 0.26 |
| Purified | 0.21 ± 0.09 | 0.25 ± 0.13 | 0.46 |
| Transplanted IEQ/kg body weight | 3142±2191 | 3241 ± 1637 | 0.81 |
LH = Liberase-HI; SN = SERVA/Nordmark; IEQ: islet equivalent. Values are means ± SD.
Percent of digest pancreas weight: (processed pancreas weight − undigested pancreas weight)/(processed pancreas weight) × 100.
Percent recovery: postpurification IEQ/postdigest IEQ × 100. FDA = fluorescein diacetate; PI = propidium iodide.
Table 4:
Islet size distribution (% of category size/total islets)
| μm | 50–100 | 100–150 | 150–200 | 200–250 | 250—300 | 300–350 |
|---|---|---|---|---|---|---|
| LH | 48.3 ± 13.3 | 26.9 ± 6.5 | 14.1 ± 5.7 | 7.5 ± 3.6 | 2.4 ± 1.9 | 0.8 ± 1.1 |
| SN | 53.5 ± 11.7 | 25.0 ± 6.1 | 12.3 ± 4.1 | 6.3 ± 2.9 | 2.2 ± 1.3 | 0.8 ± 0.9 |
| p-value | 0.06 | 0.16 | 0.10 | 0.10 | 0.42 | 0.78 |
LH = Liberase-HI; SN: SERVA/Nordmark. Values are mean ± SD. All p-values were obtained using two-sample t-test.
Figure 1: Islet yield and embadded islet percentage in each enzyme lot.
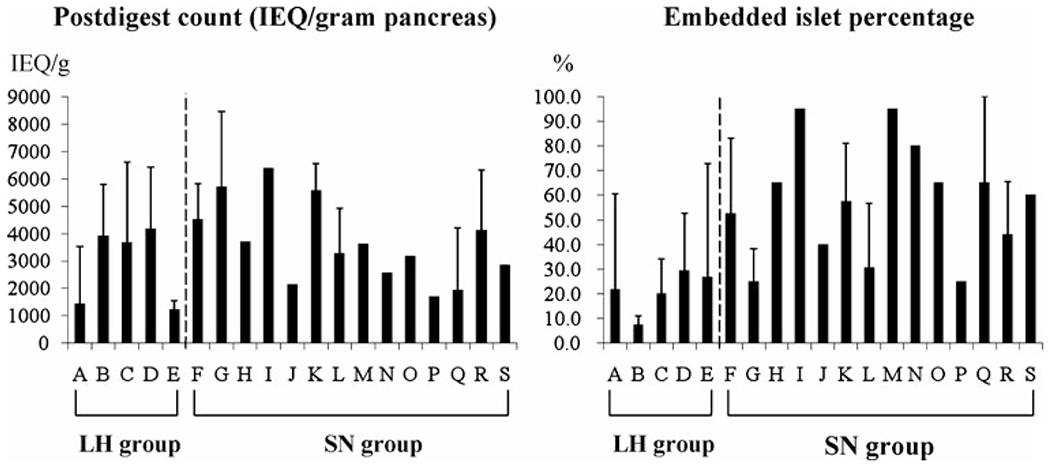
Postdigest islet count (expressed as IEQ/gram pancreas) and embedded islet percentage in five different lots of LH and 14 different combinations of SN enzyme. Islet counts were similar in both the LH (A–E) and SN (F–S) groups at the postdigest level, but a significant difference in embedded islets percentage was observed in the SN group (LH = Liberase-HI; SN = SERVA/Nordmark; IEQ = islet equivalent).
Multivariate linear regression analysis for the change in portal pressure during IAT
The change in portal pressure during IAT was monitored in 39 patients in the LH group and 46 patients in the SN group. Portal pressure was positively correlated with the amount of transplanted tissue by volume (mL) and by IEQ per kg of patient’s body weight (Figure 2). Stepwise multivariate linear regression analysis was performed to assess the effect of IAT on portal venous pressure. The factors considered were transplanted tissue volume, patient’s height, patient’s body weight, body surface area, transplanted IEQ, transplanted islet number, LH or SN group, whether purification was performed or not, and transplanted embedded islet number. Multivariate linear regression analysis revealed that transplanted tissue volume, body surface area and transplanted embedded islet number significantly predicted the change in portal venous pressure during IAT Other factors were not significant. On the basis of the difference of embedded islets in the LH and SN groups, multivariate linear regression analysis was conducted separately for the two groups. In the LH group, transplanted tissue volume was the only predictor. In contrast, the change in portal venous pressure in the SN group was predicted by transplanted tissue volume, body surface area and transplanted embedded islet number (Table 5).
Figure 2: Change in portal venous pressure after IAT.
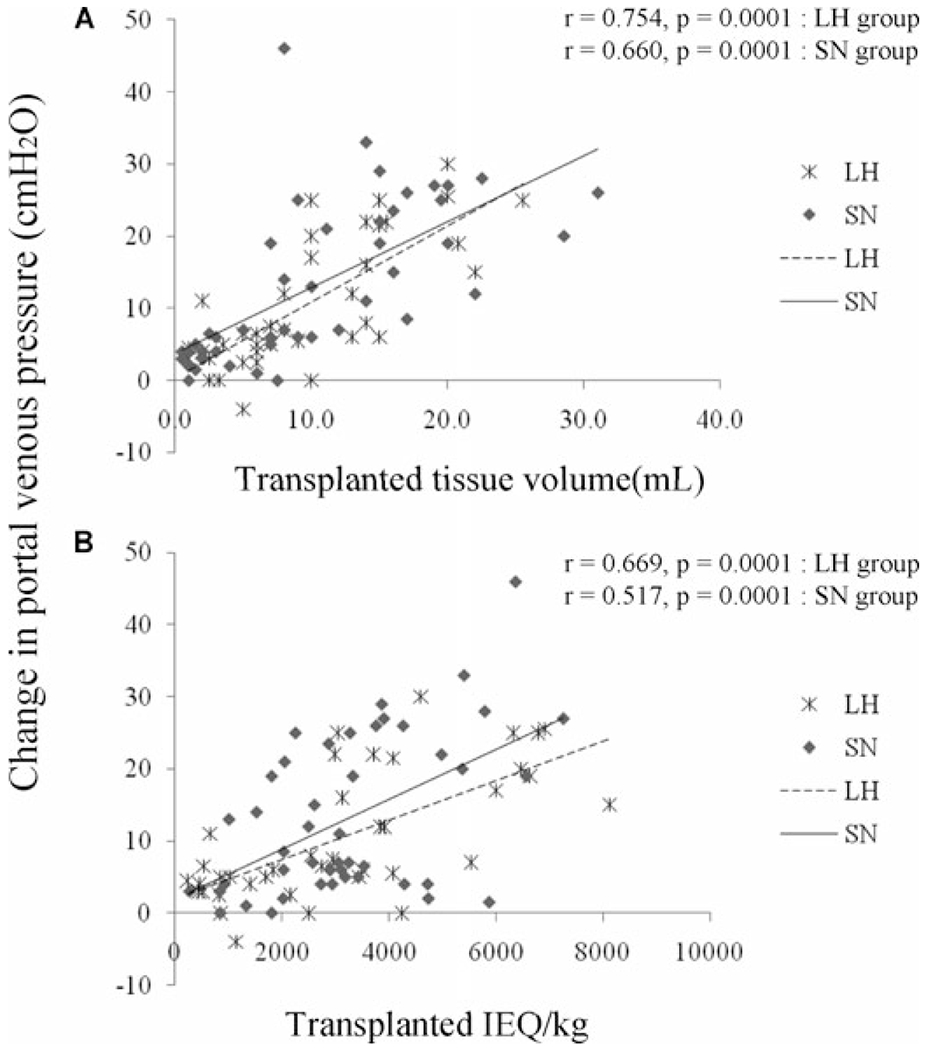
Correlation between the change in portal venous pressure at each transplant and transplanted tissue volume or transplanted IEQ/patient body weight. Statistical analyses are presented in Table 4; IEQ = islet equivalent.
Table 5:
Predictors of portal vein pressure after IAT by stepwise multivariate linear regression
| B | SE | Beta | p-Value | |
|---|---|---|---|---|
| All | ||||
| Transplanted tissue volume | 0.870 | 0.125 | 0.619 | 0.0001 |
| BSA | −8.156 | 2.267 | −0.265 | 0.001 |
| Transplanted embedded islet number | 2.689E – 05 | 0.000 | 0.208 | 0.02 |
| Constant | 15.299 | 4.005 | ||
| LH group | ||||
| Transplanted tissue volume | 1.055 | 0.154 | 0.753 | 0.0001 |
| Constant | 0.287 | 1.793 | ||
| SN group | ||||
| Transplanted tissue volume | 0.825 | 0.182 | 0.593 | 0.0001 |
| BSA | −8.715 | 3.147 | −0.295 | 0.008 |
| Transplanted embedded islet number | 3.536E – 05 | 0.000 | 0.269 | 0.038 |
| Constant | 15.925 | 5.330 |
All: R2 = 0.566, p < 0.0001. LH: R2 = 0.555, p < 0.0001. SN: R2 = 0.541, p < 0.0001.
BSA = body surface area; LH = Liberase-HI; SN = SERVA/Nordmark; SE = standard error.
Metabolic outcome according to islet yield and the probability of graft function
Follow-up data on insulin requirements existed for 59 patients at 1 year or less after IAT. In the LH group, 5 patients (15%) were insulin independent, 21 patients (64%) had partial function and 7 patients (21%) had graft failure. In the SN group, 6 patients (23%) were insulin independent, 19 patients (73%) had partial function and 1 patient (4%) had graft failure. In both groups, the mean islet yield was highest in patients with full graft function and lowest in those patients with graft failure, but there was a considerable overlap (Figure 3). The average IEQ/kg to induce insulin independence in the SN groups was significantly lower than it was in the LN group, although the average IEQ/kg to keep partial graft function was similar in both groups (p = 0.78). In the LH group, if >5000 IEQ/kg were transplanted, the probability of graft function (full and partial combined) was 100%; if 2500–5000 IEQ/kg were transplanted, it was 93% and if <2500 IEQ/kg, it was 50%. The corresponding insulin independence rates were 57%, 7% and 0%, respectively. In the SN group, if >5000 IEQ/kg were transplanted, the probability of graft function was 100%; if 2500–5000 IEQ/kg, it was100% and if <2500 IEQ/kg, it was 89%. The corresponding insulin independence rates were 40%, 25% and 11%, respectively.
Figure 3: Short-term metabolic outcome according to islet yield in the LH (n = 33) and SN groups (n = 26).
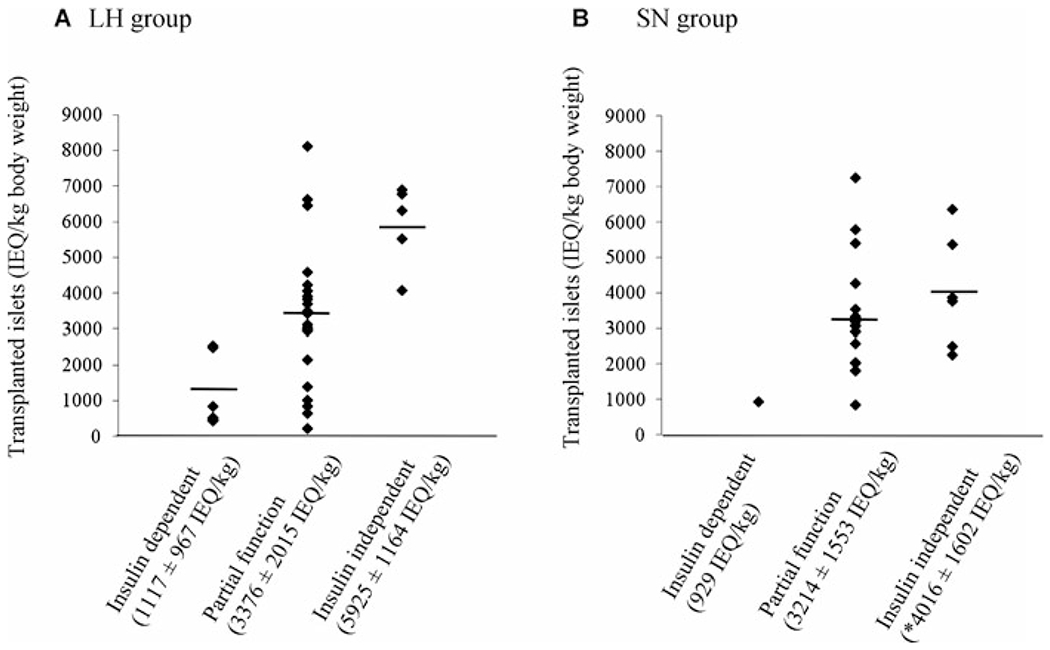
The mean islet yield (expressed as IEQ/kg ± SD) was highest in full graft function and lowest in graft failure in both groups, but there was a considerable overlap. *p < 0.05 versus insulin independent in the LH group; LH = Liberase-HI; SN = SERVA/Nordmark; IEQ = islet equivalent.
The probabilities of insulin independence or graft function in both groups were analyzed by Kaplan-Meier methods (Figure 4). Follow-up for patients in the LH group ranged from 46 days to 390 days after IAT (mean, 283 ± 99 days) and in the SN group from 9 days to 229 days after IAT (mean, 91 ± 62 days). There was no significant difference between groups with regard to the probability of insulin independence or graft function.
Figure 4: Insulin independent rate and graft function rate.
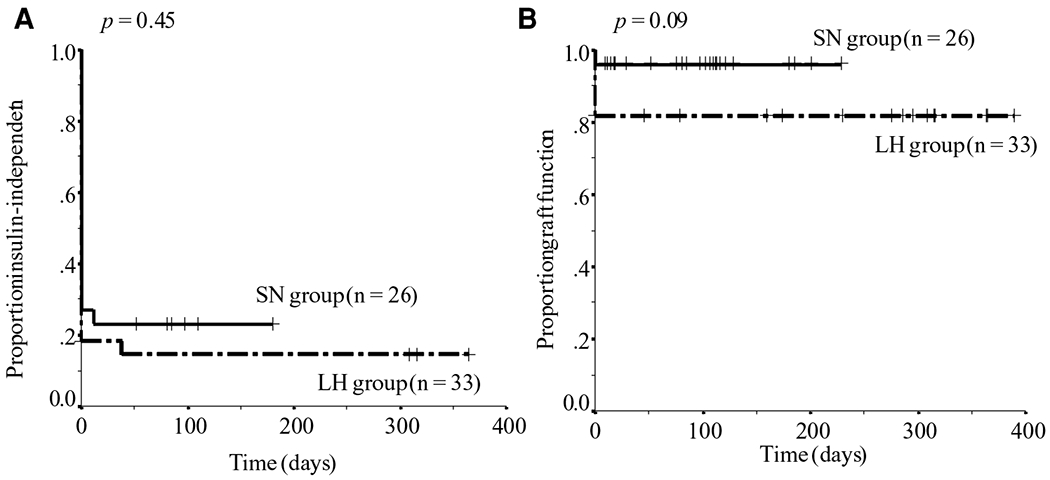
The probability of insulin independence and graft function after IAT in the LH and SN groups by the Kaplan–Meier method. All p-values were obtained using the Mantel–Cox log-rank test; IAT = islet autotransplantation; LH = Liberase-HI; SN = SERVA/Nordmark.
Discussion
We have successfully utilized the new enzyme SN for autologous islet isolation and reported the islet isolation experience and transplant outcome compared with LH. The enzyme Liberase-HI has been widely used for both human allograft and autograft islet isolations (12,14,24). However, currently this enzyme cannot be used for clinical islet isolations because of the potential TSEs risk. Since April 2007, we have been using the SN Collagenase-NB1 plus Neutral protease-NB blend (GMP grade) as an alternative enzyme for clinical autograft islet isolation. There are some reports of islet allograft isolation using these enzymes (17,25); however, the efficacy of these enzymes for autograft islet isolation has not yet been reported. This retrospective analysis on autologous islet isolations at a single center shows comparative islet yields and viability for both LH and SN enzymes. The large number of embedded islets obtained when the SN blend was used did not lead to higher tissue volume, more frequent need for purification, or lower recovery after purification. More noteworthy is that engraftment, in terms of insulin independence and graft function, was comparable to LH. These results hold promise that the SN Collagenase-NB1 plus Neutral Protease-NB blend will work as efficiently for autograft islet isolation as LH.
It has been suggested that the combination of Collagenase-NB1 plus Neutral protease-NB blend has several potential advantages. One advantage is that separate storage of individual enzyme components improves the stability of each enzyme’s activity (12). Additionally, the neutral protease to collagenase activity ratio, which is critical for successful islet isolation, can be adjusted depending on the optimal ratio (26). This allows in-process adjustment to the optimal enzymatic activity ratio for the individual characteristics of each pancreas; however, this ratio for human islet isolation has yet to be determined. Bucher and associates suggested that optimization of neutral protease to collagenase activity ratio would be critical for autograft islet isolation, where fibrous pancreata could potentially benefit from a higher proportion of neutral protease (17). We compared three different ratios of neutral protease to collagenase activity to determine the optimal ratio for autograft islet isolation; however, we could not determine the optimal ratio since there were no differences among all ratios in terms of islet yields.
Significant differences were found in pancreas digestion time, dilution time and percent of digested pancreas weight between the two groups and could be attributed to the higher proportion of embedded islets in the SN group. Digestion time is extended during the isolation procedure when many embedded islets are detected in a sample during the digestion phase. This long digestion time results in a shortened dilution time and a higher percentage of digested pancreas weight. There were more small islets in the SN group, which might be indicative of islet fragmentation. This could be attributed to the longer digestion time which potentially causes damage to the tissue. Several factors have been associated with the proportion of embedded islets occurring during isolation, including donor age (27,28), donor BMI (23), islet isolation technique (28) and type of enzyme used for pancreas dissociation (13,26). Although the mean patient age was in fact lower in the SN group, our comparison suggests that the enzyme characteristics also contributed to an increase in the number of embedded islets. Since purification declines with increasing numbers of embedded islets, the final yield is affected by the enzyme used, at least with the currently suggested ratio of Collagenase-NB to Neutral protease-NB in the SN blend. To decrease the proportion of embedded islets, optimization of enzyme activity is needed, in particular, adjustment of the amount of neutral protease (26). For example, higher neutral protease with lower collagenase ratio for younger patient may be necessary, since there were more embedded islets when pancreases from younger patient were processed.
Our results suggest that LH and the SN enzyme blend are equally effective from the perspective of posttransplant islet function. This is the first report to assess engraftment of human autologous islets obtained using the SN enzyme blend. High graft function rate has been achieved in both groups; however, the current insulin-independent rate was lower than what we achieved in the past (8) because of our recipient diabetic management. Currently, we start insulin therapy for patients who have minimally elevated blood glucose level. In the past, we did not put mildly hyperglycemic patients on insulin. Graft function of these patients should be considered as full function category. As described previously (3,8), the ability to achieve insulin independence after IAT correlates with the islet yield transplanted. No patient who received less than 2000 IEQ/kg became insulin independent in either group. In both groups, patients who received higher islet yields tended to have better short-term metabolic outcomes; however, it is difficult to determine the specific minimum islet yield needed for insulin independence. Our results suggest the possible advantage that the average IEQ/kg body weight needed to achieve insulin independence might be lower when the SN enzyme is used for islet isolation. Patient-related factors such as the intraportal microenvironment may play a significant role in islet autograft functional outcome. Longer follow-up data would be required to assess the possible advantage of SN.
An important issue in islet autotransplantation from the standpoint of risk is that transplanting impure or high volumes of tissue transiently increases portal venous pressure. The present study revealed that transplanted tissue volume, body surface area (which can predict total liver volume [29]) and transplanted embedded islet number significantly predicted the change in portal venous pressure during the IAT procedure. The difference between enzymes used for islet isolation was not a significant predictor. According to our analyses conducted separately for the LH and SN groups, transplanted tissue volume was the only predictor in the LH group. In contrast, not only tissue volume but also body surface area and transplanted embedded islet number were significantly associated with portal venous pressure changes when the SN enzyme was used for islet isolation. One can argue that these results reflect the characteristics of these enzymes.
Limitations of this study include its retrospective nature, short posttransplant follow up period, and the lack of standardized assessment of clinical outcomes. It is clear that patients who are insulin-independent after TP-IAT have definite graft function. If C-peptide levels were not documented in patients, we classified them as partial graft function on clinical course. Our previous study has showed all patients classified with partial graft function were indeed C-peptide positive (8).
In conclusion, we have shown that the SN Collagenase NB1 plus Neutral protease NB blend is equally effective as LH for human autograft islet isolation and transplantation. The data presented here suggest that the SN enzyme blend is suitable as an alternative choice for enzymatic dissociation of the pancreas for human islet autotransplantation and possibly for islet allotransplantation. However, further optimization of the collagenase/neutral protease ratio is needed to reduce the number of embedded islets obtained when utilizing the SN enzyme for islet purification.
Acknowledgments
B J.H. is the holder of the Eunice L. Dwan Chair in Diabetes Research and the McKnight Presidential Chair in Transplantation Science. D.E.R.S. is the holder of the John S. Najarian Surgical Chair in Clinical Transplantation and the Dobbs-Sutherland Chair in Diabetes Research. The authors would like to thank Thomas Gilmore, Muhamad Abdulla, Josh Wilhelm and Kate Mueller for excellent technical support, and Jeffrey Ansite, Juan Blondet, MD, and Barbara Bland for data compilation. This work was supported in part by philanthropic gifts made to the Schulze Diabetes Institute.
References
- 1.Schnelldorfer T, Lewin DN, Adams DB. Operative management of chronic pancreatitis: Long-term results in 372 patients. J Am Coll Surg 2007; 204: 1039–1045; discussion 1045–1037. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58: 365–382. [DOI] [PubMed] [Google Scholar]
- 3.Wahoff DC, Papalois BE, Najarian JS et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg 1995; 222: 562–575; discussion 575–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blondet JJ, Carlson AM, Kobayashi T et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007; 87: 1477–1501. [DOI] [PubMed] [Google Scholar]
- 5.Morrison CP, Wemyss-Holden SA, Dennison AR, Maddern GJ. Islet yield remains a problem in islet autotransplantation. Arch Surg 2002; 137: 80–83. [DOI] [PubMed] [Google Scholar]
- 6.Clayton HA, Davies JE, Pollard CA, White SA, Musto PP, Dennison AR. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: The first 40 patients at the Leicester General Hospital. Transplantation 2003; 76: 92–98. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad SA, Lowy AM, Wray CJ et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg 2005; 201: 680–687. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland DE, Gruessner AC, Carlson AM et al. Islet autotransplant outcomes after total pancreatectomy: A contrast to islet allograft outcomes. Transplantation 2008; 86: 1799–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenmochi T, Miyamoto M, Une S et al. Improved quality and yield of islets isolated from human pancreata using a two-step digestion method. Pancreas 2000; 20: 184–190. [DOI] [PubMed] [Google Scholar]
- 10.Goto M, Eich TM, Felldin M et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 2004; 78: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Okitsu T, Iwanaga Y et al. Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation 2006; 82: 460–465. [DOI] [PubMed] [Google Scholar]
- 12.Kin T, Johnson PR, Shapiro AM, Lakey JR. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation 2007; 83: 7–12. [DOI] [PubMed] [Google Scholar]
- 13.Brandhorst H, Brandhorst D, Hesse F et al. Successful human islet isolation utilizing recombinant collagenase. Diabetes 2003; 52: 1143–1146. [DOI] [PubMed] [Google Scholar]
- 14.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes 1997; 46: 1120–1123. [DOI] [PubMed] [Google Scholar]
- 15.Barnett MJ, Zhai X, LeGatt DF, Cheng SB, Shapiro AM, Lakey JR. Quantitative assessment of collagenase blends for human islet isolation. Transplantation 2005; 80: 723–728. [DOI] [PubMed] [Google Scholar]
- 16.Balamurugan AN, He J, Guo F et al. Harmful delayed effects of exogenous isolation enzymes on isolated human islets: Relevance to clinical transplantation. Am J Transplant 2005; 5: 2671–2681. [DOI] [PubMed] [Google Scholar]
- 17.Bucher P, Mathe Z, Morel P et al. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation 2005; 79: 91–97. [DOI] [PubMed] [Google Scholar]
- 18.Hering BJ, Kandaswamy R, Harmon JV et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4: 390–401. [DOI] [PubMed] [Google Scholar]
- 19.Lakey JR, Warnock GL, Shapiro AM et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant 1999; 8: 285–292. [DOI] [PubMed] [Google Scholar]
- 20.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(Suppl 1): 140–142. [DOI] [PubMed] [Google Scholar]
- 21.Lake SP, Bassett PD, Larkins A et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989; 38(Suppl 1): 143–145. [DOI] [PubMed] [Google Scholar]
- 22.Ricordi C, Gray DW, Hering BJ et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat 1990; 27: 185–195. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto I, Sawada T, Nakano M et al. Improvement in islet yield from obese donors for human islet transplants. Transplantation 2004; 78: 880–885. [DOI] [PubMed] [Google Scholar]
- 24.Lakey JR, Cavanagh TJ, Zieger MA, Wright M. Evaluation of a purified enzyme blend for the recovery and function of canine pancreatic islets. Cell Transplant 1998; 7: 365–372. [DOI] [PubMed] [Google Scholar]
- 25.Brandhorst H, Brendel MD, Eckhard M, Bretzel RG, Brandhorst D. Influence of neutral protease activity on human islet isolation outcome. Transplant Proc 2005; 37: 241–242. [DOI] [PubMed] [Google Scholar]
- 26.Bucher P, Bosco D, Mathe Z et al. Optimization of neutral protease to collagenase activity ratio for islet of Langerhans isolation. Transplant Proc 2004; 36: 1145–1146. [DOI] [PubMed] [Google Scholar]
- 27.Ihm SH, Matsumoto I, Sawada T et al. Effect of donor age on function of isolated human islets. Diabetes 2006; 55: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 28.Balamurugan AN, Chang Y, Bertera S et al. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia 2006; 49: 1845–1854. [DOI] [PubMed] [Google Scholar]
- 29.Vauthey JN, Abdalla EK, Doherty DA et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002; 8: 233–240. [DOI] [PubMed] [Google Scholar]


