Abstract
Only a fraction of the Biomphalaria and Bulinus snail community shows patent infection with schistosomes despite continuous exposure to the parasite, indicating that a substantial proportion of snails may resist infection. Accordingly, exterminating the schistosome intermediate snail hosts in transmission foci in habitats that may extend to kilometres is cost-prohibitive and damaging to the ecological equilibrium and quality of water and may be superfluous. It may be more cost effective with risk less ecological damage to focus on discovering the parameters governing snail susceptibility and resistance to schistosome infection. Therefore, laboratory bred Biomphalaria alexandrina and Bulinus truncatus snails were exposed to miracidia of laboratory-maintained Schistosoma mansoni and S. haematobium, respectively. Snails were examined for presence or lack of infection association with soft tissue and hemolymph content of proteins, cholesterol, and triglycerides, evaluated using standard biochemical techniques and palmitic, oleic, linoleic, and arachidonic acid, assayed by ultraperformance liquid chromatography-tandem mass spectrometry. Successful schistosome infection of B. alexandrina and B. truncatus consistently and reproducibly correlated with snails showing highly significant (up to P < 0.0001) decrease in soft tissue and hemolymph content of the monounsaturated fatty acid, oleic acid, and the polyunsaturated fatty acids, linoleic, and arachidonic acids as compared to naïve snails. Snails that resisted twice infection had soft tissue content of oleic, linoleic, and arachidonic acid similar to naïve counterparts. High levels of soft tissue and hemolymph oleic, linoleic, and arachidonic acid content appear to interfere with schistosome development in snails. Diet manipulation directed to eliciting excessive increase of polyunsaturated fatty acids in snails may protect them from infection and interrupt disease transmission in a simple and effective manner.
1. Introduction
Schistosomiasis caused by trematode worms of the genus Schistosoma is a debilitating disease associated with significant morbidity and mortality. Three members, Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum, are responsible for the great majority of approximately 300 million human infections and 800 million, principally children, at risk of the infection [1, 2]. Schistosomiasis is also termed snail fever as schistosomes' life cycle involves asexual reproduction in a compatible, fresh water snail whereby infection with a single miracidium results in production of thousands of infective cercariae [3]. Incidence and prevalence of schistosomiasis reflect the distribution of the freshwater intermediate host snail [1–3]. Snails Oncomelania density, rate of infection, and zones range, size, and proximity to human habitation were reported to be responsible for the high prevalence of schistosomiasis japonicum in the Philippines and southern China [4, 5]. In the Middle East and sub-Saharan Africa, Oncomelania spp. and schistosomiasis japonicum are not found while the widespread incidence of schistosomiasis mansoni and schistosomiasis haematobium is a function of the habitat range and distribution of susceptible snail species of the genus Biomphalaria and Bulinus, respectively [6–10]. In South America and the Caribbean, the distribution of Biomphalaria spp. (B. glabrata, B. straminea, B. tenagophila) is closely associated with the occurrence of schistosomiasis mansoni [11]. Accordingly, the use of chemical [12–15] and plant-derived [16–18] molluscicides was advocated for schistosomiasis control and transmission elimination, yet, was not strongly encouraged due to probable toxicity to aquaculture flora and fauna.
Habitats of the intermediate snail hosts in transmission foci within water bodies, along rivers, lakes, and ponds may extend to kilometres. Attempts at snail extermination with molluscicides may raise threats to the ecological equilibrium and quality of water for drinking and irrigation, vegetation, and animal life and could well be unnecessary. That is because only a fraction of the snail community shows patent infection despite continuous exposure to the parasite, indicating that a substantial proportion of snails may resist infection [7, 19–23]. Discovering the snail resistance underlying immunogenic mechanisms may well guide procedures and methods towards eliminating schistosomiasis [21].
The success of schistosome snail infection was reported to be a function of matching between the host and parasite phenotype [21, 24, 25]. The “matching hypothesis” claims that gene-encoded diversification and polymorphisms of the effector/antieffector systems and antigens and receptors of the parasite and the host lead to phenotype matching or mismatching between the schistosome and the snail, resulting in infection success and failure, respectively [21, 24, 25]. Mechanisms which correlate with resistance include too hemocyte abundance, rate of migration from hemolymph to soft tissues, higher cupper/zinc superoxide dismutase activity and capacity to produce hydrogen peroxide (H2O2), and more potent ability to encapsulate and kill the invading and developing schistosome larvae [26–33].
Additionally, resistance to infection was reported to reflect parasite-induced unfavorable biochemical changes in the intrasnail environment [21, 34, 35]. Resistance to infection of the water rat with S. mansoni was associated with increase in arachidonic acid content in the liver [36]. Susceptibility and resistance of the laboratory mouse, Mus musculus, and rat, Rattus norvegicus, to S. mansoni directly correlated with low and high serum arachidonic acid levels, respectively [37]. Poor development of S. mansoni in T and B lymphocytes-deficient mice appeared to be a function of elevated arachidonic acid levels in serum [38]. The antischistosome activity of sclareol and its heck-coupled derivatives was attributed to the drug-mediated enhancement of arachidonic acid metabolism in the target worms [39]. Additionally, arachidonic acid was shown to be an effective schistosomicide of S. mansoni and S. haematobium in vitro and in vivo in mice and hamsters, respectively [40–42]. Schistosoma haematobium larvae and adult worms were consistently more susceptible to the unsaturated fatty acids, namely, arachidonic acid and parasiticidal impact in vitro [40–44] and in vivo [40–42]. Arachidonic acid was also reported to be an effective therapy for schistosomiasis mansoni light infection in children [45, 46]. Recently, arachidonic acid was shown to function as a principal schistosomicidal and parasite ovocidal mediator of the adjuvant-free, cysteine peptidase-based schistosomiasis vaccine [47].
These findings together urged examining the role of intramolluscan polyunsaturated fatty acid (PUFA) levels in the susceptibility/resistance of the laboratory-bred snails Biomphalaria alexandrina and Bulinus truncatus to infection with the Egyptian laboratory strains of S. mansoni and S. haematobium, respectively. Accordingly, protein content and levels of cholesterol, total neutral triglycerides, palmitic, oleic, linoleic, and arachidonic acid levels were evaluated in the hemolymph and soft tissues of algae or lettuce-fed B. alexandrina and B. truncatus snails. The data obtained following infection with the parasites are the first, to our knowledge, to examine the effect of diet on snails' innate resistance to parasites and fully supported the influence of soft tissue PUFAs, notably arachidonic acid, levels in the snail resistance to infection.
2. Materials and Methods
2.1. Snails and Parasites
B. alexandrina and B. truncatus snails were obtained from the Schistosome Biological Supply Centre–Theodore Bilharz Research Institute (SBSC-TBRI), Giza, Egypt, where they were bred for several years under standardized laboratory conditions. Dechlorinated tap water has a pH of 7.1 ± 0.1, total dissolved solids of 340 ± 10 mg/l, and dissolved oxygen of 6.65 ± 1 mg/l. Temperature is maintained at 26 ± 1°C and light exposure at 12 h fluorescent light and 12 h dark. Miracidia of S. mansoni and S. haematobium were used for snail infection immediately after hatching of the eggs isolated from the liver of 7- and 12-week infected hamsters, respectively, as described [48].
2.2. Infection
Adult B. alexandrina and B. truncatus were both divided into two major groups each, one lettuce fed and the other algae fed. Three groups each of thirty algae-fed snails and six groups each of thirty lettuce-fed snails were exposed overnight, on an individual snail basis, to freshly hatched miracidia (8-10 miracidia/B. alexandrina snail and 15-20 miracidia/B. truncatus snail), under direct light at temperature of 26 ± 1°C. The snails were then maintained in standard aquaria with clean dechlorinated water (10 snails/l). After the prepatent period (30 days for S. mansoni and 40 days for S. haematobium), each of B. alexandrina (85.5% and 66.6% for algae and lettuce-fed snails, respectively) and B. truncatus (46.6% and 43.3% for algae and lettuce-fed snails, respectively) snail that was detected to shed cercariae was interchangeably labelled susceptible or infected. Snails that were unable to shed cercariae were subjected to an additional cycle of infection as described above, and if proven to lack cercarial shedding at the end of the second prepatent period (0% of algae-fed B. alexandrina and B. truncatus and 7.7% and 16.6% % of lettuce-fed B. alexandrina and B. truncatus, respectively), they were labelled resistant to infection.
2.3. Sample Collection
The head-foot of the snail was exposed (by cracking the shell around it without disturbing the animal inside) and pierced with a fine needle a short distance below the mouth. The exuded hemolymph was collected as quickly as was possible before it could have been contaminated by secretions from the mucous gland. Collected hemolymph for each individual snail was centrifuged at 2,000 × g for 3 min, the pellet was discarded, and the supernatant was divided into the two Eppendorf tubes each containing 50 μl of hemolymph, one for the biochemical assays and the other for the ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis. Soft tissue samples were collected by crushing the snails individually (after hemolymph collection) and divided into two Eppendorf tubes each containing 50 mg of soft tissue, one for the biochemical assays and the other for the UPLC-MS/MS analysis.
2.4. Protein, Cholesterol, and Total Neutral Triglyceride Assays
Hemocyte-free hemolymph was used. As for the soft tissue, it was homogenized in 500 μl ultrapure, pyrogen free water containing 0.05% (w/v) sodium dodecyl sulfate (SDS), centrifuged at 2,000 × g, and the clear supernatant was used. Total protein content (5 replicates per group) was analysed using Bradford Protein Assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA), following the manufacturer instructions. Samples were added in duplicate wells of 96-well plates (10 μl/well), incubated at 25 ± 1°C for 15 min with occasional light shaking and absorbance read at 630 nm (ELx808, BioTek, Bad Friedrichshall, Germany). Total protein content was expressed as mg/ml.
Cholesterol and neutral triglyceride levels were evaluated in 5 replicates per each group using colorimetric assay kits (Cholesterol assay kit, Chronolab Systems, Barcelona, Spain, and Triglycerides assay kit, Reactivos GPL, Barcelona, Spain, respectively). In 96-well plates, 200 μl of reagent was added to the wells along with 2 μl standard, 2 μl hemolymph, or 2 μl soft tissue extract samples adjusted to contain 3 mg/ml protein, i.e., 6 μg/well. The plates were incubated at 25 ± 1°C for 15 min with occasional light shaking and absorbance read at 540 nm (ELx808). Cholesterol and triglyceride content was expressed as mg/mg protein.
2.5. Fatty Acid Extraction and Assay
Fatty acids were extracted from samples of hemolymph (50 μl) and soft tissue (50 mg) on an individual snail basis, following the Folch method [49] with an added extraction step to ensure the purity of the resulted fatty acid extract. Butylated hydroxytoluene (BHT) 1 : 30 w/v was added to the chloroform/methanol mixture as an antioxidant to prevent any oxidation of the extracted fatty acids [50]. All samples and chemicals were kept on ice at all times. Extracted fatty acids were reconstituted in 100 μl hexane to chloroform (3 : 1)/HBT and stored at -20°C until assayed by UPLC-MS/MS. Samples were allowed to completely dry from hexane to chloroform (3 : 1) solvent mixture on ice in a fume hood and reconstituted in 500 μl HPLC grade methanol and membrane (0.22 μm syringe filter, Membrane Solutions, Shanghai, China) filtered in glass vials before injection in UPLC-MS/MS. Liquid chromatography was done using an UPLC system (ACQUITY UPLC; Waters Corporation, Milford, MA, USA) on Acquity BEH C18 Column (1.7 μm, 2.1 mm × 50 mm; Waters Corporation) and analysed with a Triple quadrupole MS/MS (Xevo TQ-S micro, Waters). Elution was isocratic, where mobile phase was 65% of 5 mM ammonium acetate in acetonitrile and 35% 5 mM ammonium acetate in isopropanol. Flow rate was 0.2 ml/min; column temperature was 40°C. Data were collected using MRM (Multiple Reaction Monitoring) in negative ESI (Electrospray Ionization) mode. The capillary voltage was 4 kV, cone voltage 62 V, radio frequency (RF) lens voltage 2.5 V, and source temperature 200°C. Nitrogen was used as the desolvation and cone gas at a flow rate of 650 l/hr, while argon was used as the collision gas at a pressure of approximately 3.67 × 10−3 mbar. System operation and data acquisition were controlled using the MassLynx® 4.1 software (Waters Corporation). The method was validated according to ICH guidelines of bioanalytical method validation where the range of linearity was evaluated by testing different dilutions of the four fatty acids in HPLC grade methanol (palmitic acid (Cat# P0500, Sigma, St. Louis, MO, USA), oleic acid (Cat# O1008, Sigma, USA), linoleic acid (Cat# L1376, Sigma, USA), and arachidonic acid (Cat# A3611, Sigma, USA)) ranging from 5 ng/ml to 20,000 ng/ml, where the linear dynamic range was found to be from 50 ng/ml to 20,000 ng/ml and limit of detection (LOD) and limit of quantitation (LOQ) were 3 ng/ml and 10 ng/ml, respectively. The reproducibility, accuracy, and precision were calculated by running a selected array of 6 standard concentrations as quality control samples (50 ng/ml, 500 ng/ml, 2000 ng/ml, 6000 ng/ml, 10,000 ng/ml, and 18,000 ng/ml) in between the snail samples' runs and evaluating the intra- and interday data. For calibration curves, a serial dilution (0.05-20 μg/ml in HPLC grade methanol) of the four fatty acids was used. Linear regression analysis was performed to correlate peak area ratios of fatty acids (peak area fatty acid/peak area of internal standard (docosahexaenoic acid, Sigma) to fatty acid concentrations (Supplementary Table 1A-D; Supplemental Figure 1A-E)).
2.6. Histology and Light Microscopy Studies
Twelve days after miracidium infection, snails were initially relaxed with methanol crystals. Each snail was carefully crushed between two microscope slides, and the broken shell pieces pulled away from the soft tissue with care. The columellar muscle was successfully separated from the shell, and the snail soft tissue extracted intact. Snails' soft tissue was stretched to their full length on a glass slide, fixed with a few droplets of Bouin's fixative, and transferred to Bouin in a sterile Eppendorf for at least 24 hours. Bouin-treated soft tissue samples were dehydrated in gradually increased concentrations of ethanol, cleared in xylol, and embedded in paraffin blocks. Using a microtome, 5 μm sections from the paraffin block were cut and stained with haematoxylin-eosin dye. Haematoxylin-eosin-stained 5 μm sections were examined under light microscopy for histological condition of larval trematodes, as categorized by Borges et al. [51], with 10x lens. Sections of interest were photographed using 40x lens.
2.7. Statistical Analysis
One-way ANOVA tests were used to analyse the statistical significance of differences between all groups of the same snail species and considered significant at P < 0.05. Calculations were performed using PRISM® 5.01 (GraphPad, San Diego, CA, USA).
3. Results
3.1. Biomphalaria Alexandrina
3.1.1. Effect of Diet
Two experiments were performed in tandem. In each, five healthy B. alexandrina snails maintained in parallel in identical conditions, but on lettuce and algae diet, were assessed for soft tissue and hemolymph protein and lipid content on an individual snail basis. The statistical analyses of results revealed that the diet difference had no significant impact on soft tissue and limited effect on hemolymph protein content (Supplementary Tables 2, 3). Lettuce diet elicited decrease in soft tissue cholesterol, hemolymph triglycerides (Figures 1(a)–1(d)), and soft tissue palmitic and oleic acid (Figures 2(a) and 2(b)) levels as compared to algae-fed snails, perhaps providing an explanation for detection of resistant snails only among those on lettuce diet. Otherwise, there was no differential impact of algae and lettuce diet on fatty acid levels in soft tissue and hemolymph (Figures 2(a)–2(h)).
Figure 1.
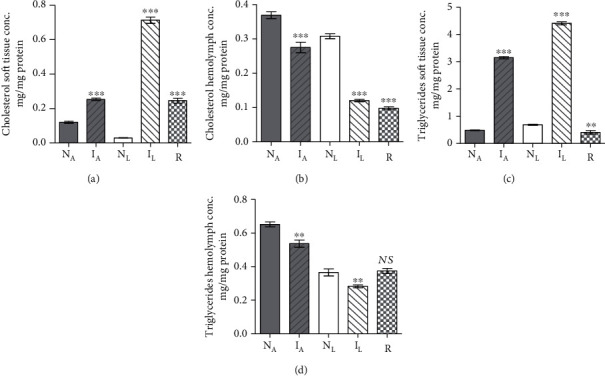
Each column represents mean B. alexandrina soft tissue cholesterol (a), hemolymph cholesterol (b), soft tissue triglycerides (c), and hemolymph triglycerides (d) content for five naïve-algae fed (NA), infected-algae fed (IA), naïve-lettuce fed (NL), infected-lettuce fed (IL), resistant snails (R), and vertical bars the SE about the mean. Statistical differences between naïve and infected or resistant same diet-fed snails are shown; ∗∗∗P < 0.001, ∗∗0.001 < P < 0.005. NS: not significant.
Figure 2.
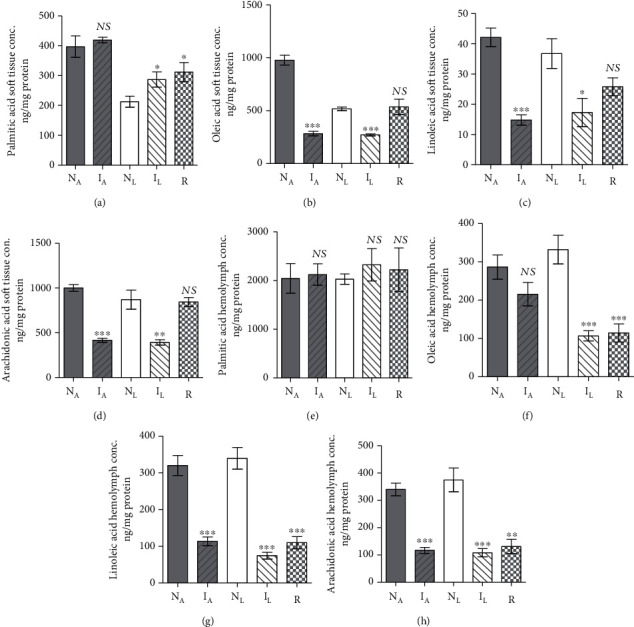
Each column represents mean B. alexandrina soft tissue and hemolymph fatty acid level content. Each column represents mean soft tissue palmitic (a), oleic (b), linoleic (c), and arachidonic (d) acid and hemolymph palmitic (e), oleic (f), linoleic (g), and arachidonic (h) acid content for five B. truncatus snails, and vertical bars the SE about the mean. NA: naïve-algae fed; IA: infected-algae fed; NL: naïve-lettuce fed; IL: infected-lettuce fed; R: resistant snails, and vertical bars the SE about the mean. Statistical differences between naïve and susceptible or resistant same diet-fed snails are shown; ∗∗0.001 < P < 0.005; ∗∗∗P < 0.001. NS: not significant.
3.1.2. Effect of Infection in Algae-Fed Snails
In two independent experiments, five B. alexandrina snails maintained on algae diet were subjected to infection with S. mansoni miracidia and assayed 30 days later for soft tissue and hemolymph protein and lipids content in parallel with entirely naïve snails. Infection failed to alter the snail soft tissue and hemolymph protein (Supplementary Tables 2, 3) or palmitic acid (Figures 2(a) and 2(e)) content. The most salient changes 30 days S. mansoni infection induced regarding cholesterol, and triglyceride parameters were highly significant (P < 0.005) increase in soft tissue accompanied with decline in hemolymph (Figures 1(a)–1(d)). Of note, infection resulted in highly significant (P < 0.0001) decrease in soft tissue oleic, linoleic, and arachidonic acid levels and in hemolymph linoleic and arachidonic acid content when compared with naïve snails (Figure 2).
3.1.3. Effect of Infection in Lettuce-Fed Snails
Snail infected with S. mansoni miracidia for 30 days did not greatly differ from their naïve counterparts regarding the protein content of soft tissue or hemolymph (Supplementary Tables 2, 3). Like algae-fed snails, 30 days S. mansoni infection induced cholesterol and triglycerides highly significant (P < 0.005) level increase in soft tissue and decline in hemolymph (Figures 1(a)–1(d)). Of note, 30 days S. mansoni infection in lettuce-fed snails led to some increase in palmitic acid content (Figures 2(a) and 2(e)), while led to highly significant (P < 0.001) decline in oleic, linoleic, and arachidonic acid level soft tissue and hemolymph, when compared with naïve snails (Figures 2(a)–2(h)).
3.1.4. Resistance versus Susceptibility Parameters
Laboratory bred naïve snails were maintained on algae or lettuce diet and unexposed to any infection. A series of two sets of five snails were exposed twice to infection with 5-10 of S. mansoni miracidia, with one-month interval. The snails, which shed cercariae either after the first or the second experimental infection, were labelled infected and considered susceptible. Resistant snails were detected uniquely among lettuce-fed hosts. All three groups (naïve, infected, and resistant) were assessed for soft tissue and hemolymph protein and lipids content on an individual snail basis. Snail resistance to infection with S. mansoni miracidia was associated with minor decrease in soft tissue and hemolymph protein content (Supplementary Tables 2, 3), and variable changes in cholesterol, triglycerides (Figures 1(a)–1(d)), and palmitic acid (Figures 2(a) and 2(e)) levels. Highly significant (P < 0.0001) decline in hemolymph oleic, linoleic, and arachidonic acid content was recorded when compared to lettuce-fed naïve snails (Figures 2(f)–2(h)). In contrast to susceptible group, resistant snails failed to decrease their soft tissue oleic (Figure 2(b)), linoleic (Figure 2(c)), and arachidonic (Figure 2(d)) acid content; mg PUFA/mg protein values were nearly similar to those of naïve snails. Resistance of snails to support S. mansoni development remarkably correlated with the inability to decrease the soft tissue content of PUFAs to levels that are not lethal to the larvae.
3.1.5. Histological Examination
Haematoxylin sections of B. alexandrina snail soft tissues at 12 days postinfection displayed limited hemocytes involvement in naïve (Figures 3(a) and 3(b)), infected (Figures 3(c) and 3(d)), resistant (Figures 3(e) and 3(f)), algae- (Figures 3(a) and 3(c)), or lettuce- (Figures 3(b) and 3(d)–3(f)) fed snails, despite that the correct timing was selected because sporocysts stay in the head-foot region of the snail for roughly 2 weeks after infection. After then, the sporocysts either migrate to the intestine of the snail (in case of susceptible snails), or some of them may veer off to the heart region (in case of resistant snails) [6, 48].
Figure 3.
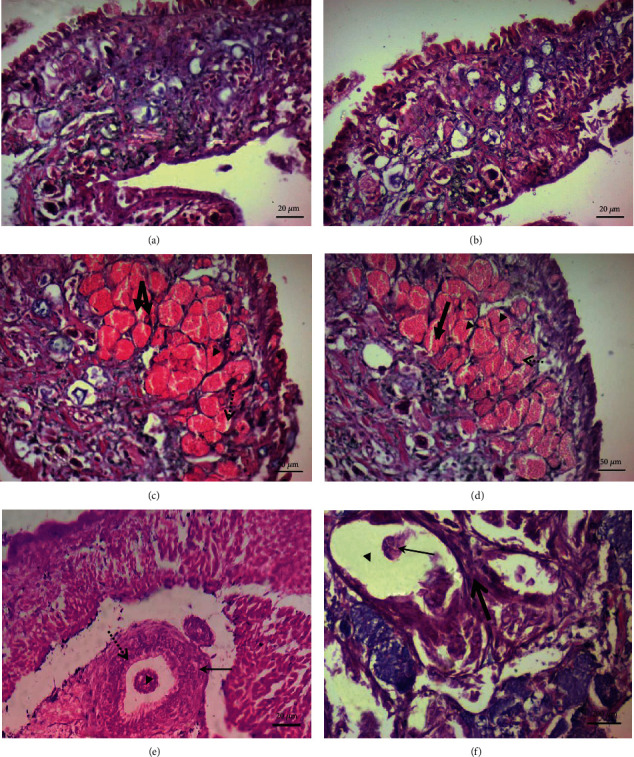
Histological transverse sections of B. alexandrina snails showing cephalopodal tissue. (a, b) are of naïve snails maintained on algae (a) and lettuce (b). (c, d) are of infected snails maintained on algae and lettuce, respectively, showing live S. mansoni mother sporocysts with normal development pattern; some of them contain single sporocyst cluster (thin arrow), and others contain multiple sporocyst clusters (thick arrow). (e, f) are of snails that resisted infection. Cephalopodal tissue is seen showing dead sporocyst (thin arrow) surrounded by granuloma-like structure consisting of layers of flattened hemocytes followed by concentric layer of fibroblasts (thick arrow) (e). The mantle collar region shows the sporocysts losing their identical cluster-like shape and presence of germinal cells lacking nucleoli. The size of the vacant space (thin arrow) is wider than in normal sporocysts. Note, presence of fibroblast lamination (thick arrow) surrounding the sporocysts' tegument (f).
3.2. Bulinus Truncatus
3.2.1. Effect of Diet
Two experiments were performed in tandem. In each, five healthy snails maintained in parallel in identical conditions, except for lettuce and algae diet, were assessed for soft tissue and hemolymph protein and lipids content on an individual snail basis. The statistical analyses of results revealed that lettuce diet elicited significant (P < 0.005) decrease in hemolymph protein (Supplementary Tables 4, 5), soft tissue cholesterol, and triglyceride content as compared to algae-fed snails (Figures 4(a)–4(d)) and increase in soft tissue palmitic and oleic acid (Figures 5(a) and 5(b)), perhaps providing an explanation for detection of resistant snails only among those on lettuce diet. There was no otherwise remarkable effect of algae versus lettuce diet (Figure 5).
Figure 4.
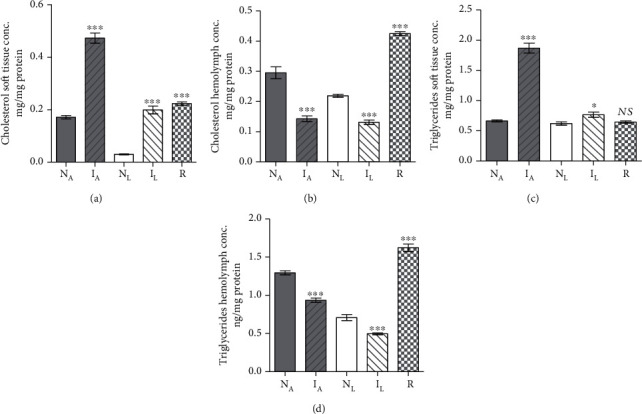
Each column represents mean soft tissue cholesterol (a), hemolymph cholesterol (b), soft tissue triglycerides (c), and hemolymph triglyceride (d) content for five B. truncatus snails, and vertical bars the SE about the mean. NA: naïve-algae fed; IA: infected-algae fed; NL: naïve-lettuce fed; IL: infected-lettuce fed; R: resistant snails. Statistical differences between naïve and infected or resistant same diet-fed snails are shown; ∗∗∗P < 0.001, ∗P < 0.05. NS: not significant.
Figure 5.
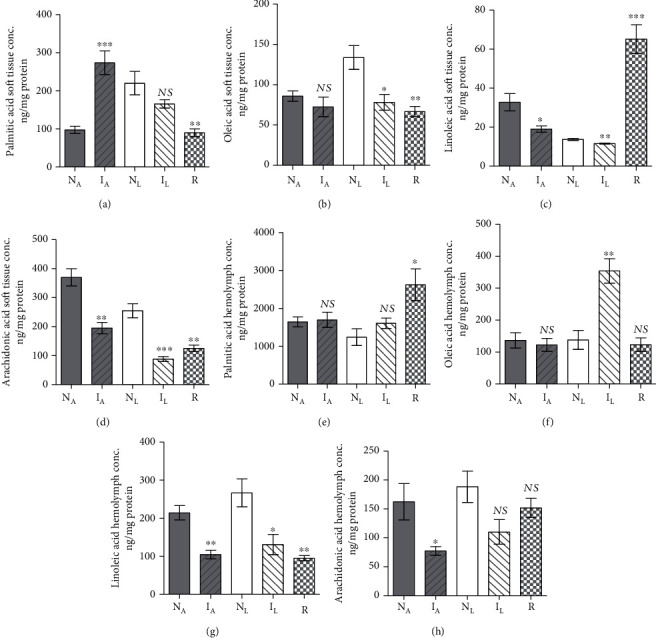
Each column represents mean soft tissue palmitic (a), oleic (b), linoleic (c), and arachidonic (d) acid and hemolymph palmitic (e), oleic (f), linoleic (g), and arachidonic (h) acid content for five B. truncatus snails, and vertical bars the SE about the mean. NA: naïve-algae fed; IA: infected-algae fed; NL: naïve-lettuce fed; IL: infected-lettuce fed; R: resistant snails (lettuce fed). Statistical differences between naïve and infected or resistant same diet-fed snails are shown; ∗∗∗P < 0.001, ∗∗0.001 < P < 0.005, ∗P < 0.05. NS: not significant.
3.2.2. Effect of Infection in Algae-Fed Snails
In two independent experiments, five B. truncatus snails maintained on algae diet were subjected to infection with S. haematobium miracidia and assayed, 40 days later, for soft tissue and hemolymph protein and lipids content in parallel with entirely naïve snails. Infection failed to alter the snail soft tissue protein content (Supplementary Table 4) but elicited highly significant (P < 0.001) increase in the hemolymph protein content (Supplementary Table 5). Changes observed after 40 days S. haematobium infection regarding cholesterol and triglyceride parameters were significant (P < 0.005) increase in soft tissue accompanied with decline in hemolymph (Figures 4(a)–4(d)). The most salient findings associated with S. haematobium infection were variable changes in palmitic acid levels in soft tissues and hemolymph (Figures 5(a) and 5(e)) and highly significant (up to P < 0.0001) decrease in soft tissue and hemolymph linoleic and arachidonic acid levels compared with naïve snails (Figure 5).
3.2.3. Effect of Infection in Lettuce-Fed Snails
Snail infection with S. haematobium larvae for 40 days led to significantly (P < 0.0001) higher soft tissue and hemolymph protein content as compared to naïve snails (Supplementary Tables 4, 5). As for algae-fed snails, 40 days S. haematobium infection induced highly significant (P < 0.0001) increase in soft tissue and decline in hemolymph cholesterol and triglyceride levels (Figures 4(a)–4(d)) and variable changes in palmitic acid content (Figures 5(a) and 5(e)). Additionally, infection of lettuce-fed B. truncatus infection was associated with highly significant (up to P < 0.0001) decrease in soft tissue and hemolymph linoleic and arachidonic acid levels, when compared to naïve snails (Figure 5).
3.2.4. Resistance versus Susceptibility Parameters
Laboratory bred naïve B. truncatus snails were maintained on algae or lettuce diet and unexposed to any infection. A series of snails were exposed twice to infection with 5-10 of S. haematobium miracidia, with one-month interval. The snails, which shed cercariae either after the first or the second experimental infection, were labelled susceptible. Resistant snails were found uniquely among the lettuce-fed hosts. All three groups (naïve, susceptible, and resistant) were assessed for soft tissue and hemolymph protein and lipids content on an individual snail basis. Snail resistance to infection with S. haematobium miracidia was associated with highly significant (P < 0.001) increase in soft tissue and hemolymph protein content (Supplementary Tables 4, 5) and cholesterol level in soft tissues and hemolymph (Figures 4(a) and 4(b)). Additionally, snail resistance versus susceptibility to infection was associated with variable changes in soft tissue and hemolymph palmitic acid content (Figures 5(a) and 5(e)). In sharp contrast to susceptible snails, soft tissues, but not hemolymph, of resistant snails displayed highly significant (P < 0.0001) increase in the levels of linoleic acid (Figures 5(c) and 5(g)) and no decrease in the levels of hemolymph oleic and arachidonic acid (Figures 5(f) and 5(h)). Snail resistance to support S. haematobium development remarkably correlated with the inability to decrease the soft tissues content of linoleic acid and hemolymph content of oleic and arachidonic acids to levels that are not lethal to the larvae.
3.2.5. Histologic Examination
Histological transverse sections showed normal cephalopodal tissue structure of naïve B. truncatus snails maintained on algae or lettuce, respectively (Figures 6(a) and 6(b)). Figures 6(c) and 6(d) are of susceptible snails showing live mother sporocysts with normal development pattern. Regarding resistant snails, sporocysts showed loss of their characteristic cluster-like shape, dissolution of the surrounding tegument (dashed arrow), and dispersion of germinal cells (Figure 6(e)). The number of hemocytes and fibroblasts lamination surrounding the sporocysts appeared negligible (Figure 6(f)).
Figure 6.
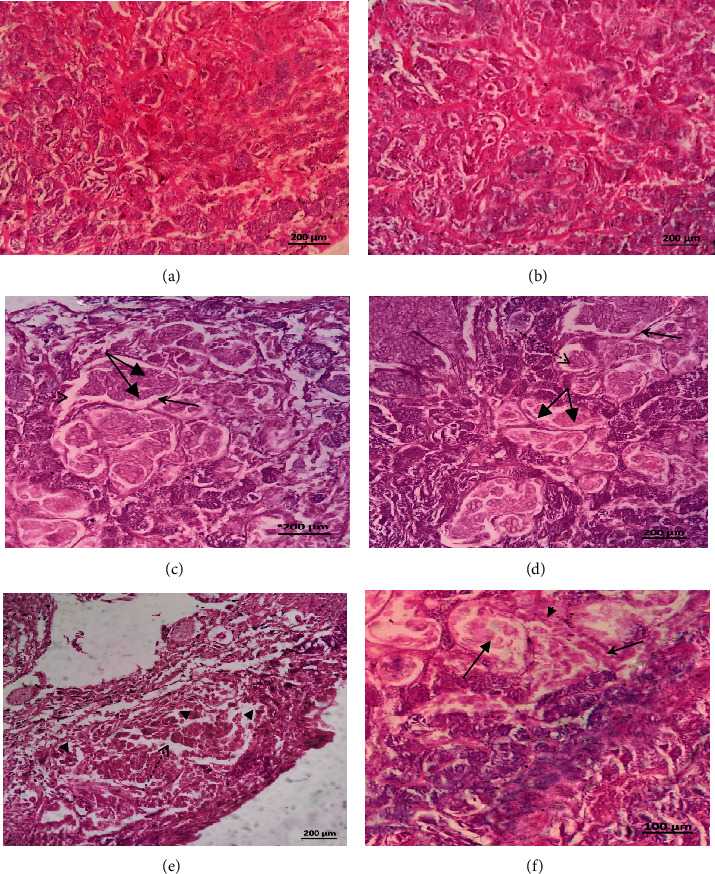
Histological transverse sections showed normal cephalopodal tissue structure of naïve B. truncatus snails maintained on algae or lettuce, respectively (a, b). (c, d) are of infected snails showing live mother sporocysts with normal development pattern; some of them contain single sporocyst cluster (arrowhead), and others contain multiple sporocyst clusters (twin thick arrows). All sporocysts contain viable germinal cells with nucleoli; the vacant space (dashed arrow) between the sporocyst and the tegument of the mother sporocyst also appears normal. Regarding resistant snails, cephalopodal tissue shows the sporocysts losing their characteristic cluster-like shape and dissolution of the surrounding tegument (dashed arrow), leading to dispersion of germinal cells (arrowhead) (e). The mantle collar region displays sporocycts have lost their cluster-like shape, and no viable germ cells are observed (thick arrow). Note, the negligible presence of hemocytes (thin arrow) and fibroblasts lamination (arrowhead) surrounding the sporocysts (f).
4. Discussion
Before exploring the possible effect of protein and lipids content on the susceptibility/resistance of snails to schistosome infection, it was essential to examine the influence of diet. Compared with lettuce, B. alexandrina and B. truncatus algae feeding led to increase in hemolymph triglycerides content, similarly to hen yolk eggs in the Biomphalaria glabrata model [52] and considerable increase in cholesterol content in soft tissue and hemolymph, while hen egg yolk led to B glabrata cholesterol increase in soft tissue but not hemolymph [53]. Hen yolk egg plus lettuce or lettuce supplemented with tetramine diet also elicited variable changes in cholesterol and triglycerides content of B. glabrata soft tissue [54]. Feeding B. alexandrina snails with algae versus lettuce led to minor increase in the palmitic and oleic acid content in soft tissue but did not affect the levels of proteins and linoleic and arachidonic acid in soft tissue and hemolymph. Similarly, lettuce and hen yolk egg diet variably affected the levels of palmitic, oleic, linoleic, arachidonic, and other fatty acids of whole B. glabrata snail body [55]. The data together indicate that diet impact on lipid content and distribution in the different body compartments varies with the diet composition and snail species. This conclusion should be taken into consideration regarding the feeding strategies of parasite vectors and edible snails.
Successful infection of algae- or lettuce-fed B. alexandrina and B. truncatus with schistosome miracidia similarly elicited dramatic increase in soft tissue cholesterol and triglycerides content versus decrease in hemolymph. Likewise, S. mansoni infection of B. glabrata led to considerable changes in soft tissue amounts of cholesterol and triglycerides [56, 57]. The results of the present study corroborate the report of lipid content reduction (80%) after 60 days infection of B. alexandrina with S. mansoni, with lipids measured involving triacylglycerols and free sterols as well as free fatty acids [58]. Indeed, the most striking and consistent parameter change in our snails study was the association of susceptibility to schistosome infection with highly significant (up to P < 0.0001) decrease in soft tissue and hemolymph oleic, linoleic, and arachidonic acid content. These results suggest that S. mansoni and S. haematobium intramolluscan development is successful provided low levels of mono- and especially polyunsaturated fatty acids in soft tissue. In support, resistance of B. alexandrina and B. Bulinus snails to schistosome infection appeared to directly correlate with inability to decrease the soft tissue content of oleic, linoleic, and arachidonic acid. Polyunsaturated acids, especially arachidonic acid, appeared thus to affect the development of schistosomes in both the intermediate snail and final mammalian [36–42] host. Successful development of S. mansoni had to be accompanied by more than 50% decrease of B. alexandrina soft tissue mono- and polyunsaturated fatty acids. Schistosoma haematobium, known for their higher sensitivity to PUFAs [40–44], would have never approached Bulinus truncatus if it carried the PUFA levels of B. alexandrina. Of note, invading schistosomes were indifferent to the levels of palmitic acid in snail soft tissue and hemolymph, while decrease in PUFAS levels strongly support theit function as endogenous antimicrobial molecules [59].
Our results document for the first time a limited, but significant, role of diet in snails innate immune responses, as snail resistant to second infection was 0% in algae versus approximately 10% in lettuce-fed snails. Additionally, our findings are in accord with both the “resistance” and the “matching” hypotheses [21, 24, 25]. Upon invasion, parasites are able to immediately dampen the snail PUFA synthesis pathways, survive, and reproduce. Parasite elimination occurs when the schistosome-host interaction fails to elicit decline in the soft tissue PUFA content, and on the contrary, leads to its increase. It might be useful to determine the mechanism(s) via which invading miracidia manipulate PUFA biosynthetic machinery [60–63]. Data have been obtained documenting the ability of the schistosome to manipulate the snail genome, including the expression of specific genes [26, 64, 65]. Yet, numerous transcripts critical to S. mansoni intra-Biomphalaria development are not identified as yet, and details on how considerable infection hurts the snail host metabolism are lacking [63, 65]. The present findings suggested that snail susceptibility/resistance to schistosomes is not uniquely dependent on hemocyte abundance and activity [26–33, 64]. Hemocyte migration and function may be easily impaired by several sporocyst-derived factors, among which antioxidant and immune-modulator molecules, and proteolytic enzymes, notably the metalloproteinase, SmLeish [66–70]. Fortunately, the parasites also fail to develop due to a plethora of intramolluscan immune factors and miracidia deterrents and biochemically unsuitable determinants [21, 26–34, 64, 71–73]. Moreover, PUFA level is a target that could be manipulated via diet to prevent snail infection, using PUFA (notably arachidonic acid) synthesizing algae, fungi, and plants [74]. It is, however, necessary to investigate whether such approach leads to snail over population or affects aquaculture fauna populations.
5. Conclusions
Laboratory-bred, algae-, or lettuce-fed Biomphalaria alexandrina and Bulinus truncatus adult snails were exposed to miracidia of long-term laboratory maintained Egyptian strains of Schistosoma mansoni and S. haematobium, respectively. Less than 50% of exposed snails showed patent infection 30 and 40 days after exposure, respectively, while around 10% of lettuce-fed snails resisted not once but twice infection with miracidia. Over two experiments, biochemical investigations of snails from each group revealed consistent and reproducible correlation between successful infection and highly significant decrease in oleic, linoleic, and arachidonic acid content in soft tissue and hemolymph, suggesting susceptibility of schistosome larvae to critical PUFA concentration. In support, the snails that resisted twice infection had soft tissue PUFA levels as high as unexposed naïve snails. The data together led to recommending manipulating the schistosome intermediate snail host diet towards excessive accumulation of PUFAs in soft tissue and hemolymph. The aim is to interfere with intrasnail schistosome development and transmission of infection, taking into consideration the effects of that approach on snail and other aquaculture fauna maturation and reproduction.
Acknowledgments
The authors wish to acknowledge the unfailing support and gift of snails and miracidia from the Schistosome Biological Supply Centre–Theodore Bilharz Research Institute (SBSC-TBRI), Giza, Egypt. This research was funded, in part, by Theodor Bilharz Research Institute (Internal project 105C).
Data Availability
All data are shown in tables, figures, and supplementary materials.
Ethical Approval
All animal experiments were performed following the recommendations of the current edition of the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, USA. Approval on experiments with hamsters and snails was obtained from the Institutional Animal and Care Committee of Theodore Bilharz Research Institute, Giza, Egypt.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
R.H., S.B., and R.R. conceived and designed the experiments. M.E., R.H., and S.B. performed the experiments. M.E., R.H., S.B., and R.R. analysed the results and wrote the manuscript. All authors read and approved the final manuscript version.
Supplementary Materials
Figure S1: Multiple Reaction Monitoring (MRM) chromatograms of DHA-the internal standard- (A), palmitic (B), oleic (C), linoleic (D) and arachidonic (E) fatty acids, using the optimized method conditions at their selective transitions (Table S1). Table S1A: method validation parameters for palmitic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision; Table 1B: method validation parameters for oleic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision; Table 1C: method validation parameters for linoleic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision; Table 1D: method validation parameters for arachidonic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision. Table S2: soft tissue protein content of Biomphalaria alexandrina snails. Table S3: hemolymph protein content of Biomphalaria alexandrina snails. Table S4: soft tissue protein content of Bulinus truncatus snails. Table S5: hemolymph protein content of Bulinus truncatus snails.
References
- 1.Barsoum R. S. Urinary schistosomiasis: review. Journal of Advanced Research. 2013;4(5):453–459. doi: 10.1016/j.jare.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsoum R. S., Esmat G., El-Baz T. Human schistosomiasis: clinical perspective: review. Journal of Advanced Research. 2013;4(5):433–444. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker A. J. Insights into the functional biology of schistosomes. Parasites & Vectors. 2011;4(1):p. 203. doi: 10.1186/1756-3305-4-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonardo L., Rivera P., Saniel O., et al. New endemic foci of schistosomiasis infections in the Philippines. Acta Tropica. 2015;141:345–360. doi: 10.1016/j.actatropica.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Ross A. G., Sleigh A. C., Li Y., et al. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clinical Microbiology Reviews. 2001;14(2):270–295. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan J. A., Dejong R. J., Snyder S. D., Mkoji G. M., Loker E. S. Schistosoma mansoniandBiomphalaria: past history and future trends. Parasitology. 2001;123(7):211–228. doi: 10.1017/S0031182001007703. [DOI] [PubMed] [Google Scholar]
- 7.Allan F., Dunn A. M., Emery A. M., et al. Use of sentinel snails for the detection of Schistosoma haematobium transmission on Zanzibar and observations on transmission patterns. Acta Tropica. 2013;128(2):234–240. doi: 10.1016/j.actatropica.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Diakité N. R., Winkler M. S., Coulibaly J. T., Guindo-Coulibaly N., Utzinger J., N'Goran E. K. Dynamics of freshwater snails and Schistosoma infection prevalence in schoolchildren during the construction and operation of a multipurpose dam in central Côte d'Ivoire. Infectious Diseases of Poverty. 2017;6(1):p. 93. doi: 10.1186/s40249-017-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allan F., Sousa-Figueiredo J. C., Emery A. M., et al. Mapping freshwater snails in north-western Angola: distribution, identity and molecular diversity of medically important taxa. Parasites & Vectors. 2017;10(1):p. 460. doi: 10.1186/s13071-017-2395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiamah O. C., Ubachukwu P. O., Anorue C. O., Ebi S. Urinary schistosomiasis in Ebonyi State, Nigeria from 2006 to 2017. Journal of Vector Borne Diseases. 2019;56(2):87–91. doi: 10.4103/0972-9062.263721. [DOI] [PubMed] [Google Scholar]
- 11.Scholte R. G., Carvalho O. S., Malone J. B., Utzinger J., Vounatsou P. Spatial distribution of Biomphalaria spp., the intermediate host snails of Schistosoma mansoni, in Brazil. Geospatial Health. 2012;6(3):S95–S101. doi: 10.4081/gh.2012.127. [DOI] [PubMed] [Google Scholar]
- 12.King C. H., Sutherland L. J., Bertsch D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Neglected Tropical Diseases. 2015;9, article e0004290 doi: 10.1371/journal.pntd.0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bing-Rong L., Wei-Si W., Jun-Min Y., et al. Molluscicidal activity of 25% wettable powder of pyriclobenzuron sulphate against Oncomelania hupensis robertsoni. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;31:115–120. doi: 10.16250/j.32.1374.2019014. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Wang W., Yao J., et al. Toxicity of a molluscicide candidate PPU07 against Oncomelania hupensis (Gredler, 1881) and local fish in field evaluation. Chemosphere. 2019;222:56–61. doi: 10.1016/j.chemosphere.2019.01.102. [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira M. A., Santos D. B. D., Silva L. D., Rocha T. L., Bezerra J. C. B. Molluscicidal activity of polyhexamethylene biguanide hydrochloride on the early-life stages and adults of the Biomphalaria glabrata (Say, 1818) Chemosphere. 2019;216:365–371. doi: 10.1016/j.chemosphere.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Whitfield P. J. Novel anthelmintic compounds and molluscicides from medicinal plants. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(6):596–600. doi: 10.1016/S0035-9203(96)90401-0. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim A. M., Ghoname S. I. Molluscicidal impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Experimental Parasitology. 2018;192:36–41. doi: 10.1016/j.exppara.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Jia T. W., Wang W., Sun L. P., et al. Molluscicidal effectiveness of Luo-Wei, a novel plant-derived molluscicide, against Oncomelania hupensis, Biomphalaria alexandrina and Bulinus truncatus. Infectious Diseases of Poverty. 2019;8:p. 27. doi: 10.1186/s40249-019-0535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sire C., Durand P., Pointier J. P., Théron A. Genetic diversity and recruitment pattern of Schistosoma mansoni in a Biomphalaria glabrata snail population: a field study using random-amplified polymorphic DNA markers. The Journal of Parasitology. 1999;85(3):436–441. doi: 10.2307/3285775. [DOI] [PubMed] [Google Scholar]
- 20.Lotfy W. M., Hanelt B., Mkoji G. M., Loker E. S. Genotyping natural infections of Schistosoma mansoni in Biomphalaria alexandrina from Damietta, Egypt, with comparisons to natural snail infections from Kenya. The Journal of Parasitology. 2011;97(1):156–159. doi: 10.1645/GE-2537.1. [DOI] [PubMed] [Google Scholar]
- 21.Mitta G., Gourbal B., Grunau C., Knight M., Bridger J. M., Théron A. The compatibility between Biomphalaria glabrata snails and Schistosoma mansoni: an increasingly complex puzzle. Advances in Parasitology. 2017;97:111–145. doi: 10.1016/bs.apar.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Zanardi V. S., Barbosa L. M., Simões F. M., et al. Prevalence of infection of Biomphalaria glabrata by Schistosoma mansoni and the risk of urban Schistosomiasis mansoni in Salvador, Bahia, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2019;52, article e20190171 doi: 10.1590/0037-8682-0171-2019. [DOI] [PubMed] [Google Scholar]
- 23.Rabone M., Wiethase J. H., Allan F., et al. Freshwater snails of biomedical importance in the Niger River Valley: evidence of temporal and spatial patterns in abundance, distribution and infection with Schistosoma spp. Parasites & Vectors. 2019;12(1):p. 498. doi: 10.1186/s13071-019-3745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Théron A., Coustau C. Are Biomphalaria snails resistant to Schistosoma mansoni? Journal of Helminthology. 2005;79(3):187–191. doi: 10.1079/JOH2005299. [DOI] [PubMed] [Google Scholar]
- 25.Mitta G., Adema C. M., Gourbal B., Loker E. S., Theron A. Compatibility polymorphism in snail/schistosome interactions: from field to theory to molecular mechanisms. Developmental and Comparative Immunology. 2012;37(1):1–8. doi: 10.1016/j.dci.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonner K. M., Bayne C. J., Larson M. K., Blouin M. S. Effects of Cu/Zn superoxide dismutase (sod1) genotype and genetic background on growth, reproduction and defense in Biomphalaria glabrata. PLoS Neglected Tropical Diseases. 2012;6, article e170 doi: 10.1371/journal.pntd.0001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender R. C., Broderick E. J., Goodall C. P., Bayne C. J. Respiratory burst of Biomphalaria glabrata hemocytes: Schistosoma mansoni-resistant snails produce more extracellular H2O2 than susceptible snails. The Journal of Parasitology. 2005;91(2):275–279. doi: 10.1645/GE-415R. [DOI] [PubMed] [Google Scholar]
- 28.Martins-Souza R. L., Pereira C. A., Coelho P. M., Martins-Filho O. A., Negrão-Corrêa D. Flow cytometry analysis of the circulating haemocytes from Biomphalaria glabrata and Biomphalaria tenagophila following Schistosoma mansoni infection. Parasitology. 2009;136(1):67–76. doi: 10.1017/S0031182008005155. [DOI] [PubMed] [Google Scholar]
- 29.Nacif-Pimenta R., de Mattos A. C., Orfanó Ada S., Barbosa L., Pimenta P. F., Coelho P. M. Schistosoma mansoni in susceptible and resistant snail strains Biomphalaria tenagophila: in vivo tissue response and in vitro hemocyte interactions. PLoS One. 2012;7(9, article e45637) doi: 10.1371/journal.pone.0045637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson M. K., Bender R. C., Bayne C. J. Resistance of Biomphalaria glabrata 13-16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. International Journal for Parasitology. 2014;44(6):343–353. doi: 10.1016/j.ijpara.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pila E. A., Gordy M. A., Phillips V. K., Kabore A. L., Rudko S. P., Hanington P. C. Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):5305–5310. doi: 10.1073/pnas.1521239113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pila E. A., Tarrabain M., Kabore A. L., Hanington P. C. A novel toll-like receptor (TLR) influences compatibility between the gastropod Biomphalaria glabrata, and the digenean trematode Schistosoma mansoni. PLoS Pathogens. 2016;12(3, article e1005513) doi: 10.1371/journal.ppat.1005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pila E. A., Li H., Hambrook J. R., Wu X., Hanington P. C. Schistosomiasis from a snail’s perspective: advances in snail immunity. Trends in Parasitology. 2017;33(11):845–857. doi: 10.1016/j.pt.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Lie K. J., Heyneman D. Schistosoma mansoni, Echinostoma lindoense, and Paryphostomum segregatum: interference by trematode larvae with acquired resistance in snails, Biomphalaria glabrata. Experimental Parasitology. 1977;42:343–347. doi: 10.1016/0014-4894(77)90091-1. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan J. T., Richards C. S. Schistosoma mansoni, NIH-SM-PR-2 strain, in susceptible and nonsusceptible stocks of Biomphalaria glabrata: comparative histology. The Journal of Parasitology. 1981;67(5):702–708. doi: 10.2307/3280445. [DOI] [PubMed] [Google Scholar]
- 36.Amaral K. B., Silva T. P., Malta K. K., et al. Natural Schistosoma mansoni infection in the wild reservoir Nectomys squamipes leads to excessive lipid droplet accumulation in hepatocytes in the absence of liver functional impairment. PLoS One. 2016;11(11, article e0166979) doi: 10.1371/journal.pone.0166979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna V. S., Gawish A., Abou El Dahab M., Tallima H., El Ridi R. Is arachidonic acid an endoschistosomicide? Journal of Advanced Research. 2018;11:81–89. doi: 10.1016/j.jare.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu R., Ye F., Zhong Q. P., et al. Comparative serum metabolomics between SCID mice and BALB/c mice with or without Schistosoma japonicum infection: clues to the abnormal growth and development of schistosome in SCID mice. Acta Tropica. 2019;200:p. 105186. doi: 10.1016/j.actatropica.2019.105186. [DOI] [PubMed] [Google Scholar]
- 39.Crusco A., Whiteland H., Baptista R., et al. Antischistosomal properties of sclareol and its heck-coupled derivatives: design, synthesis, biological evaluation, and untargeted metabolomics. ACS Infectious Diseases. 2019;5(7):1188–1199. doi: 10.1021/acsinfecdis.9b00034. [DOI] [PubMed] [Google Scholar]
- 40.El Ridi R., Aboueldahab M., Tallima H., et al. In vitro and in vivo activities of arachidonic acid against Schistosoma mansoni and Schistosoma haematobium. Antimicrobial Agents and Chemotherapy. 2010;54(8):3383–3389. doi: 10.1128/AAC.00173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Ridi R., Tallima H., Salah M., et al. Efficacy and mechanism of action of arachidonic acid in the treatment of hamsters infected with Schistosoma mansoni or Schistosoma haematobium. International Journal of Antimicrobial Agents. 2012;39(3):232–239. doi: 10.1016/j.ijantimicag.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 42.El Ridi R., Tallima H., Migliardo F. Biochemical and biophysical methodologies open the road for effective schistosomiasis therapy and vaccination. Biochimica et Biophysica Acta. 2016;1861:3613–3620. doi: 10.1016/j.bbagen.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Tallima H., Salah M., El-Ridi R. In vitro and in vivo effects of unsaturated fatty acids on Schistosoma mansoni and S. haematobium lung-stage larvae. The Journal of Parasitology. 2005;91(5):1094–1102. doi: 10.1645/GE-514R.1. [DOI] [PubMed] [Google Scholar]
- 44.El Ridi R., Tallima H. Equilibrium in lung schistosomula sphingomyelin breakdown and biosynthesis allows very small molecules, but not antibody, to access proteins at the host-parasite interface. The Journal of Parasitology. 2006;92(4):730–737. doi: 10.1645/GE-745R1.1. [DOI] [PubMed] [Google Scholar]
- 45.Selim S., El Sagheer O., El Amir A., et al. Efficacy and safety of arachidonic acid for treatment of Schistosoma mansoni-infected children in Menoufiya, Egypt. The American Journal of Tropical Medicine and Hygiene. 2014;91:973–981. doi: 10.4269/ajtmh.14-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barakat R., Abou El-Ela N. E., Sharaf S., et al. Efficacy and safety of arachidonic acid for treatment of school-age children in Schistosoma mansoni high-endemicity regions. The American Journal of Tropical Medicine and Hygiene. 2015;92(4):797–804. doi: 10.4269/ajtmh.14-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tallima H., El Dahab M. A., El Ridi R. Role of T lymphocytes and papain enzymatic activity in the protection induced by the cysteine protease against Schistosoma mansoni in mice. Journal of Advanced Research. 2019;17:73–84. doi: 10.1016/j.jare.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tucker M. S., Karunaratne L. B., Lewis F. A., Freitas T. C., Liang Y. S. Schistosomiasis. Current Protocols in Immunology. 2013;103(1):19.1.1–19.1.58. doi: 10.1002/0471142735.im1901s103. [DOI] [PubMed] [Google Scholar]
- 49.Folch J., Lees M., Sloane-Stanley G. H. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 50.Stone W. L., Farnsworth C. C., Dratz E. A. A reinvestigation of the fatty acid content of bovine, rat and frog retinal rod outer segments. Experimental Eye Research. 1979;28(4):387–397. doi: 10.1016/0014-4835(79)90114-3. [DOI] [PubMed] [Google Scholar]
- 51.Borges C. M., Souza C. P., Andrade Z. A. Histopathologic features associated with susceptibility and resistance of Biomphalaria snails to infection with Schistosoma mansoni. Memórias do Instituto Oswaldo Cruz. 1998;93(Supplement 1):117–121. doi: 10.1590/S0074-02761998000700016. [DOI] [PubMed] [Google Scholar]
- 52.Fried B., Cahn-Hidalgo D., Fujino T., Sherma J. Diet-induced differences in the distribution of neutral lipids in selected organs of Biomphalaria glabrata (Gastropoda: Planorbidae) as determined by thin-layer chromatography and light and electron microscopy. Transactions of the American Microscopical Society. 1991;110(2):163–171. doi: 10.2307/3226752. [DOI] [Google Scholar]
- 53.Shetty P. H., Fried B., Sherma J. Sterols in the plasma and digestive gland-gonad complex of Biomphalaria glabrata snails, fed lettuce versus hen’s egg yolk, as determined by GLC. Comparative Biochemistry and Physiology. B. 1990;96(4):791–794. doi: 10.1016/0305-0491(90)90233-J. [DOI] [PubMed] [Google Scholar]
- 54.Beers K., Fried B., Fujino T., Sherma J. Effects of diet on the lipid composition of the digestive gland-gonad complex of Biomphalaria glabrata (Gastropoda) infected with larval Echinostoma caproni (Trematoda) Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 1995;110(4):729–737. doi: 10.1016/0305-0491(94)00195-Z. [DOI] [PubMed] [Google Scholar]
- 55.Fried B., Rao S., Sherma J. Fatty acid composition of Biomphalaria glabrata (gastropoda: Planorbidae) fed hen’s egg yolk versus leaf lettuce. Comparative Biochemistry and Physiology Part A: Physiology. 1992;101(2):351–352. doi: 10.1016/0300-9629(92)90545-2. [DOI] [Google Scholar]
- 56.Thompson S. N. Effect of Schistosoma mansoni on the gross lipid composition of its vector Biomphalaria glabrata. Comparative Biochemistry and Physiology. B. 1987;87(2):357–360. doi: 10.1016/0305-0491(87)90152-0. [DOI] [PubMed] [Google Scholar]
- 57.Fried B., Muller E. E., Broadway A., Sherma J. Effects of diet on the development of Schistosoma mansoni in Biomphalaria glabrata and on the neutral lipid content of the digestive gland-gonad complex of the snail. The Journal of Parasitology. 2001;87(1):223–225. doi: 10.1645/0022-3395(2001)087[0223:EODOTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 58.Mahmoud M. B., El-Sayed K., El-Din A. T. Fatty acids contents in Biomphalaria alexandrina during the course of infection with Schistosoma mansoni. Journal of the Egyptian Society of Parasitology. 2013;43(2):517–527. doi: 10.12816/0006408. [DOI] [PubMed] [Google Scholar]
- 59.Das U. N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. Journal of Advanced Research. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joseph J. D. Lipid composition of marine and estuarine invertebrates. Part II: mollusca. Progress in Lipid Research. 1982;21:109–153. doi: 10.1016/0163-7827(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 61.Zhu N., Dai X., Lin D. S., Connor W. E. The lipids of slugs and snails: evolution, diet and biosynthesis. Lipids. 1994;29(12):869–875. doi: 10.1007/BF02536255. [DOI] [PubMed] [Google Scholar]
- 62.Monroig Ó., Tocher D. R., Navarro J. C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Marine Drugs. 2013;11(10):3998–4018. doi: 10.3390/md11103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buddenborg S. K., Kamel B., Hanelt B., et al. The in vivo transcriptome of Schistosoma mansoni in the prominent vector species Biomphalaria pfeifferi with supporting observations from Biomphalaria glabrata. PLoS Neglected Tropical Diseases. 2019;13(9, article e0007013) doi: 10.1371/journal.pntd.0007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodall C. P., Bender R. C., Broderick E. J., Bayne C. J. Constitutive differences in Cu/Zn superoxide dismutase mRNA levels and activity in hemocytes of Biomphalaria glabrata (Mollusca) that are either susceptible or resistant to Schistosoma mansoni (Trematoda) Molecular and Biochemical Parasitology. 2004;137(2):321–328. doi: 10.1016/j.molbiopara.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Knight M., Ittiprasert W., Arican-Goktas H. D., Bridger J. M. Epigenetic modulation, stress and plasticity in susceptibility of the snail host, Biomphalaria glabrata, to Schistosoma mansoni infection. International Journal for Parasitology. 2016;46(7):389–394. doi: 10.1016/j.ijpara.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Connors V. A., Lodes M. J., Yoshino T. P. Identification of a Schistosoma mansoni sporocyst excretory-secretory antioxidant molecule and its effect on superoxide production by Biomphalaria glabrata hemocytes. Journal of Invertebrate Pathology. 1991;58(3):387–395. doi: 10.1016/0022-2011(91)90185-S. [DOI] [PubMed] [Google Scholar]
- 67.Wu X. J., Sabat G., Brown J. F., et al. Proteomic analysis of Schistosoma mansoni proteins released during in vitro miracidium-to-sporocyst transformation. Molecular and Biochemical Parasitology. 2009;164(1):32–44. doi: 10.1016/j.molbiopara.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanington P. C., Forys M. A., Loker E. S. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Neglected Tropical Diseases. 2012;6(3, article e1591) doi: 10.1371/journal.pntd.0001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang T., Zhao M., Rotgans B. A., et al. Proteomic analysis of the Schistosoma mansoni miracidium. PLoS One. 2016;11, article e0147247 doi: 10.1371/journal.pone.0147247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hambrook J. R., Kaboré A. L., Pila E. A., Hanington P. C. A metalloprotease produced by larval Schistosoma mansoni facilitates infection establishment and maintenance in the snail host by interfering with immune cell function. PLoS Pathogens. 2018;14(10, article e1007393) doi: 10.1371/journal.ppat.1007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ittiprasert W., Knight M. Reversing the resistance phenotype of the Biomphalaria glabrata snail host Schistosoma mansoni infection by temperature modulation. PLoS Pathogens. 2012;8(4, article e1002677) doi: 10.1371/journal.ppat.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knight M., Elhelu O., Smith M., et al. Susceptibility of snails to infection with schistosomes is influenced by temperature and expression of heat shock proteins. Epidemiology (Sunnyvale) 2015;5:p. 189. doi: 10.4172/2161-1165.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fogarty C. E., Zhao M., McManus D. P., Duke M. G., Cummins S. F., Wang T. Comparative study of excretory-secretory proteins released by Schistosoma mansoni-resistant, susceptible and naïve Biomphalaria glabrata. Parasites & Vectors. 2019;12(1):p. 452. doi: 10.1186/s13071-019-3708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shanab S. M. M., Hafez R. M., Fouad A. S. A review on algae and plants as potential source of arachidonic acid. Journal of Advanced Research. 2018;11:3–13. doi: 10.1016/j.jare.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Multiple Reaction Monitoring (MRM) chromatograms of DHA-the internal standard- (A), palmitic (B), oleic (C), linoleic (D) and arachidonic (E) fatty acids, using the optimized method conditions at their selective transitions (Table S1). Table S1A: method validation parameters for palmitic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision; Table 1B: method validation parameters for oleic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision; Table 1C: method validation parameters for linoleic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision; Table 1D: method validation parameters for arachidonic acid: precision, accuracy, and recovery. ∗a: intraday precision, b: interday precision. Table S2: soft tissue protein content of Biomphalaria alexandrina snails. Table S3: hemolymph protein content of Biomphalaria alexandrina snails. Table S4: soft tissue protein content of Bulinus truncatus snails. Table S5: hemolymph protein content of Bulinus truncatus snails.
Data Availability Statement
All data are shown in tables, figures, and supplementary materials.


