Abstract
Background and aims
The small bowel has, to date, remained a difficult area to access via endoscopy. The novel motorized power spiral enteroscopy, recently introduced, has more depth of insertion and is of shorter duration. Presented here is a case series of motorized spiral enteroscopies.
Methods
Motorized spiral enteroscopy is indicated for patients requiring deep enteroscopy (eg, for a diagnosis of obscure GI bleeding, suspected inflammatory bowel disorder) and for therapeutic interventions, such as polypectomy, hemostasis, or stricture dilatation. It is contraindicated in patients who are not eligible for general anesthesia, with perforation, or with coagulopathy and in the pediatric population. The contraindications for the anterograde route are gastroesophageal varices, foregut stenosis, eosinophilic esophagitis, and so on. The retrograde approach is contraindicated in active colitis, anal stenosis, or colonic stricture. Informed consent was sought from all the patients.
Results
The procedure was successful in 13 of 14 (92.8%) in that the target site was reached or panenteroscopy was confirmed. The diagnoses were stricture and ulcers of the jejunum or ileum. The procedures performed were argon plasma coagulation and stricture dilation. The average duration of anterograde enteroscopy was 61.1 minutes and retrograde enteroscopy was 90 minutes. The major adverse events were hypothermia (3 of 14) and pancreatitis (1 of 14), from which the patients recovered fully.
Conclusions
Novel motorized power spiral enteroscopy is a great tool in small-bowel diagnostics and therapeutics. Speed and ease add to the attractiveness of the procedure.
The small bowel has until now been a difficult area to access via endoscopy. For many decades, push enteroscopy remained the only way to visualize the small bowel.1 Video capsule endoscopy, introduced in 2000, created a revolutionary change in that the entire small bowel could be visualized.2 The double-balloon enteroscope (Fujifilm, Tokyo, Japan) was introduced by Yamamoto et al in 2001.3 The double-balloon enteroscope was a lengthy procedure, involved substantial radiation exposure because of constant fluoroscopy, and did not have much stability during endoscopic procedures. Single-balloon enteroscopy (2007, Olympus corp, Tokyo, Japan) had similar drawbacks.4 Total enteroscopy rates and depth of maximum insertion with device-assisted techniques were still low.5 Laparoscopy-assisted enteroscopy was used whenever panenteroscopy was desired.6 The spiral enteroscope (Spirus Medical, Stoughton, Mass, USA) was introduced in 2008. This enteroscope required manual spiral rotation. It needed 2 operators and proved tedious. The novel motorized power spiral enteroscope (Olympus corp) has done away with the shortcomings of the predecessors.7 It is a single-operator technique.
Materials and methods
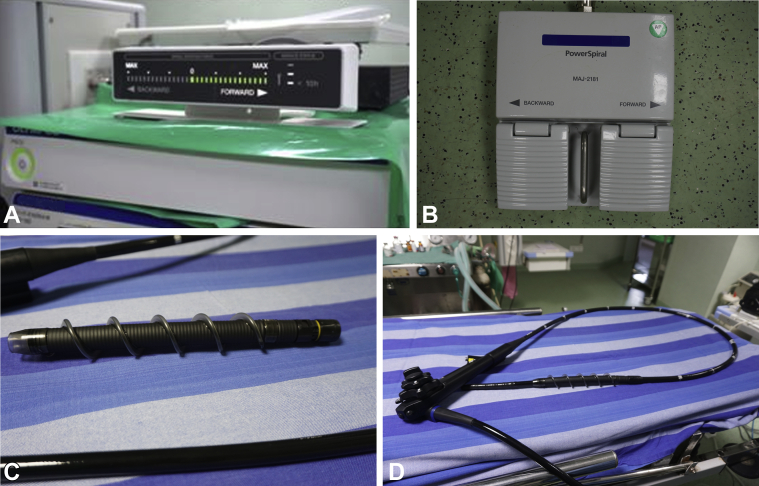
The motorized spiral enteroscope is 168 cm long (Fig. 1A and 1B) and 12.8 mm in diameter with a 3.2-mm working channel. It is compatible with standard colonoscopic accessories. It has an inbuilt motor operated by a foot pedal (Fig. 1C), which drives the rotation of the spiral segment. The forward pedal causes clockwise movement of the spiral segment. This causes pleating of the bowel and forward movement of the endoscope. The backward pedal causes the bowel to unpleat. The single-use disposable spiral overtube is 24 cm in length (Fig. 1A and 1B) and fits 16 cm from the tip of the enteroscope (behind the bending section) by a double-lock mechanism. It has a rubber spiraling fin with a total diameter of 31 mm. Although not recommended, before anterograde approach, serial bougie dilation is done up to 18 mm to ensure easy passage of the spiral segment. The neck of the patient is extended, and the endotracheal tube cuff is deflated to facilitate passage across the cricopharynx.
Figure 1.
A, Novel motorized power spiral enteroscope. B, Disposable spiral overtube. C, Foot pedal, which drives the motor. D, Force gauge.
The spiral rotation is started right at the cricopharyngeal region and continued intermittently throughout the procedure. Standard enteroscopy maneuvers are used to move forward, in addition to operation of the motorized spiral. The safety features include a force gauge that helps to prevent injury due to excessive force. The force gauge stops the spiral rotation when there is excess pressure because of undue looping or distal obstruction (Fig. 1D). This acts as a safeguard against perforation, which was reported to have a 0.27% rate with conventional spiral enteroscopy.8 Carbon dioxide and water irrigation is used during insertion. Limited water irrigation is done at sharp turns and bends; excess water irrigation hinders the pleating action. Fluoroscopy is not necessary but may be used rarely for better localization. During withdrawal, the endoscope has to be maintained at the 80-cm mark until the endoscope exits from the pylorus.
Indications and contraindications
Motorized spiral enteroscopy is indicated for patients requiring deep enteroscopy (eg, for a diagnosis of obscure GI bleeding, suspected inflammatory bowel disorder) and for therapeutic interventions, such as polypectomy, hemostasis, or stricture dilatation. The endoscope is stable while sampling tissue and while performing therapies such as argon plasma coagulation, hemoclipping, polypectomy, and stricture dilatation.
Motorized spiral enteroscopy is contraindicated in patients who are not eligible for general anesthesia or prolonged deep sedation. Other contraindications include patients with suspected perforation, uncontrolled coagulopathy, or recently placed feeding jejunostomy and the pediatric population. For the anterograde approach, additional contraindications include esophageal or gastric varices, foregut stenosis, deep mucosal laceration, suspected eosinophilic esophagitis, and inability to accept the mouthpiece. Relative contraindications are cervical disc prolapse or inability to extend the neck. Contraindications for the retrograde approach include severe active inflammation of the colon, anal stenosis, or colonic stricture. A cautious approach is advised for stricturizing diseases, previous abdominal or pelvic surgery, altered anatomy, or pregnancy.
Preparation
For the anterograde approach, overnight fasting before the procedure was sufficient. For the retrograde approach, bowel preparation similar to colonoscopy was done. Anterograde enteroscopy was done with the patient under general anesthesia. Nasotracheal intubation was used. For the solely retrograde approach, deep sedation was given. Informed consent was sought from all patients before the procedure.
Here, we present a case series of 14 patients who underwent novel motorized spiral enteroscopy at a gastroenterology referral center.
Results
The demographic characteristics, indication for enteroscopy, route of enteroscopy, procedure duration, findings, therapeutic intervention, and adverse effects are given in Table 1 and summarized in Table 2.
Table 1.
Case series of novel motorized spiral enteroscopy
| No. | Age/sex | Indication for enteroscopy | Route of enteroscopy | Successful (target reached) | Time taken | Findings | Histopathology findings | Procedures performed | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28/M | Distal jejunal and proximal ileal strictures on CT enterography | Anterograde and retrograde | Yes | 30 min 40 min |
Distal jejunal stricture, proximal ileal stricture with nodularity and ulceration | Crohn’s enteritis | Biopsy | – |
| 2 | 60/M | Short-segment wall thickening in distal jejunum and proximal ileum on CT enterography | Anterograde | Yes | 15 min | Mid-jejunal stricture with ulcers | Crohn’s enteritis | Biopsy | – |
| 3 | 64/M | Long-segment thickening in proximal ileum; multiple large mesenteric nodes on CT enterography | Retrograde and push enteroscopy anterograde | Yes | 75 min | Proximal ileal stricture | Crohn’s enteritis | Balloon dilatation and biopsy | – |
| 4 | 44/M | Thickening with mucosal enhancement of proximal jejunal loops on CECT abdomen | Anterograde | Yes | 30 min | Tight stricture in distal jejunum with preceding clean-based ulcer | Crohn’s jejunitis | Biopsy | – |
| 5 | 36/M | End-stage renal disease; retrieval of retained capsule endoscope due to proximal ileal stricture | Retrograde; patient did not consent to anterograde | Yes | 180 min | Proximal ileal stricture noted | Nonspecific ileitis | Balloon dilation; procedure abandoned because patient could not tolerate the prolonged anesthesia. Plan to repeat later. | Hypothermia |
| 6 | 25/M | Skip areas of circumferentially enhancing wall thickening in mid- and distal ileum | Anterograde and retrograde (panenteroscopy confirmed by tattooing) | Yes | 60 min 90 min |
Normal study | – | Panenteroscopy | – |
| 7 | 44/F | Subacute intestinal obstruction, with history of appendectomy and cesarean section; high CRP and fecal calprotectin | Anterograde could not be done because the spiral segment could not pass beyond the cricopharynx. Retrograde approach was difficult, probably because of adhesions—150 cm of ileum reached. | Yes | 85 min | Distal ileal narrowing with erosions | Crohn’s ileitis | Biopsy | – |
| 8 | 82/F | Recurrent obscure GI bleeding | Anterograde could not be done because of a short neck. Hence retrograde was done. Tattooing and push enteroscopy were done anterograde and panenteroscopy confirmed. | Yes | 80 min | Angiectasias in ileum and jejunum | – | APC | – |
| 9 | 63/M | Obscure GI bleeding | Anterograde—cecum reached | Yes | 105 min | Ileal ulcers with bleeding | Crohn’s ileitis | APC | – |
| 10 | 80/F | Obscure GI bleeding | Anterograde and retrograde | Yes | 90 min 110 min |
Jejunal and ileal angiectasias | – | APC | Hypothermia |
| 11 | 50/M | Midjejunal stricture | Anterograde | Yes | 30 min | Mid-jejunal stricture | Crohn’s jejunitis | Biopsy | – |
| 12 | 65/M | Short segment of distal jejunal loop adherent to anterior abdominal wall in left iliac fossa region | Anterograde and retrograde | Yes | 100 min 60 min |
Ileal ulcer | Nonspecific ileitis | Biopsy | – |
| 13 | 58/F | Long-segment wall thickening in distal jejunum and proximal ileum | Anterograde (not progressing beyond jejunum) and retrograde (nonprogression beyond ileocecal valve) | No | – | Small jejunal ulcers and erosions | Nonspecific jejunitis | Biopsy | Hypothermia and pancreatitis |
| 14 | 79/M | Obscure occult GI bleeding | Anterograde—panenteroscopy confirmed by reaching cecum | Yes | 90 min | Normal | – | – | – |
CECT, Contrast-enhanced computerized tomography; APC, argon plasma coagulation; CRP, C-reactive protein.
Table 2.
Routes of enteroscopy
| No. | Route of enteroscopy | n/N (%) |
|---|---|---|
| 1 | Anterograde only | 5/14 (35.71) |
| 2 | Retrograde only | 4/14 (28.57) |
| 3 | Both anterograde and retrograde | 5/14 (35.71) |
The indications for the enteroscopy in this case series were small-bowel pathology needing histopathology and obscure GI bleeding. All of our patients with abdominal pain had undergone CT enterography with definite findings before enteroscopy. One patient had undergone capsule endoscopy, which showed ulcers in the mid-ileum, and was scheduled for spiral enteroscopy for biopsy. Informed consent was obtained before the procedure. In the previous case reports, the indication for motorized spiral enteroscopy was obscure GI bleeding.7,9 In the current case series, 10 of 14 procedures were indicated for suspected thickening/stricture of ileal and jejunal segments; obscure GI bleeding was the indication in 4 of 14 patients. In the patients with suspected stricture, the site of pathology was reached and biopsy specimens were taken. The biopsy and procedures were performed during insertion because it was possible to miss lesions during withdrawal.
The procedural yield and the route taken are shown in Table 3. The duration of anterograde and retrograde enteroscopy is shown in Table 4. Panenteroscopy was confirmed on entering the cecum in 2 patients (Video 1, available online at www.VideoGIE.org). The duration of panenteroscopy was 90 minutes in 1 patient and 105 minutes in another. In other patients (3 of 14) it was confirmed by tattooing and reaching the tattooed segment via the opposite route. In most patients, panenteroscopy was not attempted because of strictures. Even if the stricture was crossed, care was taken that the spiral segment did not cross the stricture.
Table 3.
Procedural yield
| No. | Procedural yield | n/N (%) |
|---|---|---|
| 1 | Target lesion reached or total enteroscopy achieved | 13/14 (92.8) |
| 2 | Total enteroscopy | 5/14 (35.71) |
| 3 | Total enteroscopy by anterograde route alone (complete anterograde enteroscopy) | 2 |
| 4 | Total enteroscopy with anterograde and retrograde routes combined | 1 |
| 5 | Total enteroscopy with retrograde route and push enteroscopy anterograde | 2 |
Table 4.
Time taken for enteroscopy
| No. | Route of enteroscopy | Average duration (min) |
|---|---|---|
| 1 | Anterograde enteroscopy | 61.1 |
| 2 | Retrograde enteroscopy | 90 |
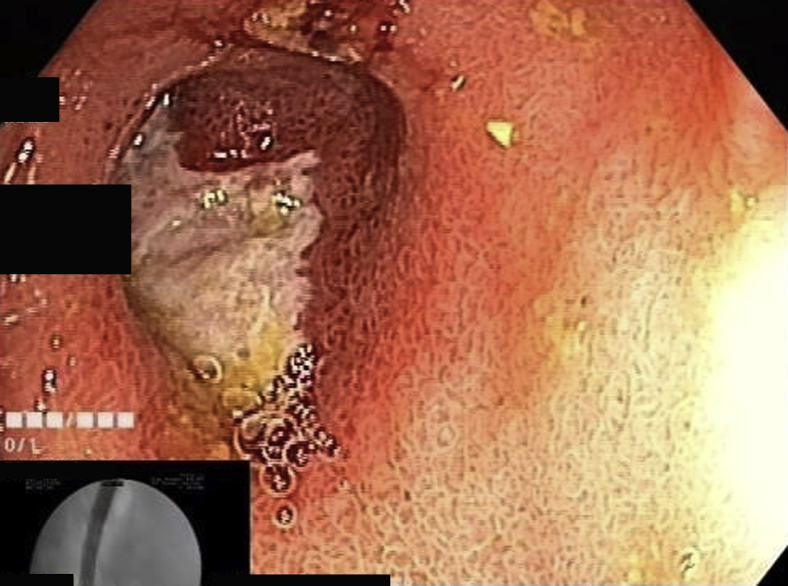
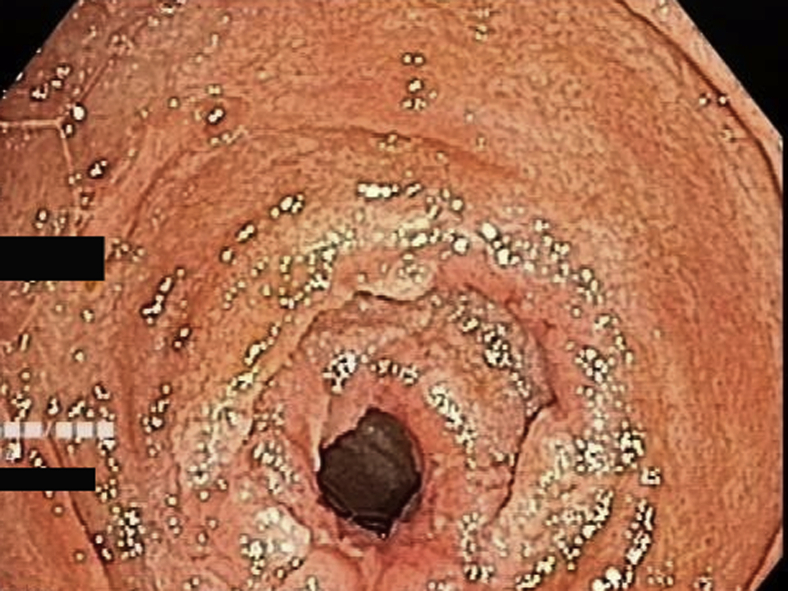
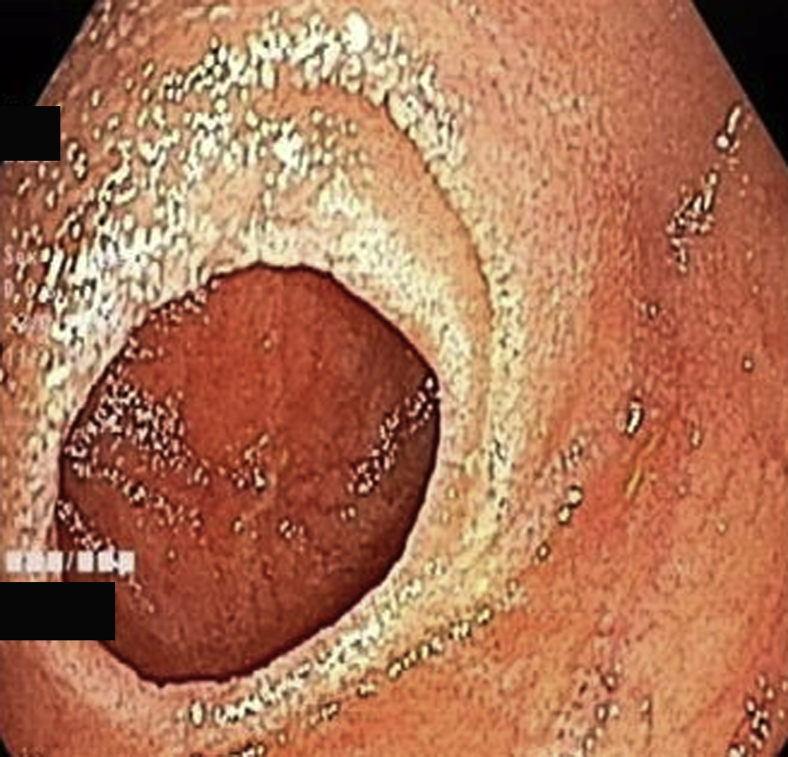
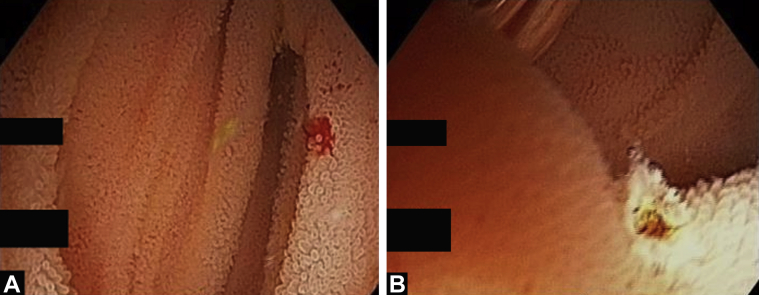
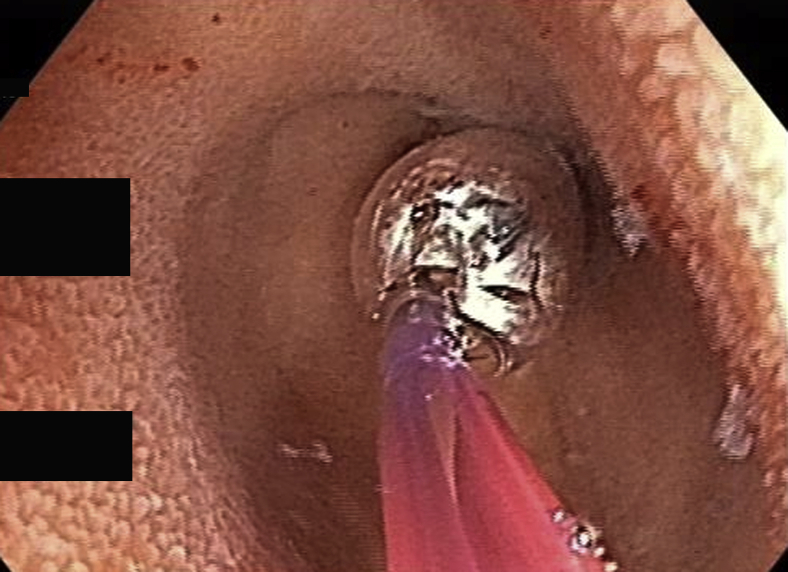
The target lesion was reached or total enteroscopy was achieved in 13 of 14 of cases (92.8%). The most common diagnoses were strictures or ulcers of the jejunum or ileum (Figure 2, Figure 3, Figure 4). In obscure GI bleeding, the diagnoses were small-bowel angiectasias and ileal ulcers with ooze. In the initial learning curve, having a roadmap like a previous capsule endoscopy or CT enterography can be very useful. In most patients with small-bowel strictures and ulcers, the histopathologic diagnosis was Crohn’s disease (7 of 10 patients). Therapeutic procedures performed were argon plasma coagulation for angiectasia (Fig. 5A and B), argon plasma coagulation for oozing ulcer, and balloon dilation of the stricture (Fig. 6).
Figure 2.
Distal jejunal stricture.
Figure 3.
Mid-jejunal stricture.
Figure 4.
Distal jejunal stricture.
Figure 5.
A and B. Argon plasma coagulation of ileal angiectasia.
Figure 6.
Balloon dilatation of proximal ileal stricture.
Previous case reports on use of novel motorized spiral enteroscopy report success in reaching the targeted lesion, short duration, and no serious adverse events.7,9 The issues faced with motorized spiral enteroscopy in our series were mucosal abrasions to the cricopharyngeal region because of the large spiral tube. However, none of the patients had perforation, and they were all given liquids within 12 hours. Some (3 of 14) had mild odynophagia, which resolved with oral sucralfate suspension. In 2 patients, the spiral segment could not be passed across the cricopharynx. Both of the patients were female and had a short neck. The thick ring-like stub that projects from the site where the spiral overtube locks onto the tip of the endoscope is difficult to pass across the cricopharynx. Both of the patients underwent retrograde spiral enteroscopy, and the target lesion was reached successfully. Some patients (3 of 14) had superficial mucosal abrasions in the small intestine. The patients were asymptomatic.
Three patients had hypothermia during the procedure and recovered within 6 hours. Hypothermia was probably due to a large volume of water infusion and prolonged anesthesia. This prompted us to use warm water as a protocol, after which we did not find hypothermia. One patient developed pancreatitis and recovered with conservative management.
Conclusion
Novel motorized spiral enteroscopy is a great tool for diagnostic and therapeutic enteroscopy. Panenteroscopy is easily achieved with this. Speed and ease add to the attractiveness of the procedure.
Disclosures
All authors disclosed no financial relationships.
Footnotes
If you would like to chat with an author of this article, you may contact Dr Prasad at drvgm@rediffmail.com.
Disclosure: All authors disclosed no financial relationships.
Supplementary data
A spiraling journey into the small bowel. A case series of novel motorized power spiral enteroscopy.
References
- 1.Foutch P.G., Sawyer R., Sanowski R.A. Push-enteroscopy for diagnosis of patients with gastrointestinal bleeding of obscure origin. Gastrointest Endosc. 1990;36:337–341. doi: 10.1016/s0016-5107(90)71060-7. [DOI] [PubMed] [Google Scholar]
- 2.Iddan G., Meron G., Glukhovsky A. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H., Sekine Y., Sato Y. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2010;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann D., Eickhoff A., Tamm R. Balloon-assisted enteroscopy using a single-balloon technique. Endoscopy. 2007;39S1:E276. doi: 10.1055/s-2007-966616. [DOI] [PubMed] [Google Scholar]
- 5.Schneider M., Hollerich J.J., Beyna T. Device-assisted enteroscopy: a review of available techniques and upcoming new technologies. World J Gastroenterol. 2019;25:3538–3545. doi: 10.3748/wjg.v25.i27.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meister T.E., Nickl N.J., Park A. Laparoscopic-assisted panenteroscopy. Gastrointest Endosc. 2001;53:236–239. doi: 10.1067/mge.2001.108300. [DOI] [PubMed] [Google Scholar]
- 7.Neuhaus H., Beyna T., Schneider M. Novel motorized spiral enteroscopy: first clinical case. VideoGIE. 2016;1:32–33. doi: 10.1016/j.vgie.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akerman P. Severe complications of spiral enteroscopy in the first 1750 patients. Gastrointest Endosc. 2009;69:127–128. [Google Scholar]
- 9.Mans L., Arvanitakis M., Neuhaus H. Motorised spiral enteroscopy for occult bleeding. Dig Dis. 2018;36:325–327. doi: 10.1159/000488479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A spiraling journey into the small bowel. A case series of novel motorized power spiral enteroscopy.