To the editor:
We read the recent study by Detterich et al.1 on oxygen supply-demand mismatch in adults with sickle cell disease (SCD) with and without strokes receiving regular blood transfusion therapy. The investigators evaluated flow-mediated-dilation (FMD) with laser Doppler flowmetry in 26 participants and revealed no acute change in regional oxygen saturation or perfusion prior to, and following, blood transfusion. Blood transfusion therapy was found to improve FMD and oxygen supply-demand matching but not the hyperemic response, which was blunted in adults with SCD and proportional to resting perfusion.
In patients with SCD, cerebral blood flow (CBF) is elevated to offset reduced oxygen delivery from anemia. However, many clinical studies reveal an absence of even partial normalization of CBF following blood transfusion therapies. We postulate that the lack of detectable changes following blood transfusion is attributable to additional relevant factors that contribute to oxygen delivery.
Specifically, changes in hemoglobin (Hb) levels and reduced Hb-S (HbS) fraction secondary to transfusion can alter microvascular flow velocities and capillary transit time (i.e., time required for blood to traverse the capillary network). In patients with arterio-venous capillary shunting, in whom capillary transit time is reduced, there may be insufficient time to offload oxygen and as such oxygen delivery to tissue may be sub-optimal despite elevated CBF. Previous work has provided evidence from blood-based markers that arterio-venous shunting is present in SCD2, and here we tested whether increases in Hb secondary to blood transfusion can lead to an increase in capillary transit time and corresponding increase in tissue oxygen extraction in the absence of a CBF change.
A 9-year-old African American male with SCD (HbSS) provided written, informed consent/assent and was enrolled in a larger IRB-approved prospective study to assess cerebral hemo-metabolic changes in children with SCD. The child was followed in a SCD clinic for approximately monthly blood transfusions initiated at 3-years-of-age for primary stroke prevention. The child’s provider reported two imaging-type transcranial doppler ultrasounds (TCDs) completed within one week that showed time-averaged mean middle cerebral artery (MCA) maximum velocity >190 cm/s (above 185 cm/s threshold for transfusion with imaging TCD). He was treated with blood transfusions and showed no stroke-like symptoms. MRI and MR angiography (MRA) completed at 9-years-of-age revealed unchanged prior right anterior cerebral artery (ACA)-MCA watershed infarcts and left MCA-posterior cerebral artery watershed infarcts, 6-10 chronic infarct lesions greater than 3 mm in maximal cross-sectional diameter in the subcortical white matter and 5 smaller bilateral white matter lesions less than 3 mm. Detailed neurological examination and history obtained from the mother during the study visit confirmed that infarcts met the definition of silent cerebral infarcts (SCIs). MRA showed intracranial stenosis of the bilateral first segments of the ACA of approximately 90%.
For CBF assessment, arterial spin labeling (ASL) MRI using a multi-compartment quantification procedure that accounted for the measured blood T1 and labeling efficiency (labeling efficiency=0.72) was applied3. For oxygen-extraction-fraction (OEF) assessment, the T2-relaxation-under-spin-tagging (TRUST) MRI approach was utilized with effective-echo-time range=0-160 ms, inter-pulse-spacing=10 ms, and post-labeling-delay=1022 ms4. Images were pair-wise subtracted and supra-tentorial OEF calculated as the fractional difference in arterial oxygenation measured from pulse-oximetry and venous oxygenation measured in the superior sagittal sinus from TRUST. As there is debate regarding optimal calibration, we applied three human blood models derived from hemoglobin-A, hemoglobin-F, and hemoglobin-S blood (Supplementary Material).
On ASL, a CBF of 76.0 ml/100g/min was found as well as venous endovascular signal extending from the superior sagittal sinus to the confluence of the sagittal and transverse sinuses, a phenomenon previously shown to be associated with high flow velocities and consistent with cerebral capillary shunting5 (Figure). A global OEF of 36.1% (HbF model), 32.6% (HbA model), and 23.1% (HbS model) was calculated.
Figure.
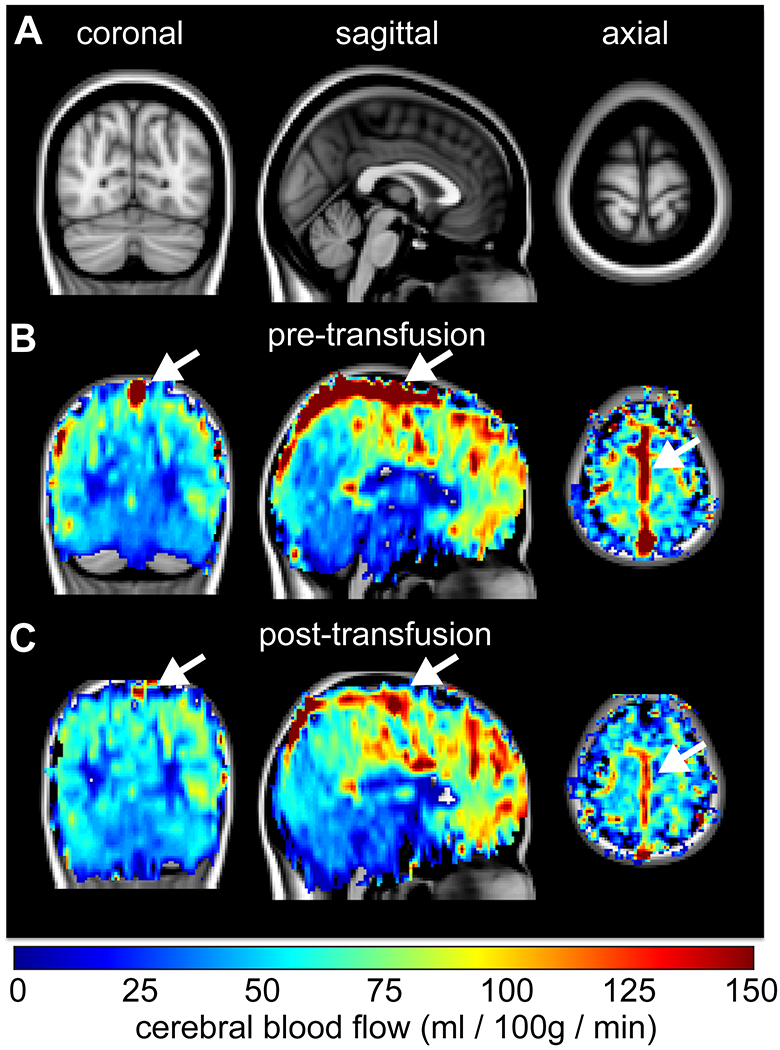
A 9-year-old male with sickle cell disease (SCD) scanned before and approximately two hours after exchange transfusion. A standard 2 mm T1-weighted brain atlas (A) is shown together with the cerebral blood flow (CBF)-weighted arterial spin labeling maps before (B) and after (C) transfusion. While the cortical CBF is largely unchanged after transfusion (mean=70-80 ml/100g/min), signal in the superior sagittal sinus (white arrow) is markedly reduced after transfusion. This is consistent with an increase in capillary transit times after transfusion, which paralleled an increase in tissue oxygen extraction of 1-9%. These data highlight that transfusions can alter oxygen delivery in patients with SCD even in the absence of changes in CBF.
On the same day, the patient underwent a modified manual exchange blood transfusion. Two units of blood (525 mL each), were removed and transfused. Following transfusion, Hb and hematocrit increased from 9.1 g/dL and 28% to 9.9 g/dL and 30%, respectively. This paralleled a reduction in HbS from 42% to 27%.
Following transfusion, neuroimaging was repeated on the same day which, consistent with the study by Detterich et al.1, revealed that CBF remained unchanged within error at 79.7 ml/100g/min. However, CBF-weighted ASL signal in the superior sagittal sinus decreased in intensity by 102.2% (Figure), consistent with a reduction in capillary shunting and increase in capillary transit time. Arterial oxygenation (SaO2) increased from 98% pre-transfusion to 99% post-transfusion (fractional oxygen carrying capacity, 1.34 mlO2/g*SaO2*[Hb] g/dl, increase of 9.9%). Post-transfusion, the calculated OEF was 36.3% (HbF model), 33.8% (HbA model), and 25.2% (HbS model), resulting in OEF increases for all models of 0.73% (HbF model), 3.7% (HbA model), and 8.9% (HbS model). Therefore, capillary transit time increases, which can manifest as a venous signal reduction on ASL scans, were associated with an OEF increase despite no significant CBF change. We postulate that the origin of overt strokes and SCIs may be partly attributable to impaired oxygen delivery according to this arterio-venous shunting mechanism.
Prior work has shown that OEF can be pathologically increased in patients with SCD and infarcts4,6. OEF elevations are typically explained by a mismatch in oxygen consumption and delivery and are logical in some moderate-to-severely impaired patients with preserved metabolic activity but reductions in total oxygen delivery. Here, the OEF was observed to be in a typical range for healthy tissue (23-36%). It is possible that in mildly affected patients, where oxygen delivery is largely maintained by adjustments in arteriolar autoregulation, the impact of capillary shunting on OEF may be more prominent.
These data indicate that blood transfusions in patients with SCD can alter tissue oxygenation even in the absence of measurable differences in CBF. The proposed mechanism is an increase in pathologically reduced and possibly heterogeneous, capillary transit times, allowing for improved distribution of blood through capillary beds and therefore increased offloading of oxygen to tissue. As SCD pathophysiology is heterogeneous with competing effects from anemia, vasculopathy, and autoregulation, studies that quantify these factors together may be required to provide a comprehensive perspective on impairment.
Supplementary Material
Acknowledgments
Funding provided by: National Institute of Neurological Disorders and Stroke [1R01NS07882801]; National Institute of Nursing Research [1R01NR01507901]; and National Institute of Neurological Disorders and Stroke [1R01NS097763].
References
- 1.Detterich JA, Kato R, Bush A, et al. Sickle cell microvascular paradox-oxygen supply-demand mismatch. Am J Hematol. 2019;94(6):678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahavandi M, Millis RM, Tavakkoli F, et al. Arterialization of peripheral venous blood in sickle cell disease. Journal of the National Medical Association. 2002;94(5):320–326. [PMC free article] [PubMed] [Google Scholar]
- 3.Juttukonda MR, Jordan LC, Gindville MC, et al. Cerebral hemodynamics and pseudo-continuous arterial spin labeling considerations in adults with sickle cell anemia. NMR Biomed. 2017;30(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan LC, Gindville MC, Scott AO, et al. Non-invasive imaging of oxygen extraction fraction in adults with sickle cell anaemia. Brain. 2016;139(Pt 3):738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juttukonda MR, Donahue MJ, Waddle SL, et al. Reduced oxygen extraction efficiency in sickle cell anemia patients with evidence of cerebral capillary shunting. Journal of cerebral blood flow and metabolism. 2020:271678X20913123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields ME, Guilliams KP, Ragan DK, et al. Regional oxygen extraction predicts border zone vulnerability to stroke in sickle cell disease. Neurology. 2018;90(13):e1134–e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


