Abstract
Purpose
To report a case of neovascularization of posterior capsule (NVPC) successfully treated with intravitreal ranibizumab (Lucentis) and neodymium:YAG (Nd:YAG) capsulotomy, followed by phacogenic uveitis.
Observations
We report a systemically otherwise healthy 81-year-old male presenting with unilateral NVPC and iris (NVI) occurring five years after a central retinal vein occlusion. A single intravitreal injection of ranibizumab led to complete regression of NVPC and NVI within three weeks after which a Nd:YAG capsulotomy was performed. Two weeks later, the patient returned with a severe inflammatory reaction diagnosed as phacogenic uveitis and treated with surgical capsular bag/intraocular lens complex removal and peripheral pan-retinal photocoagulation. One-year follow-up demonstrated no recurrence of NVPC. Visual acuity remained at baseline of light perception.
Conclusions
We acknowledge that intravitreal anti-vascular endothelial growth factor treatment with Nd:YAG capsulotomy for NVPC is a rational option, but raise awareness to the rare possibility of phacogenic uveitis.
Keywords: Neovascularization, Neovascularization of posterior capsule, Phacogenic uveitis, Lens-induced uveitis, Central retinal vein occlusion
1. Introduction
Neovascularization of the posterior capsule (NVPC) is a rarely reported occurrence characterized by vascular proliferation behind the posterior lens capsule causing opacification and decreased visual acuity.1, 2, 3, 4, 5, 6, 7 Neovascularization in various areas of the eye is a common phenomenon caused by the secretion of vascular endothelial growth factor (VEGF) driven by ischemia. Therapeutic inhibition of VEGF is one treatment option which leads to regression of neovascular vessels, reducing the downstream complications such as intraocular hemorrhage, neovascular glaucoma, and tractional retinal detachments.
Lens-induced uveitis (LIU) is autoimmune-mediated attack of crystalline lens proteins, which are immune privileged under healthy physiological conditions. Phacogenic uveitis is a subcategory of LIU caused by rupture of the lens capsule (e.g. trauma, surgery), leading to exposure of lens material and causing autosensitization. Decrease in the prevalence of LIU is correlated to increased availability of safe and affordable cataract surgeries in preventing advanced cataracts.8,9
We present a case of NVPC successfully treated with intravitreal ranibizumab (Lucentis) and Nd:YAG capsulotomy that resulted in phacogenic uveitis.
2. Case report
The patient is a systemically healthy 81-year-old male with an ocular history of an uncomplicated bilateral phacoemulsification with a one-piece acrylic intraocular lens (IOL) (SA60, Alcon) completed in the 2000s. In addition, he had an initially ischemic left central retinal vein occlusion (CRVO) with central cystoid macular edema (CME) in 2011. Given the severity of the ischemia and decrease in visual acuity (VA), treatment with intravitreal ranibizumab injections administered in a treat-and-extend protocol over two years in an effort to treat the CME. Neovascularization was not observed during the follow-up period. Final VA at that time in the left eye was counting fingers at one foot with an atrophic retina, and a central retinal thickness (CRT) of 154 μm, as measured by standard domain optical coherence tomography (sdOCT: Carl Zeiss, Cirrhus 4000). He was observed until 2016 with no recurrent CME and was then referred back to his optometrist for routine care.
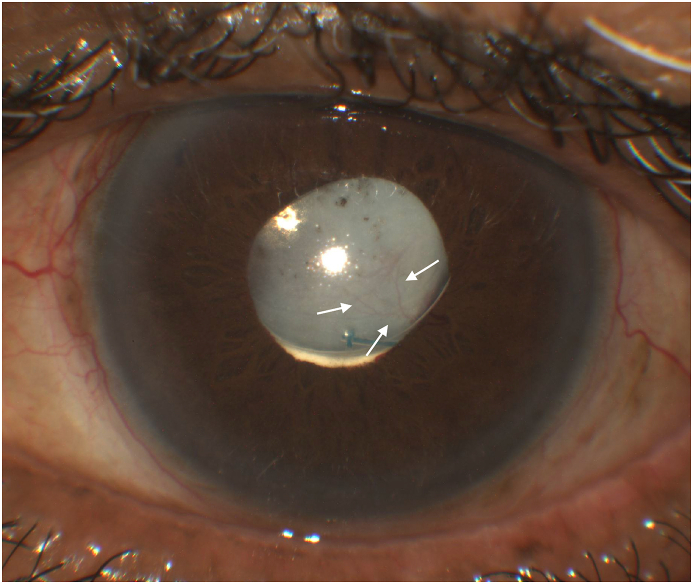
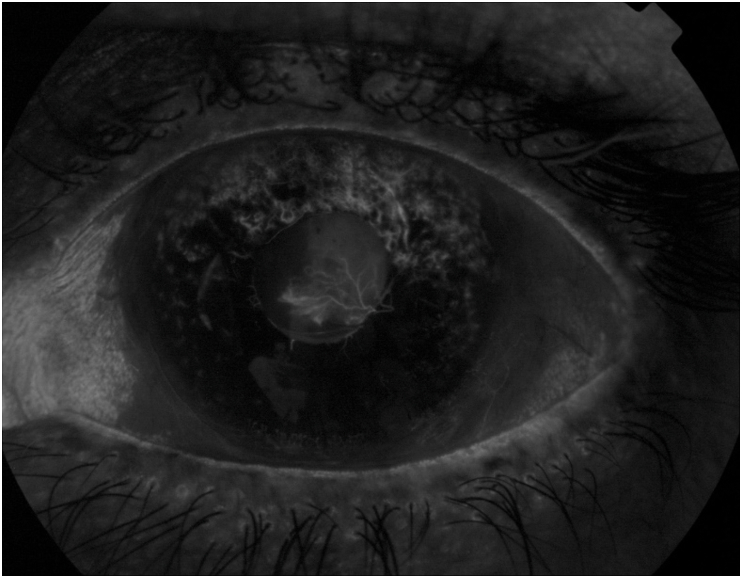
In 2018, the patient presented with self-reported decreased VA in the left eye from counting fingers to light perception (LP). Anterior segment examination of his left eye demonstrated a clear cornea, a quiet anterior chamber, and a white conjunctiva and sclera. Dense posterior capsule opacification (PCO) covered the one-piece IOL with extensive neovascularization of the superior and temporal iris (NVI) extended onto the inferior temporal portion of the posterior capsule (Fig. 1). Intraocular pressure (IOP) was 9 mmHg. Visualization or OCT imaging of the posterior segment was not possible due to the dense PCO. A B-scan revealed an attached retina with a clear vitreous. Imaging with intravenous fluorescein angiography confirmed NVPC (Fig. 2). Examination of the right eye revealed VA of 20/50, IOP of 8 mmHg, an intact IOL and patent capsule, and a normal posterior segment exam.
Fig. 1.
Colour image depicting neovascularization of posterior capsule. Patient's left eye with neovascular vessels in inferior-temporal posterior lens capsule. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Intravenous fluorescein angiography of neovascular vessels. Visualization of neovascularization of posterior capsule and iris in inferior-temporal area of left eye.
The patient was diagnosed with left eye NVPC secondary to a remote history of ischemic CRVO. The patient consented for the following procedures in a staged format: anti-VEGF injection, Nd:YAG capsulotomy, and pan-retinal photocoagulation (PRP).
The first stage treatment was a single dose of 0.5mL intravitreal ranibizumab (Lucentis) injection carried out aseptically without complication. At the three-week follow-up, there was evidence of complete regression of both NVI and NVPC, and an absence of cells in the anterior chamber. The second stage treatment was an uncomplicated Neodyminum:YAG (Nd:YAG) capsulotomy (Ellex, Tango, 2.0mJ, 15 pulses). Lotemax Gel QID (Bausch and Lomb) was initiated for one week. VA in left eye remained at LP post-capsulotomy.
Fourteen days following the Nd:YAG capsulotomy, and prior to the scheduled PRP appointment, the patient presented to the emergency department with left eye pain, a 3+ injected eye, a 4+ cell and flare in the AC, 2mm hypopyon, IOP of 35 mmHg, and an unchanged VA (LP). The posterior segment was not visible through the patent capsulotomy. B-scan revealed an attached retina with moderate vitreous debris. Topical Moxifloxacin (Vigamox) and PredForte 1% (Allergan Pharmaceutical) eye drops were initiated hourly. The patient was referred to surgical retina for further management. A presumptive diagnosis of infectious endophthalmitis was made, and a pars plana vitrectomy (PPV) was carried out, successfully alleviating the patient from pain.
Intraoperatively, a significant amount of residual cataract material was noted around the IOL in a Soemmering ring configuration with surrounding inflammatory infiltrate. Given this finding, the working diagnosis was modified to phacogenic uveitis. The IOL and capsular bag complex were completely removed in toto to remove the nidus of the severe intraocular inflammatory reaction. On inspection, there was no suspicious areas typical of P.acnes on examination of the explanted IOL-capsular complex. A small area of NVI was noted intraoperatively as well, and peripheral PRP was completed followed by injection of intravitreal vancomycin (1mg), ceftrazidine (2mg), dexamethasone (400 mg), and bevacizumab (1.25mg). The rest of the procedure was completed without intraoperative complications. The patient was put on topical Tobradex QID (Novartis Pharmaceuticals) for one month and healed without complications. Culture of the aspirated vitreous and entire large pupillary vascular capsular membrane was negative for organism growth.
Prompt resolution to the left phacogenic uveitis was noted at the subsequent follow up visits. One-week post-operatively, VA remained at LP, a quiet aphakic anterior and posterior segment with evidence of full PRP was noted, and IOP was 15 mmHg. This ocular stability persisted and was noted on his one-year follow-up with no evidence of recurrent NVPC or NVI.
3. Discussion
The earliest case of NVPC was reported by Erig et al., in 1990, who coined the term “rubeosis capsulare” in two diabetic patients following cataract extraction.6 While most NVPC cases since have reported the same etiology1,2,4, other etiologies include idiopathic ciliary body inflammation3, surgical interventions (pneumatic retinopexy and pars plana vitrectomy)5, and traumatic microcapsular breach.7 Cases of NVPC occurring with NVI2,6 and in isolation3,5 have both been reported.
Previous studies have reported successful management of NVPC with intravitreal bevacizumab (Avastin) injections followed by Nd:YAG capsulotomy.1,2,4 Other treatments that have been reported include photodynamic therapy followed by Nd:YAG capsulotomy5 and vitrectomy.6 In all these reported cases, treatment restored VA close to baseline, and no recurrence of NVPC noted on their latest follow-up visits, which ranged upwards of nine months.5 In our case, VA was restored to the baseline of LP. We suspect there was no further improvement in VA due to the history of ischemic CRVO.
The rationale for using an anti-VEGF agent in the treatment of NVPC is to decrease the risk of vitreous hemorrhage during or following a Nd:YAG capsulotomy which has been successfully established with the use of bevacizumab in three cases reports.1,2,4 In our case report, we chose to use ranibizumab, a recombinant humanized anti-VEGF-A monoclonal antibody in an off-label fashion, as this drug was used in this patient's prior history for the treatment of CRVO. We report a successful regression of the NVPC as noted at the three-week follow-up, allowing the Nd:YAG capsulotomy to be performed without any hemorrhagic complications. To our knowledge, this is the first reported case of NVPC with a post-CRVO etiology, and furthermore, the first to describe the management using ranibizumab.
Another unique element of this case report is represented by the phacogenic uveitis manifestation two-weeks following Nd:YAG capsulotomy. No prior associations between phacogenic uveitis and Nd:YAG capsulotomy were found to be previously reported. The other differential diagnoses to be considered include Propionibacterium acnes endophthalmitis, post-injection infectious endophthalmitis, or severe inflammatory reaction from capsulotomy. Although we do not have histological confirmation, our clinical diagnosis is supported by the absence of microbiologic growth and clinical time-course most supportive of phacogenic uveitis. While P. acnes endophthalmitis may mimic a toxic lens syndrome10, this was less likely in this case based on the lack of an indolent presentation11 coupled with the absence of typical clinical or intraoperative features.12,13 Other differential diagnoses of phacogenic uveitis which are not relevant to our case include: post-traumatic or post-operative endophthalmitis, sterile endophthalmitis, anterior uveitis, herpetic uveitis, and traumatic iritis.9
Phacogenic uveitis is a form of lens-induced uveitis (LIU) where a ruptured lens capsule creates an autoimmune reaction to lens proteins.14 The lens is known to be immune privileged because it is isolated from fetal circulation during embryonic development, is avascular, and not innervated in adults.9 In trauma or with advanced cataracts, crystallin proteins of the lens may be exposed, causing autosensitization and subsequent inflammatory reaction.15 Phacogenic uveitis was previously known as phacoanaphylactic endophthalmitis or phacotoxic uveitis and was renamed after mast cell actions including immunoglobulin E and histamine release were not found to be involved.9 The average time of phacogenic uveitis is within two weeks of trauma or surgery to the lens, but cases taking up to 59 years have been reported.16 Confirmatory assessment with histopathology acquired from needle biopsy demonstrate granulomatous reaction surrounding lens fragments which may help confirm the diagnosis in cases of clinical ambiguity.9 Evaluating a breach in the integrity of the lens capsule is critical to the diagnosis, and the use of the anterior segment OCT has been reported as one ancillary test in this regard.17 Definitive management requires lens extraction, in conjunction with steroid anti-inflammatories, and IOP management, if indicated.9
4. Conclusion
We present the first reported case of NVPC occurring post-CRVO, and the first reported case successfully treated with intravitreal ranibizumab (Lucentis) in an off-label fashion. Furthermore, we uniquely describe the development of phacogenic uveitis post-YAG capsulotomy. We acknowledge that anti-VEGF treatment for NVPC is a rational option, but raise awareness to the rare possibility of phacogenic uveitis after Nd:YAG capsulotomy.
Patient consent
The patient verbally consented to publication of this case.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding Support
No funding or grant support.
Declaration of competing interest
The following authors have no financial disclosures: F.T., E.M., S.S.
Acknowledgements
None to be declared.
References
- 1.Al-Mohaimeed M., Al-Gehedan S., Dhibi H.A. Intravitreal bevacizumab for posterior capsule neovascularization. Saudi J Ophthalmol. 2010;24(2):63–65. doi: 10.1016/j.sjopt.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eren E., Kucukerdonmez C., Yilmaz G., Akova Y.A. Regression of neovascular posterior capsule vessels by intravitreal bevacizumab. J Cataract Refract Surg. 2007;33(6):1113–1115. doi: 10.1016/j.jcrs.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S., Gogia V., R T., Sen S., Venkatesh P. Posterior lens capsular neovascularization of young: management using endodiathermy assisted biopsy. Can J Ophthalmol. 2015;50(1):e4–7. doi: 10.1016/j.jcjo.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Castro G.Y., Hitos-Fajer A., Mendoza-Schuster E., Velez-Montoya R., Velasco-Barona C.F. Posterior capsule opacification and neovascularization treated with intravitreal bevacizumab and Nd:YAG capsulotomy. Clin Ophthalmol. 2008;2(3):657–660. doi: 10.2147/opth.s2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayata A., Unal M., Ersanli D., Gulecek O., Sonmez M. Photodynamic therapy for posterior capsule neovascularization. J Cataract Refract Surg. 2007;33(6):1131–1132. doi: 10.1016/j.jcrs.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Eifrig D.E., Hermsen V., McManus P., Cunningham R. Rubeosis capsulare. J Cataract Refract Surg. 1990;16(5):633–636. doi: 10.1016/s0886-3350(13)80783-3. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S., Gogia V., Ramya A., Sihota R. Capsular neovascularisation: case report and review of literature. Eye. 2014;28(3):358–359. doi: 10.1038/eye.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitoula R.P., Sarkar I., Nayak D., Singh S.K. Lens induced glaucoma: an experience in tertiary eye care center in eastern Nepal. Nepal J Ophthalmol. 2016;8(16):161–166. doi: 10.3126/nepjoph.v8i2.17006. [DOI] [PubMed] [Google Scholar]
- 9.Nche E.N., Amer R. Lens-induced uveitis: an update. Graefes Arch Clin Exp Ophthalmol. 2020;258(7):1359–1365. doi: 10.1007/s00417-019-04598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piest K.L., Kincaid M.C., Tetz M.R., Apple D.J., Roberts W.A., Price F.W., Jr. Localized endophthalmitis: a newly described cause of the so-called toxic lens syndrome. J Cataract Refract Surg. 1987;13(5):498–510. doi: 10.1016/s0886-3350(87)80103-7. [DOI] [PubMed] [Google Scholar]
- 11.Hall G.S., Pratt-Rippin K., Meisler D.M., Washington J.A., Roussel T.J., Miller D. Growth curve for Propionibacterium acnes. Curr Eye Res. 1994;13(6):465–466. doi: 10.3109/02713689408999875. [DOI] [PubMed] [Google Scholar]
- 12.Clark W.L., Kaiser P.K., Flynn H.W., Jr., Belfort A., Miller D., Meisler D.M. Treatment strategies and visual acuity outcomes in chronic postoperative Propionibacterium acnes endophthalmitis. Ophthalmology. 1999;106(9):1665–1670. doi: 10.1016/S0161-6420(99)90348-2. [DOI] [PubMed] [Google Scholar]
- 13.Zambrano W., Flynn H.W., Jr., Pflugfelder S.C. Management options for Propionibacterium acnes endophthalmitis. Ophthalmology. 1989;96(7):1100–1105. doi: 10.1016/s0161-6420(89)32768-0. [DOI] [PubMed] [Google Scholar]
- 14.Wirostko E., Spalter H.F. Lens-induced uveitis. Arch Ophthalmol. 1967;78(1):1–7. doi: 10.1001/archopht.1967.00980030003001. [DOI] [PubMed] [Google Scholar]
- 15.Halbert S.P., Manski W. Biological aspects OF autoimmune reactions IN the lens. Invest Ophthalmol. 1965;4:516–530. [PubMed] [Google Scholar]
- 16.Thach A.B., Marak G.E., Jr., McLean I.W., Green W.R. Phacoanaphylactic endophthalmitis: a clinicopathologic review. Int Ophthalmol. 1991;15(4):271–279. doi: 10.1007/BF00171031. [DOI] [PubMed] [Google Scholar]
- 17.Dhami A., Dhami A.S., Singh H., Dhami G.S. Role of anterior segment optical coherence tomography for safer management of mature white cataracts. J Cataract Refract Surg. 2019;45(4):480–484. doi: 10.1016/j.jcrs.2018.11.009. [DOI] [PubMed] [Google Scholar]