Abstract
Neuropsychiatric systemic lupus erythematosus is an autoimmune disorder characterized by an irregular exchange between the central nervous system and the immune system, leading to the outbreak of neurological conditions with possible disabling effects. Although neuropsychiatric systemic lupus erythematosus is the most common expression of lupus condition, it is still poorly understood. In this study, we focus on the development of an advantageous method based on the application of synthetic nucleic acids and protein-based antigen arrays in order to characterize autoreactive antibodies in neuropsychiatric systemic lupus erythematosus. We confirmed the benefits of using synthetic oligonucleotides such as assay reproducibility, elevated affinity and specificity to autoreactive antibodies. We also demonstrated presence of autoantibodies towards three particular synthetic double stranded antigens and verify similarity of antinuclear antibody patterns in ordinary lupus and neuropsychiatric systemic lupus erythematosus.
Keywords: Neuropsychiatric systemic lupus erythematosus, Synthetic Antigens, Oligonucleotides, Autoreactive antibodies, Enzyme-Linked Immunosorbent assay
Highlights
-
•
Neuropsychiatric systemic lupus erythematosus is the most common expression of lupus condition.
-
•
Neuropsychiatric systemic lupus erythematosus is still poorly understood.
-
•
Antibodies to synthetic nucleic acid antigens differentiate systemic lupus erythematosus.
-
•
Autoantibody levels in NPSLE correlate with the disease activity but not with the organic brain dysfunction.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by dysfunction of body’s self-tolerance, resulting in systemic inflammation and organ failure [1]. There has been significant improvement in prognosis of patients with SLE over the last 20–30 years [1,2]. Nevertheless, a crucial complication of SLE involves both the central nervous system (CNS) and peripheral nervous system (PNS) with disabling effects such as neuropsychiatric SLE (NPSLE) [3], comprising 93% CNS, 7% PNS involvement and 12%–95% prevalence in SLE cases [4].
NPSLE is a challenging autoimmune disease, responsible for high morbidity and mortality including a great economic and social burden [4]. The complexity of NPSLE involves a various range of symptoms including focal symptoms, diffuse disorders, thrombotic events and many more. These varieties of symptoms frequently present diagnostic and therapeutic challenges [4]. Nowadays, there is no single test enabling the specific diagnosis of NPSLE [5]. The diagnostic methodology for NPSLE comprehends an inspection of patient’s history together with physical examination, neurologic and mental status evaluation [[6], [7], [8]].
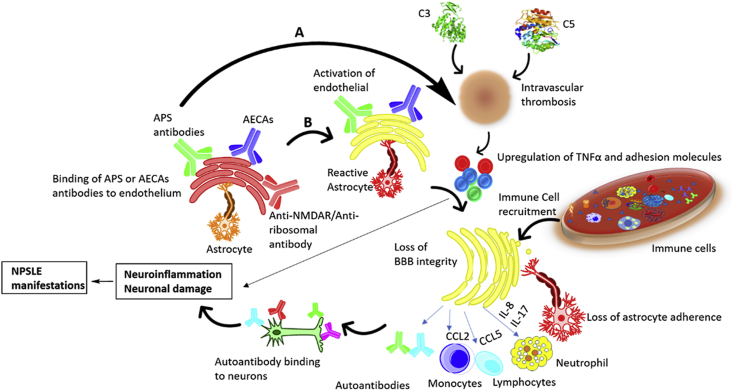
A growing number of self-reactive antibodies including antiphospholipid antibodies, anti-dsDNA antibodies, anti-ribonucleoprotein (RNP1), anti-SSA, anti-P ribosomal antibodies and many more play key roles in mediating both ischemic and inflammatory disease mechanisms (Fig. 1). Many of these antibodies are directed to brain antigens [9], while the others are systemic autoantibodies. Unfortunately, the majority of these autoantibodies are not used in clinical work, but they can be employed as additional tests to better monitor the patients.
Fig. 1.
Role of cytokines and autoantibodies in NPSLE pathogenesis. A. Focal NPSLE: Antiphospholipid (APS) antibodies and complement components mediates the vascular mechanism resulting in the development of intravascular thrombosis and contribute to blood brain barrier dysfunction by upregulating pro-inflammatory cytokines, adhesion molecules and promoting reactive oxygen species formation. B. Diffuse NPSLE: Different antibodies, primarily anti-NMDAR, anti-ribosomal and anti-endothelial cell antibodies (AECAs) promote the upregulation of pro-inflammatory cytokines and endothelial cell adhesion molecules that could result in the disruption of the endothelium of the blood brain barrier which allows extravasation of leukocytes to the central nervous system (CNS) ultimately inducing neural apoptosis and/or altered synaptic function.
The disease activity in SLE and NPSLE correlates with complement (C3/C4, or CH50) determinations, anti-dsDNA antibodies and anti-Smith (anti-Sm) antibodies and checking the presence of antiphospholipid antibodies [10].
So called anti-DNA antibodies, i.e. antibodies that are able to recognize and bind to DNA, are widely used as serological tests for diagnosis and disease activity of SLE [11]. Specifically, anti-DNA antibodies are ANA (antinuclear antibodies), which include anti-ssDNA (anti single-stranded DNA) and anti-dsDNA (anti-double stranded DNA). ANA are present in 70–90% SLE and NPSLE subjects [12]. According to the American College of Rheumatology (ACR) 2019 guideline, ANA positivity is an entry criterion for SLE [12]. Disease specificity of anti-ss and anti-ds DNA is different: for the former the test sensitivity is variable (30–70%) [13], in contrast the test sensitivity for the anti-dsDNA antibodies is 60% [[14], [15], [16]].
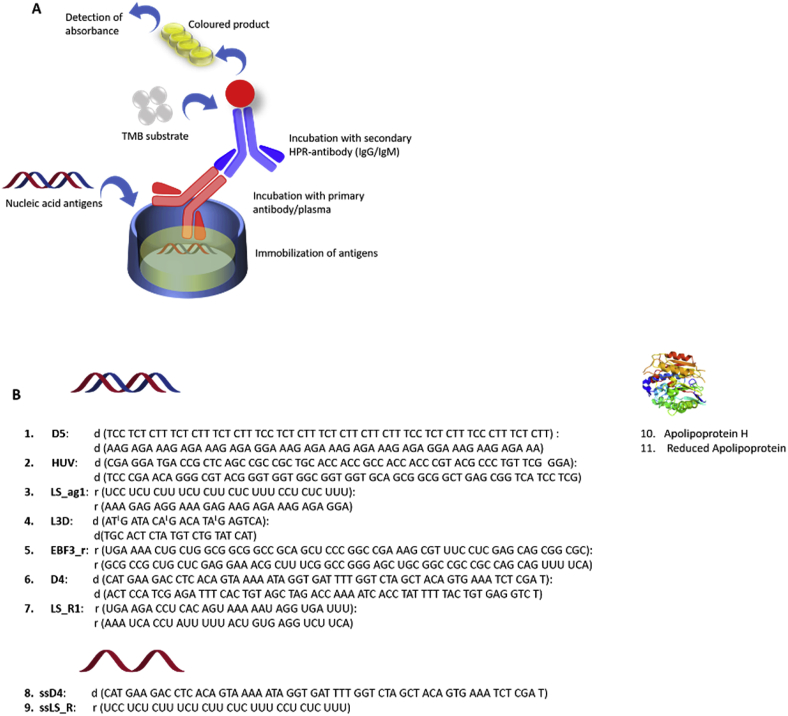
In our previous studies on a-DNA in SLE and linear scleroderma (LS) [17], a series of synthetic oligonucleotides were developed in order to explore the antigen recognition by autoimmune antibodies (Fig. 2). The novel antigens showed up to 10-fold higher analytical sensitivity over the control Calf Thymus DNA and commercial anti-ssDNA kit [17]. We provided significant evidence of an effect of the clinical features of LS and SLE subjects on anti-nucleic acid antibodies. We also confirmed an association of particular a-dsDNA, D5, with SLE disease activity index SLEDAI [17].
Fig. 2.
A. General principle of an indirect enzyme-linked immunosorbent assay (ELISA). B. Sequences of synthetic antigens used in this study [17]. Locked nucleic acid (LNA) in antigen L3D, Entry 4, is shown as an upper case letter L after the corresponding nucleotide letter. ELISA was performed for each antigen individually.
In this study, we hypothesized that there is a difference in autoantibody reactivity between SLE and NPSLE. We also hypothesized that a particular subset of autoantibodies might be related to specific clinical features of NPSLE. To evaluate our hypothesis, we screened a series of synthetic antigens in a cohort of 73 NPSLE individuals and controls by enzyme-linked immunosorbent assay (ELISA). In this paper, we describe the application of a nucleic acid (DNA, RNA and locked nucleic acid, LNA) and protein-based antigen array that comprises well described autoantibody targets previously identified by us in an autoantibody profiling study of SLE [18]. We observed high binding levels for three antigens in IgG ELISA. There was a statistically significant difference in autoantibody profiles for SLE vs NPSLE, as well, however with not confirmed relationship with particular clinical features of NPSLE.
2. Methods
2.1. Patients
This research was approved by the local ethical committee of Hokkaido University Hospital No.018–0384, received on the June 19th, 2019, and by the institutional ethical committee of Kitasato University School of Medicine, permit no. B09-55 issued in April 4th, 2017.
All patients with NPSLE were recruited in Japan, from the Kitasato University School of Medicine (n = 60) or at the Hokkaido University Graduate School of Medicine (n = 13). Patients with NPSLE fulfilled the ACR 1999 criteria for NPSLE. Demyelination in NPSLE was identified on the basis of MRI findings.
Healthy (n = 16) samples were provided by the Kitasato University School of Medicine, Japan (n = 10) and Stanford University Hospital (n = 6). The SLE samples (n = 20) were received from Stanford University School of Medicine (SU), USA. All sera were stored at – 20 °C until being analyzed.
Details on the subjects are given in Table 1. NPSLE subjects were of Asian ethnicity, mostly females (over 75%), actively treated with diverse medications, and in remission for 79% of the cohort. The NPSLE subjects were compared to 20 SLE cohort, Caucasian ethnicity, 100% females, similar age range as NPSLE (median ages were 42 and 46.6 years old, respectively). NPSLE and SLE had similar median SLEDAI index of 2.5–3. Medication for SLE was more active than for NPSLE; anti-malarial drugs and HDSC being more actively applied for SLE. Matched healthy controls (n = 16) were 40% Caucasian and 60% Asian, age median value: 44, 87% females.
Table 1.
Demographic and clinical characteristics of subjects applied in this study.∗
| Variable | Diseased samples: |
Controls |
|
|---|---|---|---|
| NPSLE | SLE | Healthy (HC) | |
| N (subjects) | 73 | 20 | 16 |
| Female/Male | 56/8 | 20/0 | 14/2 |
| Gender Unknown | 9 | – | |
| Ethnicity | Asian | Caucasian | Caucasian 40%, Asian 60% |
| Median age (yr) at sample collection (range) | 46.6 (14–61.2) | 42 (20–56) | 44 (30–65) |
| Median age (yr) at disease onset (range) | 43 (9–75.59) | 34 (17–45) | – |
| OBD classification ACR1999, code (% patients), out of 57 in total | 1 (54%), 2 (37%), 3 (9%) | n/a | n/a |
| Disease phase at sampling Remission/relapse | 58/15 | 18/2 | – |
| CS + IS + PE treatment/total no. samples (%) | 5 (6.85%) | 4 (20%) | – |
| CS + IS treatment/total no. samples (%) | 11 (15.06%) | 4 (20%) | – |
| CS treatment/total no. samples (%) | 13 (17.80%) | 5 (25%) | – |
| IS treatment/total no. samples (%) | 1 (1.37%) | 2 (10%) | – |
| CS + IVIG then RTX treatment/total no. samples (%) | 1 (1.37%) | – | – |
| CS + PE treatment/total no. samples (%) | 2 (2.74%) | – | – |
| HDCS (prior to sampling)/total no. samples (%) | 6 (8.22%) | 8 (40%) | – |
| Antimalarial treatment/total no. samples (%) | 3 (4.11%) | 8 (40%) | – |
| Median (range) SLEDAI/no. samples | 2.5 (0–44)/24 | 3 (1–11)/20 | – |
| Median (range) ANA ELISA | 32.13 (2.80–127.7) | 50 (11–156) | – |
| Mean (range) ESR | Nd | 25 (22–34) | 26 (20–37) |
∗ Abbreviations: NPSL E = Neuropsychiatric systemic lupus erythematosus, SLE = Systemic lupus erythematosus, HC = Healthy control, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index, ESR = Erythrocyte sedimentation rate, ELISA = Enzyme linked immunosorbent assay, OBD = organic brain dysfunction. IgG = Immunoglobulin G, CS= Corticosteroid, IS=Immunosupressive, PE = plasma exchange, IVIG= Intravenous immunoglobulin, RTX = Rituximab, ANA = Antinuclear antibody, HDCS= Human Diploid Cell Strain, yr = Year, ND= No data, n/a = not applied.
2.2. ELISA
The procedure for enzyme-linked immunosorbent assay (ELISA) was adopted from our recently published paper [18]. Positive and negative controls were commercial anti-dsDNA (Sigma MAB129) and anti-gp120 (SAB3500931), respectively.
Maxisorb 96 well plates (NUNC Thermofisher, Germany) were coated with individual antigen [3.5 μg/mL in 1 X PBS (100 μL/well)] and incubated overnight at +4 °C. The plates were washed two times with washing buffer (1X PBS; 300 μL/well) and blocked for 1 h at 37 °C with 1X PTB buffer (20 g BSA, 50 μL Tween 20, 1 L 1X PBS; 100 μL/well). After washing the plates 2 times with the washing buffer, the plates were incubated with diluted plasma/control [1 μL plasma in 100 μL diluent (2 g BSA, 50 μL Tween-20, 1 L 1X PBS; 100 μL/well)] for 1.5 h at 37 °C. The plates were washed three times with washing buffer and incubated with secondary antibody or HPR-Ab (100 μL/well) in the ratio of (1:20000) diluted with previous diluent for 1.5 h at 37 °C. The plates were subsequently washed three times with washing buffer and incubated with freshly prepared TMB substrate solution (3 mg TMB, 5 mL DMSO diluted to 50 mL with 0.1 M acetate buffer, 3 μL H2O2; 100 μL/well). The reaction was stopped with 1 M H2SO4 (50 μL/well) and the absorbance of the plates was analyzed in Magellan Tecan microplate reader at 450 nm. Linear range for each antigen was determined via testing series of control dilutions (dilutions 1:50 to 1:2000). According to the results plasma dilutions 1:100–1:500 were within linear range of the assay for each antigen (R2 > 0.95).
Intra- and inter-assay coefficient of variance (CV) values for IgG and IgM ELISA were determined by a triplicate measurement, and were 8.4%–13.4% for IgG, and 7.0%–15.0% for IgM (Supplementary Table 1). The results for all antigens have been presented as boxplots, see.Supplementary Table 2.
For converting the absorbance value to concentrations, we used a commercial anti-dsDNA (Sigma MAB1293). Resulting calibration curves are given in. Supplementary Figure 1.
Cut-off values have been determined as we previously described, using a 2xSD and 3xSD above mean value for 20 healthy controls, used in same ELISA setting and same antigen, as an elevated and positive level, respectively. The obtained 2xSD and 3xSD values were as follows: D4, 42 nM, 72 nM; D5, 46 nM, 70 nM; HUV, 48 nM, 85 nM.
2.3. Affinity chromatography
Manual affinity chromatography was conducted using GE Healthcare protocol, STP sephadex, glass column, corresponding antigens and sera samples. 5′-Amine modified antigen strands were ordered from IDT, and annealed at equimolar ratio in 1x PBS with complementary strands. Conditions for annealing were: 92 °C 10 min - > room temperature for 1 h.
Five SLE and five NPSLE patients were selected for the affinity chromatography based on the positivity on all three antigens (D4, D5, HUV), determined by the initial ELISA.
5 mL Sephadex was equilibrated in a glass column, then 6 mL antigen was applied at concentration 200 μg/mL using 0.1 M bicarbonate buffer, pH 8.3. Incubation was carried out for 30 min at room temperature, followed by washing the column with 20 mL 1x PBS buffer. 0.1 mL Serum was diluted up to 5 mL with 1xPBS, and applied to the corresponding column (D4, D5 or HUV immobilized). Incubation was carried out for 30 min at +4 °C, followed by washing with 20 mL 1x PBS. Elution buffer was applied (100 mM Glycine-HCl, 10% dioxane, pH 2.5–3.0); the elution was followed by Bradford assay. When the elution was complete, the column was washed with 20 mL 1x PBS, and reused for the next sample.
Fractions of immunoglobulins were subjected to buffer exchange using Amicon spin tubes with MWCO 30 kDa, and resolved by PAGE.
Native PAGE was carried out at + 4 °C using precast 8% Biorad gel cassettes, SDS free TRIS HCL running buffer and low current running protocol (20 mA for 4 h).
Reducing denaturing PAGE was carried out for samples preheated with 2-mercaptoethanol containing loading buffer, at room temperature using precast 8% Biorad gel cassettes, SDS-TRIS HCL running buffer and high current running protocol (100 mA for 40 min).
The gels were fixed in 1 M AcOH, washed, followed by visualization using Coomassie Blue stain and BioRad gel imaging equipment.
Epitope recognition by the purified IgG’s was studies by ELISA, using plates pre-coated with individual D4, D5 or HUV, using the method described above and a serial dilution of the antibodies.
Concentration of purified immunoglobulins was measured using Qiagen nanodrop equipment.
2.4. Statistical analysis
Patient grouping has been conducted for 57 patients with available complete clinical data, using ACR recommendation for NPSLE, 1999, see Supplementary Information, Supplementary Table 3.
For the multivariate statistical analyses of IgG and IgM data, we adopted the statistical modelling framework of multivariate covariance generalized linear models (McGLMs) as proposed by Bonat and Jørgensen [19] and implemented in the R package mcglm [20,21], where statistical models are fitted using an estimating function approach based on second-moment assumptions only. For the present paper, we consider a special case of multivariate covariance generalized linear models with identity link and constant variance for all response variables. The IgG and the IgM data were analyzed separately. Two observations in each of the data sets were eliminated by list-wise deletion of missing data prior to fitting the models. Response variables were absorbance values obtained for D4, D5, HUV, Ds_L3D, Ds_LS_R1, Ds_LS_R2, Ds_EBF3_r, D4, and LS_R, all measured on a logarithmic scale. Covariates included demographic and clinical features of the patients, their serology and treatment status. In the full models, age at diagnosis, gender, ANA titer (fold), anti-ds DNA IgG (IU/ml), anti-Sm, SLEDAI, and organic brain dysfunction (OBD) ACR code served as covariates for all response variables. Ultimately, to select the best models from the all possible subsets of covariates, exhaustive searches for the best subsets of covariates in each linear predictor were performed using the pseudo Akaike information criterion (pAIC). Univariate Wald chi-square tests of fixed effects in the resulting simplified models are presented in Supplementary Tables 5-6; regression coefficients for simplified IgG and IgM models are given in. Supplementary Tables 7-8.
3. Results
The major goal for this study was to develop a sensitive, specific and reproducible method to define autoreactive autoantibodies in NPSLE patients.
We selected high throughput sensitive ELISA assay for measuring the amount of a-DNA, a-RNA, a-ApoH IgG and IgM (Fig. 2A). ELISA is a straightforward and highly sensitive method that allowed us to detect the binding of an antigenic epitope by an antibody in a time and cost-effective manner [18]. Oligonucleotides shown in Fig. 2B, Entry 1–9, were designed by considering different variables including nucleotide composition, length and sequence polarity. These rationally designed oligonucleotides showed improved binding in ELISA assay using human samples for SLE and LS [17,18,22]. LNA modified sequence in Fig. 2B, Entry 4, was used to maintain DNA topology and to increase the duplex stability. RNA sequences LS-ag1, EBF3_r and LS_R1, were RNA antigens. ssD4 and ssLS_R were single stranded DNA and RNA antigens, respectively. Last, antibodies to apolipoprotein and its reduced variant, Fig. 2B, Entry 10–11, are associated with lupus and sclerosis and therefore, also included into the study group [18].
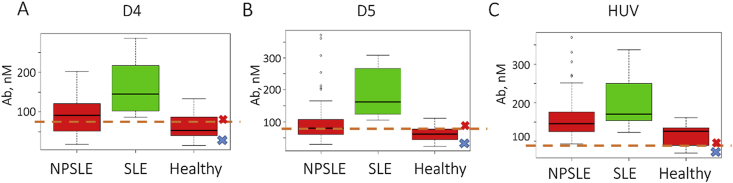
ELISA was performed for each antigen individually. The results of ELISA are presented in Fig. 3 and in the. Supplementary Table 2 One of the most striking findings from our study is that three particular antigens, D4, D5 and HUV, showed high IgG binding levels in both SLE and NPLSE (Fig. 3). Remarkably, the highest levels of IgG antibodies in NPSLE were directed to cytomegalovirus-derived dsDNA HUV, whereas for SLE, the highest binding was in D5 test (Fig. 3B and C). Similarly to previous studies, single stranded antigens and RNA were elevated in healthy samples, pointing on lack of clinical specificity (Supplementary Table 2) [18,22]. Notably, a-ApoH and a-r. ApoH IgG’s were elevated in SLE but not in NPSLE. For IgM a-ApoH and a-r. ApoH, there was statistically significant difference between NPSLE and Healthy controls (Supplementary Table 2).
Fig. 3.
Representative box-plot for relative antibody values across applied antigens, IgG study. A. D4 B. D5 C. HUV; Negative and positive control concentrations were determined to be 81 nM and 28 nM (D4); 73 nM and 15 nM (D5); 87 nM and 40 nM (HUV). The negative and positive control results are shown on each graph as blue and red crosses, respectively. Human monoclonal anti-dsDNA and anti-gp120 Abs were used as a positive and negative controls, respectively. For conversion of A450 to Ab amounts, see calibration curves given in Supplementary Fig. 1. Cut-off value for the positive result is shown as a dashed orange line. For complete results of the study, see Supplementary Table 2.
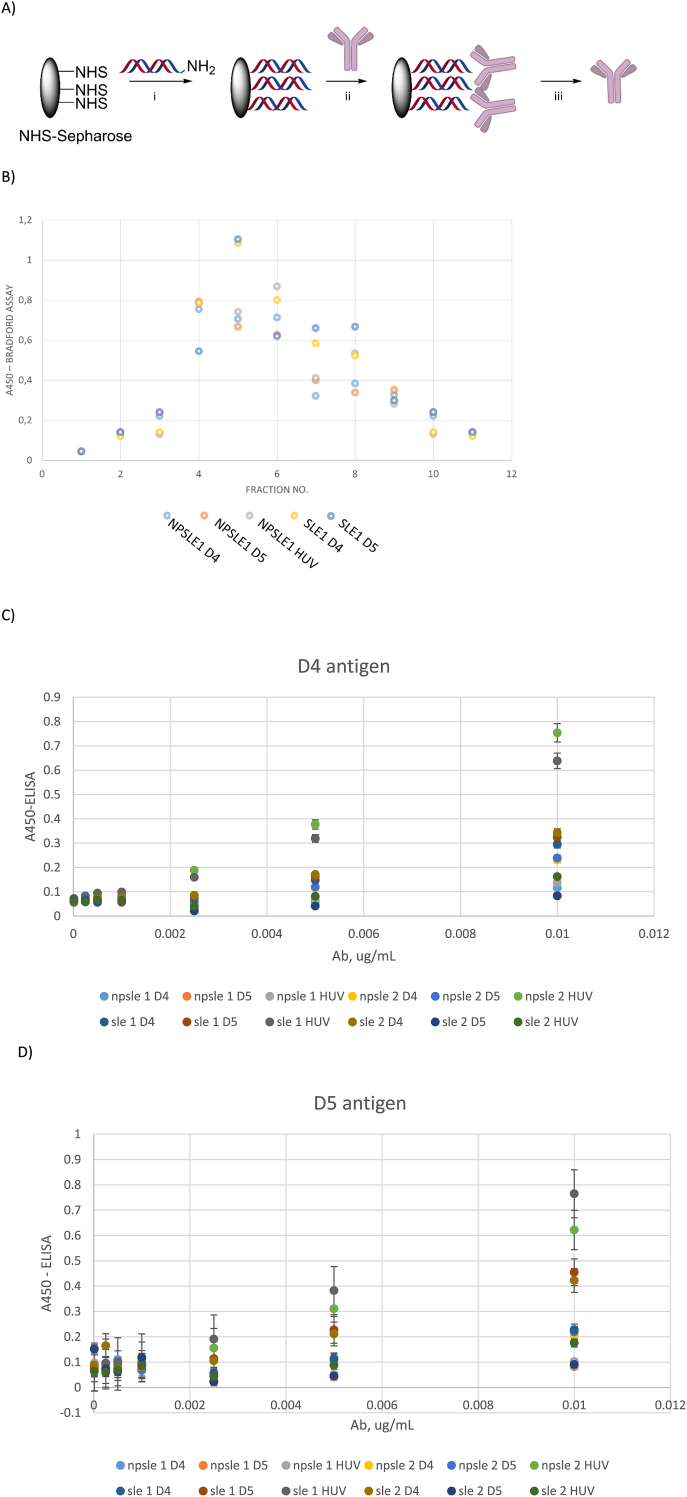
As a next step, we covalently attached antigens D4, D5 and HUV to Sephadex (GE) via NHS coupling chemistry [26]. For this, the lead sequence for each antigen (shown in Fig. 2), was obtained as a 5′-amino-labelled variant. The duplex of each antigen was annealed and attached to NHS sephadex following the manufacturer’s protocol (GE) [27]. The immobilized solid phase has been used to purify antibodies from antibody positive sera of five SLE and five NPSLE subjects (Fig. 4; see Methods for details).
Fig. 4.
Affinity chromatography and analyses of immunoglobulins from NPSLE and SLE sera. A) General scheme for affinity chromatography using synthetic dsDNA antigens: i) antigen immobilization; ii) incubation with sera; iii) immunoglobulin elution. NHS = N-hydroxysuccinimide; B) Representative elution profile followed by Bradford assay; C-D) ELISA of obtained Abs using D4 and D5 pre-coated plates. In Figure legends, the name of the Ab sample is given for the patient no. applied and the antigen used for the purification.
Elution kinetics was followed by Bradford assay (Fig. 4B) [28]. As can be seen, elution profiles were somewhat similar for both SLE and NPSLE on all three antigens, pointing on similar epitope-antibody binding kinetics for SLE and NPSLE anti-dsDNA’s [29].
We obtained 1.4–27.5 μg antibodies per SLE or NPSLE individual sample (Supplementary Table 4). The fractions with high protein content according to Bradford assay were subjected to buffer exchange and analyzed by PAGE. Native PAGE showed a single band at approx. 150 kDa in immunoglobulin fractions from both SLE and NPSLE subjects, on all three antigens (Supplementary Figs. 2&3). This indicates that the eluted antibodies were mainly of IgG type. Reducing denaturing PAGE gels of the same fractions showed two main bands at approx. 70 kDa and 20 kDa, confirming that the eluted proteins were IgG antibodies (Supplementary Figs. 4&5).
To evaluate the epitope recognition properties by the purified antibodies, we conducted ELISA. Herein, we observed similar antigen recognition trends by the purified immunoglobulins as in the whole sera ELISA experiments (Fig. 4C and D; Supplementary Fig 4 and Supplementary Table 4). Antibodies from SLE samples had higher binding levels towards dsDNA antigens than those purified from NPSLE. Notably, Abs purified on HUV antigen cross-bound to D4 and D5 for both SLE and NPSLE. However, the cross binding between purified anti-D4 to D5 and vice versa, anti-D5 to antigen D4, was lower than for Abs purified using HUV (Fig. 4C and D). For HUV, Ab recognition patterns was very much similar to D4, though with approx. 30% higher binding levels for HUV than D4 (Supplementary Fig 6 and Supplementary Table 4).
We proceeded with a detailed statistical analysis of the results for all applied antigens. Multivariate Wald chi-square tests of fixed effects in the full IgG model (see Table 2) revealed that the effects of age at diagnosis, gender, anti-ds DNA IgG, and SLEDAI are statistically significant (p < 0.05).
Table 2.
Multivariate Wald chi-square tests of fixed effects in the full IgG model.
| df | Hotelling-Lawley trace | p-value | ||
|---|---|---|---|---|
| Intercept | 9 | 1.8856 | 79.1952 | <0.001 |
| Age at diagnosis | 9 | 0.6068 | 25.4846 | 0.0025 |
| Gender | 9 | 0.4649 | 19.5254 | 0.0211 |
| ANA titer | 27 | 0.7105 | 29.8430 | 0.3213 |
| anti-ds DNA IgG | 27 | 1.2709 | 53.3797 | 0.0018 |
| anti-Sm | 9 | 0.1604 | 6.7363 | 0.6645 |
| SLEDAI | 9 | 0.5104 | 21.4370 | 0.0108 |
| OBD ACR code | 18 | 0.5180 | 21.7573 | 0.2429 |
ANA = antinuclear antibodies, OBD = organic brain dysfunction.
A specific feature of NPSLE is organic brain dysfunction (OBD). Elevated a-Sm antibodies is also an indication for neurological complications in NPSLE [[3], [4], [5]]. Interestingly, neither OBD ACR code nor anti-Sm seem to have a statistically significant effect on the logarithmically transformed absorbance values at the 0.05 significance level. However, univariate Wald chi-square tests of fixed effects in the simplified IgG model (see Supplementary Table 5) suggest that SLEDAI has a statistically significant effect on the logarithmically transformed absorbance values obtained for D4, Ds_L3D, Ds_LS_R1, Ds_LS_R2, and D5. Moreover, age at diagnosis has a statistically significant effect on the logarithmically transformed absorbance values obtained for HUV, Ds_L3D, Ds_LS_R1, Ds_LS_R2, and D4. Gender has a statistically significant effect on the logarithmically transformed absorbance values obtained for Ds_L3D. Finally, anti-ds DNA IgG has a statistically significant effect on the logarithmically transformed absorbance values obtained for Ds_LS_R2 and Ds_EBF3_r.
Likewise, multivariate Wald chi-square tests of fixed effects in the full IgM model (see Table 3) reveal that the effects of gender, ANA, and SLEDAI are statistically significant (p < 0.05). On the other hand, the effects of age at diagnosis, anti-dsDNA IgG, anti-Sm, and OBD ACR code are not statistically significant at the 0.05 significance level. Remarkably, univariate Wald chi-square tests of fixed effects in the simplified IgM model (see Supplementary Table 6) suggest that ANA titer has a statistically significant effect on all response variables, i.e. the logarithmically transformed absorbance values obtained for D4, D5, HUV, Ds_L3D, Ds_LS_R1, Ds_LS_R2, Ds_EBF3_r, D4, and LS_R. SLEDAI has a statistically significant effect on the logarithmically transformed absorbance values obtained for D4.
Table 3.
Multivariate Wald chi-square tests of fixed effects in the full IgM model.
| df | Hotelling-Lawley trace | p-value | ||
|---|---|---|---|---|
| Intercept | 9 | 0.7014 | 29.4585 | <0.001 |
| Age at diagnosis | 9 | 0.3708 | 15.5717 | 0.0764 |
| Gender | 9 | 0.6945 | 29.1676 | <0.001 |
| ANA titer | 27 | 1.6393 | 68.8506 | <0.001 |
| anti-ds DNA IgG | 27 | 0.7873 | 33.0648 | 0.1949 |
| anti-Sm | 9 | 0.2720 | 11.4233 | 0.2478 |
| SLEDAI | 9 | 0.5377 | 22.5828 | 0.0072 |
| OBD ACR code | 18 | 0.5730 | 24.0672 | 0.1528 |
4. Discussion
For decades, researchers and clinicians have been searching for definite serological biomarkers for NPSLE patients. More than 100 autoantibodies have been described for SLE. However, there is limited knowledge and test which can specifically discriminate between SLE and NPSLE on serological level, and to distinguish SLE-related and unrelated NP manifestations. Different literature have reported numerous SLE autoantibodies including amyloid, cardiolipin, glycoprotein 2, glycoprotein 210, heparin, histone H2A, prothrombin, centromere protein A, collagen II and many more [[30], [31], [32], [33]]. However, most of these autoantibodies are also related to other autoimmune diseases and only limited numbers of them such as heparin sulphate, histone H2B, anti-GluRϵ2, anti-NMDA and vimentin could differentiate NPSLE from SLE [[30], [31], [32], [33]].
Short synthetic oligonucleotides with advantages of high homogeneity, controlled purity and known sequences [[22], [23], [24], [25]], have been repeatedly reported to bind a-DNA/RNA antibodies [17,18]. Furthermore, ANAs are frequently associated with different autoimmune diseases including SLE and chronic uveitis [8].
In our present work, we took a next step towards better understanding of autoantibodies in NPSLE using synthetic nucleic acids and proteins antigens. Double stranded DNA and RNA showed high reproducibility and specificity in correlation with NPSLE samples. TC-dinucleotide rich D5 and mixmer DNA duplex D4 were reported as most reactive antigens in binding DNA in pediatric and adult SLE [22]. Our data showed similar pattern for SLE and NPSLE samples tested herein, attributing the overlap of some ANAs in SLE and NPSLE patients.
Evidence from the literature suggests a pathological role of bacteria and viruses such as Epstein-Barr virus, cytomegalovirus, Parvovirus B19, Cryptococcus, mycobacteria and Listeria monocytogenes in SLE autoimmunity including CNS infections [34]. Given this, our study also aimed to investigate the significance of cytomegalovirus specific sequence, HUV, in NPSLE and SLE patients. We have been successful to establish a strong relationship of cytomegalovirus specific DNA HUV with SLE and mostly with NPSLE in comparison to the healthy controls.
Previously HUV antigen showed recognition of antibodies in multiple sclerosis [35]. Recognition of synthetic HUV antigen by antibodies in NPSLE and SLE reported herein additionally points on the potential link between neurological autoimmune diseases and immune hyper-activation by viral infections.
According to our results for purified antibodies, anti-HUV cross-react more to D4 and D5 antigens than anti-D4 and anti-D5. This points on the fact that anti-HUV are highly reactive, with epitope recognition pattern spread across different dsDNA sequences, while anti-D4 and anti-D5 have a more conserved epitope recognition pattern. One potential explanation to this could be appearance of anti-HUV in an acute infection and/or inflammation state, vs. a more chronic nature of a-D4 and a-D5 in SLE and NPSLE.
5. Conclusion
In this study, we applied a panel of synthetic antigens (DNA, RNA and ApoH) as reagents for ELISA of NPSLE and SLE cohorts. Although epitope recognition pattern was similar for NPSLE and SLE, we observed statistically significant differences in binding levels. The highest difference between binding levels in NPSLE vs SLE was observed for three dsDNA antigens: D4, D5 and HUV. When purified by affinity chromatography, autoantibodies in NPSLE also demonstrated more active epitope sharing among the synthetic dsDNA’s than those purified from SLE controls.
Autoantibody levels in NPSLE correlated positively with SLEDAI and for some, with age, however there were no correlations for the particular antigens we used, with NPSLE-specific CNS features. Nevertheless, our study for the first time applied sequence-defined ANA to the comparative investigation of SLE and NPSLE. More studies need to be conducted to establish potential correlations with CNS features in NPSLE, by e.g. using new generation of sequence defined synthetic antigens.
Altogether, our study confirms utility of synthetic antigens in NPSLE studies and potentially, in clinical work, and opens up a new path for further discoveries of autoantibody biomarkers. Moreover, our results point on serological overlap and differences between NPSLE and non-NPSLE disease at the molecular level, opening up new possibilities for personalized improved diagnosis and follow up.
Author statements
SK, NP and KA planned and conducted experiment. YA, YF and SM provided with NPSLE subjects, healthy controls and their clinical parameter description. SK, CDJ and KA conducted the statistical analyses. SK and AF prepared an initial paper draft and graphical images. All the authors analyzed data, reviewed the paper draft and contributed to preparing the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We acknowledge Prof Dr Elizabeth D Mellins and Prof Dr PJ Utz, Stanford University, USA, for providing with SLE control samples. We acknowledge Konsul George Jorck og Hustru Emma Jorck’s Fond research prize, award no. 40934, for supporting the study. The funder had no input into neither the study nor preparing this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2020.100068.
Contributor Information
Sangita Khatri, Email: sakhat@kemi.dtu.dk.
Nikos Psaraftis, Email: nikos.psaraftis@gmail.com.
Alessia Funaro, Email: alessiafunaro95@gmail.com.
Yoshiyuki Arinuma, Email: y-arinuma@med.kitasato-u.ac.jp.
Yuichiro Fujieda, Email: edaichi@med.hokudai.ac.jp.
Simone Mader, Dr., Email: simone.mader@med.uni-muenchen.de.
Christian Damsgaard Jørgensen, Email: chdj@sdu.dk.
Kira Astakhova, Email: kiraas@kemi.dtu.dk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jia J., Xie J., Li H., Wei H., Li X., Hu J., Meng D., Zhang Y., Zhang X. Cerebral blood flow abnormalities in neuropsychiatric systemic lupus erythematosus. Lupus. 2019;28:1128–1133. doi: 10.1177/0961203319861677. [DOI] [PubMed] [Google Scholar]
- 2.Hanly J.G., Kozora E., Beyea S.D., Birnbaum J. Review: nervous system disease in systemic lupus erythematosus: current status and future directions. Arthritis Rheum. 2019;71:33–42. doi: 10.1002/art.40591. [DOI] [PubMed] [Google Scholar]
- 3.Duarte-Delgado N.P., Vásquez G., Ortiz-Reyes B.L. Blood-brain barrier disruption and neuroinflammation as pathophysiological mechanisms of the diffuse manifestations of neuropsychiatric systemic lupus erythematosus. Autoimmun. Rev. 2019;18:426–432. doi: 10.1016/j.autrev.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Marín J.D., Posso-Osorio I., Vargas S., Nieto-Aristizábal I., Ríos-Serna L.J., Tobón G.J. Antibodies associated with neuropsychiatric lupus: pathophysiological role, prevalence and diagnostic usefulness. Rev. Colomb. Reumatol. 2019;26:111–117. doi: 10.1016/j.rcreu.2018.11.002. [DOI] [Google Scholar]
- 5.Hermosillo-Romo D., Brey R.L. Diagnosis and management of patients with neuropsychiatric systemic lupus erythematosus (NPSLE) Best Pract. Res. Clin. Rheumatol. 2002;16:229–244. doi: 10.1053/berh.2001.0223. [DOI] [PubMed] [Google Scholar]
- 6.Hanly J.G. Diagnosis and management of neuropsychiatric SLE. Nat. Rev. Rheumatol. 2014;10:338–347. doi: 10.1038/nrrheum.2014.15. [DOI] [PubMed] [Google Scholar]
- 7.Perez O., Dave K., Almanzar A., Prodhan T., Concepion L. Primary psychiatric disorder masking the diagnosis of neuropsychiatric lupus in a patient with altered mental status: a case report. Cureus. 2017;9 doi: 10.7759/cureus.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinclair K., Miner J., Kim A. Immunological mechanisms of neuropsychiatric lupus. Curr. Immunol. Rev. 2015;11:93–106. doi: 10.2174/1573395511666150707181943. [DOI] [Google Scholar]
- 9.Lefranc D., Launay D., Dubucquoi S., De Seze J., Dussart P., Vermersch M., Hachulla E., Hatron P.-Y., Vermersch P., Mouthon L., Prin L. Characterization of discriminant human brain antigenic targets in neuropsychiatric systemic lupus erythematosus using an immunoproteomic approach. Arthritis Rheum. 2007;56:3420–3432. doi: 10.1002/art.22863. [DOI] [PubMed] [Google Scholar]
- 10.Hirohata S. Neuropsychiatr. Syst. Lupus Erythematosus Pathog. Clin. Asp. Treat. Springer International Publishing; 2018. Pathology of neuropsychiatrie systemic lupus erythematosus; pp. 43–58. [DOI] [Google Scholar]
- 11.Pisetsky D.S. Anti-DNA antibodies - quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 2016;12:102–110. doi: 10.1038/nrrheum.2015.151. [DOI] [PubMed] [Google Scholar]
- 12.Kimura A., Kanoh Y., Sakurai T., Koumura A., Yamada M., Hayashi Y., Tanaka Y., Hozumi I., Takemura M., Seishima M., Inuzuka T. Antibodies in patients with neuropsychiatric systemic lupus erythematosus. Neurology. 2010;74:1372–1379. doi: 10.1212/WNL.0b013e3181dad590. [DOI] [PubMed] [Google Scholar]
- 13.Teodorescu M. Clinical value of anti-ssDNA (denatured DNA) autoantibody test: beauty is in the eyes of the beholder. Clin. Appl. Immunol. Rev. 2002;2:115–128. doi: 10.1016/S1529-1049(01)00042-3. [DOI] [Google Scholar]
- 14.M P., A K., M C., R C., Jx H., Y S. Pathogenic and epiphenomenal anti-DNA antibodies in SLE. Autoimmune Dis. 2010;2011 doi: 10.4061/2010/462841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodkey L.S., Gololobov G., Rumbley C.A., Rumbley J., Schourov D.V., Makarevich O.I., Gabibov A.G., Voss E.W. DNA hydrolysis by monoclonal autoantibody BV 04-01. Appl. Biochem. Biotechnol. Part A Enzyme Eng. Biotechnol. 2000;83:95–105. doi: 10.1385/abab:83:1-3:95. [DOI] [PubMed] [Google Scholar]
- 16.Hirohata S., Sakuma Y., Yanagida T., Yoshio T. Association of cerebrospinal fluid anti-Sm antibodies with acute confusional state in systemic lupus erythematosus. Arthritis Res. Ther. 2014;16:450. doi: 10.1186/s13075-014-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelsen S., Jørgensen C.D., Mellins E.D., Torok K.S., Astakhova K. Detection of autoimmune antibodies in localized scleroderma by synthetic oligonucleotide antigens. P L o S One. 2020;13 doi: 10.1371/journal.pone.0195381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatri S., Mellins E.D., Torok K.S., Bukhari S.A., Astakhova K. Methods Mol. Biol. Humana Press Inc.; 2020. Combined assay for detecting autoantibodies to nucleic acids and apolipoprotein H in patients with systemic lupus erythematosus; pp. 57–71. [DOI] [PubMed] [Google Scholar]
- 19.Bonat W.H., Jørgensen B. Multivariate covariance generalized linear models. J. R. Stat. Soc. Ser. C (Applied Stat. 2016;65:649–675. doi: 10.1111/rssc.12145. [DOI] [Google Scholar]
- 20.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2019. https://www.R-project.org/ [Google Scholar]
- 21.Bonat W.H. Multiple response variables regression models in R: the mcglm package. J. Stat. Software. 2018;84:1–30. doi: 10.18637/jss.v084.i04. [DOI] [Google Scholar]
- 22.Klecka M., Thybo C., Macaubas C., Solov’yov I., Simard J., Balboni I.M., Fox E., Voss A., Mellins E.D., Astakhova K. Autoantibody profiling in lupus patients using synthetic nucleic acids. Sci. Rep. 2018;8:5554. doi: 10.1038/s41598-018-23910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junager N.P.L., Kongsted J., Astakhova K. Revealing nucleic acid mutations using Förster resonance energy transfer-based probes. Sensors. 2015;16(8):1173. doi: 10.3390/s16081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S., Myznikova A., Samokhina E., Astakhova I.K. Rapid genotyping using pyrene− perylene locked nucleic acid complexes. Artif. DNA PNA XNA. 2014;4(2):58–68. doi: 10.4161/adna.25903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taskova M., Mantsiou A., Astakhova K. Synthetic nucleic acid analogues in gene therapy: an update for peptide–oligonucleotide conjugates. Chembiochem. 2013;18(17):1671–1682. doi: 10.1002/cbic.201700229. [DOI] [PubMed] [Google Scholar]
- 26.Besselink G., de Korte D. Sephadex-based cell-affinity adsorbents: preparation and performance. Biotechnol. Appl. Biochem. 2002;35:55. doi: 10.1042/ba20010062. [DOI] [PubMed] [Google Scholar]
- 27.Healthcare G. 2002. Antibody Purification-Handbook.www.gelifesciences.com/protein-purification [Google Scholar]
- 28.Kruger N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-x:9. [DOI] [PubMed] [Google Scholar]
- 29.Kummitha C.M., Shirure V.S., Delgadillo L.F., Deosarkar S.P., Tees D.F.J., Burdick M.M., Goetz D.J. HECA-452 is a non-function blocking antibody for isolated sialyl Lewis x adhesion to endothelial expressed E-selectin under flow conditions. J. Immunol. Methods. 2012;384:43–50. doi: 10.1016/j.jim.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Meulen P.M., Barendregt A.M., Cuadrado E., Sar Magro-Checa C., Steup-Beekman G.M., Schonenberg-Meinema D., Merlijn Van Den Berg J., Li Q.-Z., Baars P.A., Wouters D., Voskuyl A.E., Ten Berge I.R.J.M., Huizinga T.W.J., Kuijpers T.W. Protein array autoantibody profiles to determine diagnostic markers for neuropsychiatric systemic lupus erythematosus. Rheumatology. 2017;56:1407–1416. doi: 10.1093/rheumatology/kex073. [DOI] [PubMed] [Google Scholar]
- 31.Pradhan V., Patwardhan M., Rajadhyaksha A., Dhawale N., Ghosh K. Neuropsychiatric manifestations and associated autoantibodies in systemic lupus erythematosus patients from Western India. Rheumatol. Int. 2015;35:541–545. doi: 10.1007/s00296-014-3114-z. [DOI] [PubMed] [Google Scholar]
- 32.Conti F., Alessandri C., Bompane D., Bombardieri M., Spinelli F.R., Rusconi A.C., Valesini G. Autoantibody profile in systemic lupus erythematosus with psychiatric manifestations: a role for anti-endothelial-cell antibodies. Arthritis Res. Ther. 2004;6:R366. doi: 10.1186/ar1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu C., Huang W., Chen H., Song G., Li P., Shan Q., Zhang X., Zhang F., Zhu H., Wu L., Li Y. Autoantibody profiling on human proteome microarray for biomarker discovery in cerebrospinal fluid and sera of neuropsychiatric lupus. PloS One. 2015;10:126643. doi: 10.1371/journal.pone.0126643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang M., Shi X., Gao X., Niu J., Hu X., Zhao L., Zhang X. Clinical features of central nervous system infections and experience in differential diagnosis from neuropsychiatric lupus erythematosus in a cohort of 8491 patients with systemic lupus erythematosus. Arthritis Res. Ther. 2019;21:189. doi: 10.1186/s13075-019-1971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langer-Gould A., Wu J., Lucas R. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: a multiethnic study. Neurology. 2017;89(13):1330–1337. doi: 10.1212/WNL.0000000000004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.