Abstract
Background
It is unclear whether bisphosphonates are associated with risk of cancers. Therefore, this meta-analysis aimed to evaluate the effect of bisphosphonates on overall cancers.
Methods
A search in Pubmed, Embase, Cochrane Library and Web of Science databases was conducted, from the inception date of each resource to September 26, 2019. The summarised effect estimates with 95% CIs were calculated using a random-effect model. Heterogeneity and publication bias were explored.
Results
Thirty-four articles were included in this study (4,508,261 participants; 403,196 cases). The results revealed that bisphosphonates significantly decreased the risk of colorectal cancer (RR = 0.89, 95% CI: 0.81–0.98), breast cancer (RR = 0.87, 95% CI: 0.82–0.93) and endometrial cancer (RR = 0.75, 95% CI: 0.61–0.94), but no significant association was observed in all-cause cancer. Furthermore, nitrogen-containing bisphosphonates only had protective effects both on breast cancer (RR = 0.94, 95% CI: 0.90–0.99) and endometrial cancer (RR = 0.70, 95% CI: 0.54–0.92). Non-nitrogen-containing bisphosphonates tended to increase the risk of liver cancer (RR = 2.14, 95% CI: 1.23–3.72) and pancreas cancer (RR = 1.75, 95% CI: 1.32–2.33).
Conclusion
Bisphosphonates are significantly associated with risk reduction of colorectal, breast and endometrial cancer, especially nitrogen-containing bisphosphonates. It should be noted that non-nitrogen-containing bisphosphonates might increase the risk of liver and pancreas cancer. Large prospective cohort studies are needed to find the causal association between bisphosphonates and risk of cancers.
Subject terms: Cancer prevention, Cancer prevention
Background
Cancer is an important public health problem in the world, and the incidence and mortality of global cancer have grown rapidly in recent years.1 According to the Global Burden of Disease study (GBD) 2017, the most common incident cancers were non-melanoma skin cancer (NMSC, 7.7 million incident cases), tracheal, bronchus and lung (TBL) cancer (2.2 million incident cases), breast cancer (2.0 million incident cases) and colorectal cancer (1.8 million incident cases).2 In terms of women, breast cancer was the most commonly diagnosed cancer, followed by colorectal, lung and cervical cancer.3 Prevention of cancers has become a great public health importance.
In recent years, preclinical studies have suggested that bisphosphonates have direct and indirect antitumour properties, including inhibition of tumour cell adhesion and proliferation,4,5 induction apoptosis of tumour cells,6 prevention of angiogenesis7,8 as well as activation of immune cells.9 Wysowski et al.10 reported that the US Food and Drug Administration (FDA) received 23 cases of oesophageal cancer after the use of bisphosphonates. Since then, some epidemiological studies were also conducted on the association between bisphosphonates and the risk of some types of cancers, but the results of these studies were controversial.11–14 To date, most meta-analyses were focused on the association between the use of bisphosphonates and the risk of some specific types of cancers.15–24 For instance, Yang et al.19 suggested that the use of bisphosphonates might decrease the risk of colorectal cancer by 11% (RR = 0.89, 95% CI: 0.80–0.99), while Oh et al.25 found no significant association between the use of bisphosphonates and colorectal cancer (RR = 0.62, 95% CI: 0.30–1.29). To the best of our knowledge, there was currently only one meta-analysis by Deng et al.26 on the use of bisphosphonates and the risk of all-cause cancers, which only included 13 cohort studies and analysed the association in mixed genders and females.
Bisphosphonates are widely prescribed for preventing and treating osteoporosis,27,28 but the number of bisphosphonate users is expected to increase globally. For example, in the United States alone, there are approximately 40 million bisphosphonate prescriptions each year. Considering the widespread use of bisphosphonates, it is essential to explore the association between bisphosphonates and cancers.
This systematic review and meta-analysis was intended to (1) analyse possible association between the use of bisphosphonates and the risk of overall cancers and individual types of cancers based on observational studies, (2) stratify analysis by different types and duration of bisphosphonates.
Methods
Literature search
This systematic review and meta-analysis was conducted following the PRISMA guidelines.29 We firstly searched relevant studies in the databases of Pubmed, Embase, Cochrane Library and Web of Science from the inception date of each resource to September 26, 2019, by two study investigators (Li and Cheng) independently. Detailed search terms were shown in Supplementary Table S1. Then, before the statistical analysis of the data, we manually searched from lists of references cited by the published studies, or updated our studies from other sources upto December 7, 2019, to identify whether there was new literature published (PROSPERO registration number is CRD4-2014014901).
Selection criteria
The selection criteria of this meta-analysis were composed of inclusion and exclusion criteria. Studies were included in this meta-analysis if they complied with the following criteria:
Study design was observational study (cohort study, case–control study, nested case–control study or case cohort study) addressing the association between the use of bisphosphonates and risk of any type of cancers
The exposure was defined as one or more prescriptions of bisphosphonates
The outcome was the incidence of cancers
Studies reported effect estimates, including odds ratios (ORs), relative risks (RRs) or hazard ratios (HRs) with 95% confidence intervals (CIs), or provided sufficient data to calculate them
If there were duplicate articles, the most recent published or the most complete data would be included
Language was restricted to English.
Accordingly, these studies were excluded:
Cross-sectional studies, reviews, comments or conference abstracts
Studies were in vitro or animal experiments
Studies without enough data to calculate the effect estimates
Data extraction and quality assessment
Data extraction and quality assessment were independently implemented by two researchers (Li and Liu) by using standardised forms, and any disagreement was resolved through discussion until consensus was reached. The following information was extracted from each study: first author, year of publication, population location, study design, study period, sample size, the number of cases, participants’ age and sex, type and duration of bisphosphonates, type of cancers, adjusted confounding factors and the available effect estimates with the corresponding 95% CIs. The original authors of studies would be contacted, if the required information was missing.
The quality of each included study was assessed using the Newcastle–Ottawa Scale (NOS).30 The NOS assessed quality from the following three aspects: selection, comparability and exposure (case–control studies) or outcome (cohort studies). The total score of NOS was nine stars. Studies with a score of more than 6 stars were considered as relatively high quality. Conversely, studies with a score of less than 6 stars were considered as relatively low quality.
Data synthesis and analysis
RRs were often used as indicators to assess the association between the use of bisphosphonates and risk of cancers, and HRs were similar to RRs. We pooled the risk estimates of case–control and cohort studies in the primary meta-analysis because ORs and RRs could provide similar risk estimates when the outcomes were rare.31 The maximally adjusted risk estimates with 95% CIs were pooled by using random-effect models to obtain a more conservative outcome. Then, the heterogeneity among studies was evaluated by using the Cochrane’s Q statistic and the I2 statistic.32 The low, moderate and high degrees of heterogeneity in this study corresponding to that of the I2 cut-offs were 25%, 50% and 75%, respectively.
To explore the sources of heterogeneity among studies, random-effect meta-regression analysis based on the residual maximum likelihood (REML) method and subgroup analysis for all-cause cancers were conducted by study design, population region and sample size. According to the different molecular modes, bisphosphonates were classified into two groups, including nitrogen-containing bisphosphonates (such as alendronate, ibandronate, pamidronate, risedronate and zoledronate) and non-nitrogen-containing bisphosphonates (such as clodronate and etidronate).33 Then, the subgroup analysis was carried out to study the association between the use of bisphosphonates and various types of cancers based on different types (nitrogen- and non-nitrogen-containing bisphosphonates) and duration (<1 year and ≥1 year) of bisphosphonates.
The sensitivity analysis was performed by omitting one study and calculating the pooled risk estimates with 95% CIs of the remaining studies. Furthermore, the potential publication bias was evaluated with funnel plots,34 and was quantitatively examined by Egger’s linear regression tests.35 If there was publication bias, we would adjust the effect by using the trim-and-fill method.36 For statistical tests, a two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 15.1 (Stata Corporation, College Station, TX, USA).
Results
Literature search and study characteristics
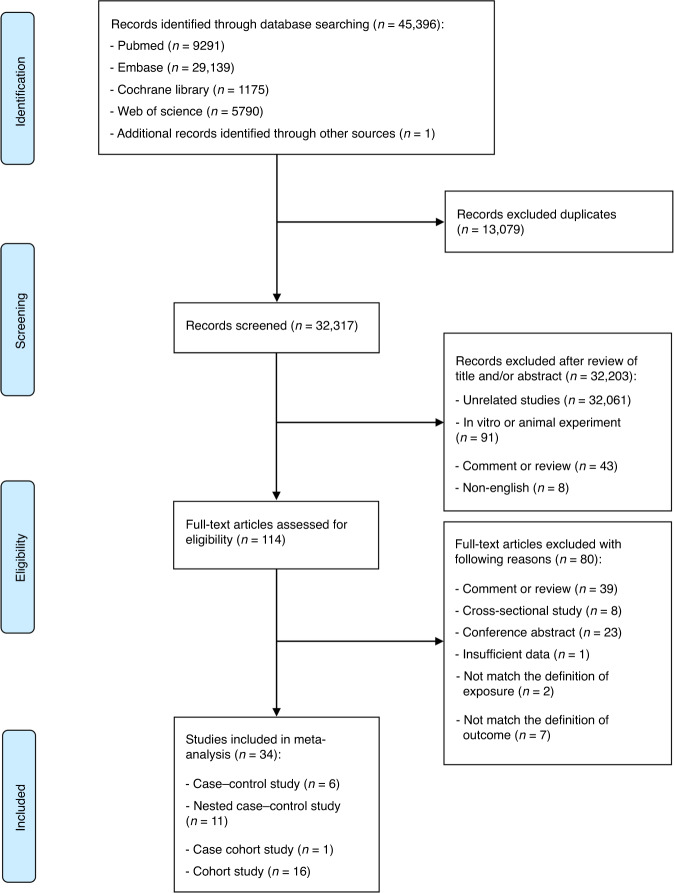
The flow diagram describing detailed literature searches and selection process is shown in Fig. 1. In brief, we identified 45,396 articles through systemically searching in the databases, of which 34 eligible articles (16 cohort studies,11,13,14,37–49 11 nested case–control studies,12,50–59 6 case–control studies60–65 and 1 case cohort study66) were included in the current meta-analysis.
Fig. 1. Flow diagram of study selection in this meta-analysis.
The update search was carried out on December 7, 2019, also manually searched lists of references cited by the published studies to examine for any additional studies reporting primary data.
The main characteristics of the included studies are summarised in Table 1. In total, 14 researches originated from Europe, 12 from America and 8 from Asia. Most of the papers (21/34) were published from 2010 to 2013. Of these, the data were available from 4,508,261 participants, including 403,196 cases originating from 19 types of cancers. Some articles focused on multiple types of cancers: 24 papers reported gastrointestinal cancers (stomach, small bowel, colon and rectum), 16 papers reported gynaecological cancers (endometrium, cervix and ovary), 14 papers reported oesophageal cancer, 10 papers reported breast cancer, 7 papers reported hepatobiliary cancers (liver and bladder), 5 papers reported lung cancer, 3 papers reported prostate cancer and 14 papers reported other cancers. Most of the participants were women, and the mean age ranged from 54.5 to 74.3 years. These studies indicated that alendronate was one of the most commonly used bisphosphonates. In addition, all studies adjusted confounding factors, and the quality assessment of the overall studies was high. Details of the adjusted confounding factors and quality assessment can be seen in Supplementary Tables S2–S4.
Table 1.
The main characteristics of 34 included studies.
| Study [year] | Population location | Study design | Study period | Sample size | Number of cases | Mean age (years)a |
Sex, female (%) | Types of bisphosphonates | Duration of bisphosphonates | Types of cancers |
|---|---|---|---|---|---|---|---|---|---|---|
| Fortuny et al. 63 | USA | Case–control study | 2001–2005 | 936 | 469 | 63.6 ± NA | 100 | NA | NA | Endometrial cancer |
| Newcomb et al.61 | USA | Case–control study | 2003–2006 | 5911 | 2936 | 54.5 ± 8.9 | 100 | NA |
3–12 months; 13–24 months; ≥25 months |
Breast cancer |
| Rennert et al.65 | Northern Israel | Case–control study | 2000–2010 | 4039 | 1832 | 64.7 ± NA | 100 | Alendronate: 86.7% |
<1 year; 1–2 years; 2–3 years; 3–4 years; 4–5 years; >5 years |
Breast cancer |
| Rennert et al.64 | Northern Israel | Case–control study | 2000–2006 | 1866 | 933 | 71.6 ± NA | 100 | Alendronate: 94.7% |
<1 year; 1–2 years; 2–3 years; >3 years |
Colorectal cancer |
| Wright et al. 60 | UK | Case–control study | 1995–2007 | 43,180 | 8636 | 64.7 ± NA | 36.4 | NA | NA | Oesophageal and gastric cancer |
| Rennert et al.62 | Israel | Case–control study | 2003–2010 | 805 | 464 | 67.2 ± NA | 100 | Alendronate and risedronate |
<1 year; 1–2 years; 2–3 years; 3–4 years; 4–5 years; >5 years |
Endometrial and ovarian cancer |
| Green et al.59 | UK | Nested case–control study | NA | 93,678 | 15,613 | ≥40 | NA | NA | NA | Oesophageal, gastric and colorectal cancer |
| Nguyen et al.56 | USA | Nested case–control study | 2000–2002 | 812 | 116 | 64.7 ± 10.3 | 2.6 | Alendronate, risedronate, etidronate, tiludronate and ibandronate | ≥1 prescription | Oesophageal cancer |
| Chen et al. 50 | Taiwan, China | Nested case–control study | 2001–2008 | 3093 | 282 | NA | NA | Alendronate | <1 year; ≥1 year | Oesophageal cancer |
| Singh et al. 52 | Canada | Nested case–control study | 2000–2009 | 59,667 | 5425 | ≥50 | 46.0 | Alendronate, risedronate, etidronate, pamidronate, clodronate and zoledronate |
<210 days; 210–572 days; 573–1250 days; >1250 days |
Colorectal cancer |
| Vinogradova et al.53 | UK | Nested case–control study | 1997–2011 | 1,023,458 | 180,401 | 69.5 ± 10.0 | 49.7 | Alendronate, risedronate, etidronate and ibandronate | <1 year; ≥1 year | Non-gastrointestinal cancersb |
| Vinogradova et al.54 | UK | Nested case–control study | 1997–2011 | 217,569 | 28,625 | ≥50 | 49.7 | Alendronate, etidronate, ibandronate and risedronate | <1 year; ≥1 year | Oesophageal, gastric and colorectal cancer |
| Vogtmann et al.55 | California | Nested case–control study | 1997–2011 | 146,567 | 2934 | 67.2 ± 12.1 | 30.8 | NA | <1 year; ≥1 year | Oesophageal and gastric cancer |
| Jung et al. 12 | Korea | Nested case–control study | 2002–2013 | 8540 | 1708 | ≥40 | 66.6 | Alendronate, risedronate, etidronate, ibandronate, clodronate and pamidronate | NA | Oesophageal and gastric cancer |
| Busby et al.57 | Scotland | Nested case–control study | 1993–2011 | 18,035 | 3098 | 69.2 ± 11.2 | 35.4 | NA | NA | Oesophageal and gastric cancer |
| Vogtmann et al.58 | California | Nested case–control study | 1997–2011 | 612,039 | 12,505 | 65.9 ± 12.9 | 48.5 | Alendronate, risedronate, etidronate, ibandronate and tiludronate | <1 year; ≥1 year | Colorectal cancer |
| Chung et al.51 | Denmark | Nested case–control study | 1996–2013 | 30,228 | 2748 | NA | 37.4 | Alendronate, risedronate, etidronate, ibandronate, clodronate and zoledronate |
≤3 months; 3–12 months; >12 months |
Renal cell carcinoma |
| Chiang et al.66 | Taiwan, China | Case cohort study | 1998–2009 | 27,603 | 3467 | 73.5 ± 8.4 | 100 | Alendronate | ≤2 years; >2 years | All types of cancer |
| Abrahamsen et al.40 | Denmark | Cohort study | 1995–2005 | 41,034 | 85 | 74.3 ± 8.8 | 89.1 | Alendronate, risedronate, etidronate, ibandronate and clodronate | Mean 2.1 years | Oesophageal and gastric cancer |
| Cardwell et al.38 | UK | Cohort study | 1996–2006 | 83,652 | 314 | 70.0 ± 11.4 | 81.0 | Alendronate, risedronate, etidronate, ibandronate, tiludronate and clodronate | NA | Oesophageal cancer |
| Chlebowski et al.41 | USA | Cohort study | 1993–2005 | 154,768 | 6276 | 50–79 | 100 | Alendronate, etidronate, tiludronate and pamidronate |
<1 year; 1–3 years; ≥3 years |
Breast cancer |
| Vestergaard et al.11 | Denmark | Cohort study | 1996–2006 | 414,245 | 103,562 | 70.5 ± 11.4 | 84.7 | Alendronate, etidronate and clodronate | NA | Digestive system cancersc |
| Vestergaard et al.48 | Denmark | Cohort study | 1996–2006 | 348,426 | 884 | 71.1 ± 10.7 | 100 | Alendronate, risedronate, etidronate, ibandronate, clodronate, pamidronate and zoledronate | NA | Breast cancer |
| Abrahamsen et al.37 | Denmark | Cohort study | 1996–2005 | 153,030 | 318 | 71.9 ± 10.0 | 100 | Alendronate | NA | Oesophageal and gastric cancer |
| Cardwell et al.13 | UK | Cohort study | 1996–2006 | 83,652 | 5956 | 70.0 ± 11.4 | 81.0 | NA |
< 1 year; 1–2 years; 2–3 years; 3–4 years |
All types of cancer |
| Khalili et al.45 | USA | Cohort study | 1998–2008 | 86,277 | 801 | 64.7 ± 7.1 | 100 | Alendronate, risedronate, etidronate and other bisphosphonates |
1–2 years; 3–4 years; ≥5 years |
Colorectal cancer |
| Lee et al.39 | Taiwan, China | Cohort study | 1998–2009 | 21,918 | 873 | NA | 84.4 | Alendronate | NA | All types of cancer |
| Pazianas et al.47 | Denmark | Cohort study | 1996–2005 | 153,030 | 1683 | 71.9 ± 10.0 | 100 | Alendronate | NA | Colon cancer |
| Passarelli et al.14 | USA | Cohort study | 1993–2009 | 143,335 | 1931 | 63.2 ± 7.2 | 100 | Alendronate, risedronate, etidronate and tiludronate |
<1 year; 1–3 years; ≥3 years |
Colorectal cancer |
| Alford et al.46 | USA | Cohort study | 1993–2001 | 23,485 | 97 | 55–74 | 100 | Alendronate, risedronate, etidronate and ibandronate | NA | Endometrial cancer |
| Newcomb et al.43 | USA | Cohort study | 1993–2010 | 83,286 | 1123 | 63.0 ± 7.2 | 100 | Alendronate, risedronate, etidronate and tiludronate |
<1 year; 1–3 years; ≥3 years |
Endometrial cancer |
| Fournier et al.49 | France | Cohort study | 2004–2011 | 64,438 | 2407 | 62.8 ± 6.4 | 100 | Alendronate, risedronate, etidronate, ibandronate, tiludronate and zoledronate |
<0.5 year; 0.5–1 years; 1–3 years; ≥3 years |
Breast cancer |
| Tao et al.42 | USA | Cohort study | 1993–2013 | 151,134 | 2511 | 50–79 | 100 | Alendronate, risedronate, etidronate, tiludronate, pamidronate and zoledronate |
<0.67 years; 0.67–1.49 years; 1.50–2.99 years; ≥3 years |
Lung cancer |
| Bae et al.44 | Korea | Cohort study | 2003–2013 | 204,525 | 2183 | 57.2 ± NA | 100 | Alendronate and risedronate | NA | Female cancerd |
NA not available.
aIf mean values of age were unavailable, median or range was extracted.
bNon-gastrointestinal cancers: bladder, breast, cervical, endometrial, lung, melanoma, ovarian, pancreas and prostate cancer.
cDigestive system cancers: oesophageal, liver, pancreas, colon and small intestinal cancer.
dBreast, endometrial and ovarian cancer.
Bisphosphonates and risk of cancers
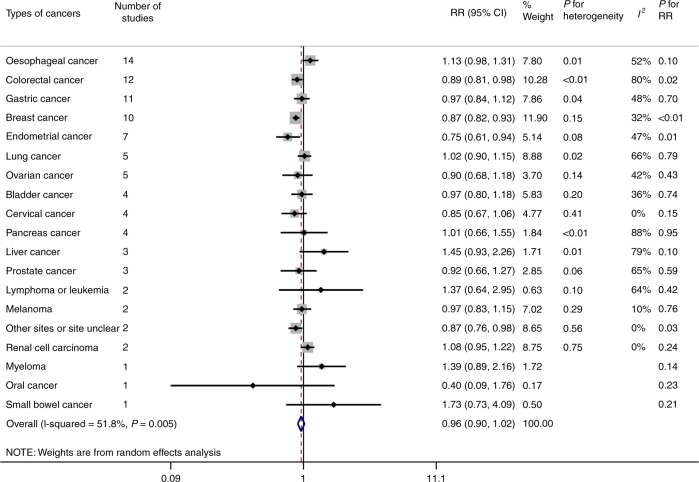
Fig. 2 shows the association between the use of bisphosphonates and risk of different types of cancers. Based on the estimates of the random-effect model, for specific types of cancers, the use of bisphosphonates was strongly associated with colorectal cancer (pooled RR = 0.89, 95% CI: 0.81–0.98, P = 0.02), breast cancer (pooled RR = 0.87, 95% CI: 0.82–0.93, P < 0.01) and endometrial cancer (pooled RR = 0.75, 95% CI: 0.61–0.94, P = 0.01). There was no statistically significant association between the use of bisphosphonates and oesophageal cancer (pooled RR = 1.13, 95% CI: 0.98–1.31, P = 0.10) as well as other types of cancers. The heterogeneity between studies was high in colorectal cancer (P < 0.01, I2 = 80%), pancreas cancer (P < 0.01, I2 = 88%) and liver cancer (P = 0.01, I2 = 79%), while the heterogeneity of the remaining studies was moderate and low. We found that the association between the use of bisphosphonates and risk of all cancers was not statistically significant (pooled RR = 0.96, 95% CI: 0.90–1.02, P = 0.18), with moderate heterogeneity between studies (P < 0.01, I2 = 52%). Detailed meta-analyses for each type of cancers are in Supplementary Fig. S1.
Fig. 2. Forest plot of summary risk estimates in different types of cancers.
The forest plot shows the RRs of different types of cancers comparing individuals using bisphosphonates to those without bisphosphonates. The types of cancers and the number of corresponding studies are shown in the figure. Each small rhombuses represents the RR for each type of cancer, with the location of the rhombuses representing both the direction and magnitude of the effect size and the horizontal line representing their 95% CIs. The square represents the weight of studies of each type of cancer. The hollow rhombus represents the pooled RRs. The maximally adjusted risk estimates with 95% CIs were pooled by using random-effect models to obtain a more conservative outcome. The heterogeneity among studies was evaluated by using the Cochraneʼs Q statistic and the I2 statistic, corresponding to the values of P for heterogeneity and I2 in the figure. RR risk ratio; CI confidence interval.
Meta-regression and subgroup analysis
Random-effect meta-regression model and subgroup analysis were used to explore the primary heterogeneity in regard to population region, study design and sample size. The association between the use of bisphosphonates and risk of all cancers was weaker in the group of sample size ≥ 5000 than in the group of sample size < 5000 (P < 0.01). We found no evidence that population region (Asia: P = 0.88; Europe: P = 0.22) and study design (P = 0.65) influenced the association between the use of bisphosphonates and risk of all cancers (Table 2).
Table 2.
Meta-regression and subgroup analyses with respect to all cancers.
| Subgroups | Number of studies | RR (95%CI) | I2 | P* |
|---|---|---|---|---|
| Overall | 34 | 0.96 (0.90–1.02) | 52% | |
| Population region | ||||
| America | 14 | 0.88 (0.82–0.95) | 38% | Ref. |
| Asia | 8 | 0.88 (0.74–1.03) | 75% | 0.88 |
| Europe | 12 | 0.96 (0.89–1.03) | 88% | 0.22 |
| Study design | ||||
| Case–control | 18 | 0.95 (0.89–1.01) | 70% | Ref. |
| Cohort | 16 | 0.92 (0.88–0.97) | 79% | 0.65 |
| Sample size | ||||
| <5000 | 6 | 0.71 (0.62–0.83) | 0% | Ref. |
| ≥5000 | 28 | 0.94 (0.90–0.99) | 81% | <0.01 |
*P values were estimated by meta-regression.
The bold values represent P value < 0.05.
The subgroup analysis was conducted based on different types of bisphosphonates for various types of cancers (Table 3). Nitrogen-containing bisphosphonates had a protective effect on breast cancer (RR = 0.94, 95% CI: 0.90–0.99, I2 = 0%) and endometrial cancer (RR = 0.70, 95% CI: 0.54–0.92, I2 = 33%), but this effect was only observed in non-nitrogen-containing bisphosphonates on breast cancer (RR = 0.88, 95% CI: 0.81–0.95, I2 = 39%). Notably, non-nitrogen-containing bisphosphonates tended to increase the risk of liver cancer (RR = 2.14, 95% CI: 1.23–3.72) and pancreas cancer (RR = 1.75, 95% CI: 1.32–2.33).
Table 3.
Subgroup analyses with respect to types of bisphosphonates.
| Subgroupsa | Nitrogen-containing bisphosphonates | Non-nitrogen-containing bisphosphonates | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of studies | RR (95% CI) | I2 | P for heterogeneity | Number of studies | RR (95% CI) | I2 | P for heterogeneity | |
| Oesophageal cancer | 8 | 1.10 (0.88–1.38) | 53% | 0.04 | 3 | 1.36 (0.97–1.90) | 57% | 0.10 |
| Gastric cancer | 6 | 0.91 (0.75–1.10) | 30% | 0.21 | 2 | 1.07 (0.53–2.17) | 87% | <0.01 |
| Small-bowel cancer | 1 | 2.19 (0.46–10.41) | NA | NA | 1 | 1.56 (0.56–4.36) | NA | NA |
| Colorectal cancer | 7 | 0.93 (0.79–1.09) | 87% | <0.01 | 4 | 0.95 (0.85–1.07) | 35% | 0.20 |
| Liver cancer | 3 | 1.36 (0.90–2.04) | 65% | 0.06 | 1 | 2.14 (1.23–3.72) | NA | NA |
| Bladder cancer | 2 | 1.18 (0.60–2.29) | 47% | 0.17 | 1 | 1.41 (0.79–2.53) | NA | NA |
| Pancreas cancer | 2 | 1.11 (0.77–1.62) | 7% | 0.30 | 1 | 1.75 (1.32–2.33) | NA | NA |
| Renal cell carcinoma | 2 | 1.15 (0.77–1.72) | 0% | 0.99 | 1 | 1.18 (0.94–1.49) | NA | NA |
| Breast cancer | 6 | 0.94 (0.90–0.99) | 0% | 0.68 | 2 | 0.88 (0.81–0.95) | 39% | 0.20 |
| Cervical cancer | 3 | 0.75 (0.55–1.01) | 0% | 0.47 | NA | NA | NA | NA |
| Endometrial cancer | 5 | 0.70 (0.54–0.92) | 33% | 0.20 | 1 | 0.40 (0.06–2.74) | NA | NA |
| Ovarian cancer | 3 | 0.89 (0.47–1.69) | 47% | 0.15 | NA | NA | NA | NA |
| Prostate cancer | 2 | 1.16 (0.56–2.39) | 78% | 0.03 | 1 | 0.98 (0.84–1.14) | NA | NA |
| Lung cancer | 4 | 1.05 (0.91–1.22) | 70% | 0.02 | 2 | 1.40 (0.77–2.54) | 80% | 0.02 |
NA not available.
aLymphoma or leukaemia and oral cancer were not listed in the table because the number of studies was too few to analyse.
The bold values represent P value < 0.05.
Regarding the association between the duration of bisphosphonates and cancers, the use of bisphosphonates upto at least 1 year (RR = 0.78, 95% CI: 0.63–0.98, I2 = 92%) had a greater protective effect on breast cancer than their use of less than 1 year (RR = 0.90, 95% CI: 0.84–0.97, I2 = 0%). Moreover, we observed a significant risk reduction for the use of bisphosphonates upto at least 1 year on prostate cancer (RR = 0.85, 95% CI: 0.76–0.95). However, there was no significant association on other types of cancers (Table 4).
Table 4.
Subgroup analyses with respect to duration of bisphosphonates.
| Subgroupsa | <1 year | ≥1 year | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of studies | RR (95% CI) | I2 | P for heterogeneity | Number of studies | RR (95% CI) | I2 | P for heterogeneity | |
| Oesophageal cancer | 5 | 1.22 (0.83–1.78) | 66% | 0.02 | 5 | 0.99 (0.79–1.24) | 26% | 0.25 |
| Gastric cancer | 3 | 1.11 (0.91–1.34) | 0% | 0.56 | 3 | 1.15 (0.75–1.75) | 76% | 0.02 |
| Colorectal cancer | 5 | 1.04 (0.88–1.24) | 65% | 0.02 | 7 | 0.84 (0.71–1.04) | 89% | <0.01 |
| Bladder cancer | 2 | 1.59 (0.53–4.75) | 74% | 0.05 | 2 | 0.98 (0.64–1.48) | 23% | 0.25 |
| Pancreas cancer | 2 | 1.25 (0.44–3.52) | 89% | <0.01 | 2 | 0.99 (0.58–1.68) | 65% | 0.09 |
| Renal cell carcinoma | 1 | 1.06 (0.73–1.53) | NA | NA | 1 | 1.10 (0.78–1.56) | NA | NA |
| Breast cancer | 4 | 0.90 (0.84–0.97) | 0% | 0.88 | 5 | 0.78 (0.63–0.98) | 92% | <0.01 |
| Endometrial cancer | 2 | 0.89 (0.61–1.30) | 0% | 0.34 | 2 | 0.55 (0.27–1.12) | 94% | <0.01 |
| Ovarian cancer | 2 | 0.75 (0.21–2.73) | 43% | 0.18 | 2 | 0.73 (0.41–1.30) | 90% | <0.01 |
| Prostate cancer | 1 | 0.90 (0.79–1.02) | NA | NA | 1 | 0.85 (0.76–0.95) | NA | NA |
| Lung cancer | 1 | 1.10 (1.00–1.21) | NA | NA | 1 | 1.01 (0.92–1.10) | NA | NA |
| Melanoma | 1 | 0.90 (0.73–1.10) | NA | NA | 1 | 1.09 (0.92–1.29) | NA | NA |
NA not available.
aOther cancers (small-bowel, liver, cervical cancer, lymphoma or leukaemia and oral cancer) were not listed in the table because the number of studies was too few to analyse.
The bold values represent P value < 0.05.
Sensitivity analysis
We performed a sensitivity analysis by removing one study at each turn and calculating the pooled risk estimates with 95% CIs of the remaining studies. Two studies that were conducted by Abrahamsen et al.37,40 had an impact on the results in oesophageal cancer (pooled RR = 1.13, 95% CI: 0.98–1.31; removed Abrahamsen et al. 2012: RR = 1.17, 95% CI: 1.01–1.35; removed Abrahamsen et al. 2009: RR = 1.17, 95% CI: 1.03–1.33). Besides, the association was not materially changed in this analysis in other types of cancers (Supplementary Figs. S2–S5).
Publication bias
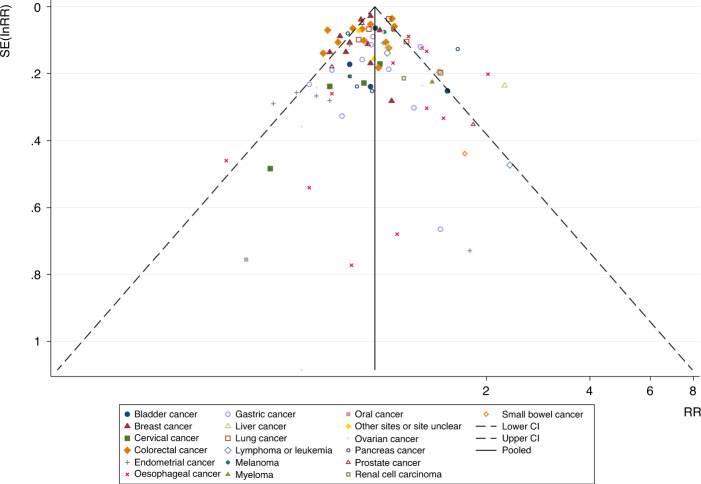
The funnel plot of the association between the use of bisphosphonates and the risk of overall cancers as well as each type of cancer did not indicate substantial asymmetry (Fig. 3). For a specific type of cancers (including more than 10 articles), the Egger’s liner regression test also implied no evidence of publication bias (all cancers: Egger’s P = 0.83; oesophageal cancer: Egger’s P = 0.45; colorectal cancer: Egger’s P = 0.13; gastric cancer: Egger’s P = 0.92; breast cancer: Egger’s P = 0.26) (Table 5). Potential publication bias was not found through the funnel plot and the Egger’s liner regression test, so we did not carry out the trim-and-fill method.
Fig. 3. Funnel plot of the meta-analysis of the association between bisphosphonates and risk of all cancers.
The abscissa is the effect size (RR), and the ordinate is the standard error of the effect size (SE (InRR)). Small symbols of different shapes represent the studies involved in different cancers. The vertical line in the middle represents the combined RR value, and the two oblique dashed lines represent the 95% confidence intervals of the funnel graph. The funnel plot of the association between the use of bisphosphonates and the risk of overall cancers as well as each type of cancer did not indicate substantial asymmetry.
Table 5.
The bias examination of the association between bisphosphonates and risk of cancers.
| Types of cancers | Number of studies | P value for Egger’s liner regression test |
|---|---|---|
| All cancers | 34 | 0.83 |
| Oesophageal cancer | 14 | 0.45 |
| Colorectal cancer | 12 | 0.13 |
| Gastric cancer | 11 | 0.92 |
| Breast cancer | 10 | 0.26 |
Discussion
Summary of the main results
The major findings of this meta-analysis are (1) the use of bisphosphonates might have a protective effect on colorectal, breast and endometrial cancer, but no significant association was observed with respect to all-cause cancer as well as other types of cancers. (2) Moreover, nitrogen-containing bisphosphonates could reduce the risk of breast cancer by 6% and endometrial cancer by 30%; non-nitrogen-containing bisphosphonates could also reduce the risk of breast cancer by 12%. However, non-nitrogen-containing bisphosphonates might be associated with an increased risk of liver and pancreas cancer. (3) The use of bisphosphonates upto at least 1 year had a greater protective effect on breast cancer than their use for less than 1 year, but such result was not found in other cancers.
Consistent and inconsistent with current studies
With regard to all-cause cancer, our results are largely consistent with previous meta-analyses in similar contexts, supporting that the association between bisphosphonates and the risk of all-cause cancer is not statistically significant.26 The difference is that their study only included 13 cohort studies. Because this study had more sample size and the total number of cancer cases, we could examine more types of cancers and obtain higher statistical power. As we found that the sample size might be one of the sources of heterogeneity, we speculate that the use of bisphosphonates has a weak protective effect on all-cause cancer, which needs more sample size to explore this association.
With regard to female cancers, our findings are consistent with previous studies that bisphosphonates could reduce the risk of breast23,26,67,68 and endometrial cancer,22,24 but no significant link was observed in ovarian cancer.24 This might be due to the oestrogen receptor (ER) involved in the anticancer effect of bisphosphonates.69 Furthermore, the study by Chlebowski et al.41 showed that bisphosphonates reduced the risk of ER-positive breast cancer by 30% (HR = 0.70, 95% CI: 0.52–0.95, P = 0.02). However, the data on the association between bisphosphonates and ER-positive female cancers are poor, as we could not conduct subgroup analysis based on ER. Further studies are needed to focus on the use of bisphosphonates and their protective effect on the different subtypes of female cancers to confirm these findings.
The effect of bisphosphonates on gastrointestinal cancers is controversial. The study by Oh et al.25 showed that there was no significant association between bisphosphonates and the risk of oesophageal cancer (RR = 0.96, 95% CI: 0.65–1.42), which was consistent with our results and other three meta-analyses.20,21,26 However, Andrici et al.70 found that bisphosphonates might increase the risk of oesophageal cancer (OR = 1.74, 95% CI: 1.19–2.55). Similarly, the results regarding the association between bisphosphonates and the risk of gastric cancer are inconsistent.12,54,55 Clinical reports have found that bisphosphonates can cause gastrointestinal problems, such as erosive oesophagitis and gastric ulcers;71,72 these patients are more likely to receive the upper gastrointestinal endoscopy to accelerate the discovery of upper gastrointestinal cancer. In addition, because oesophageal adenocarcinoma of the distal oesophagus is very similar to the adenocarcinoma at the junction of the gastro-oesophagus, it is difficult to accurately distinguish them in clinical diagnosis. Original studies rarely report the results of subgroup analysis of the precise site of upper gastrointestinal cancer, as we are unable to perform pool analysis. We should focus on distinguishing the particular subtypes of oesophageal and gastric cancer when analysing the effects of bisphosphonates on gastric and oesophageal cancer in future studies. With respect to colorectal cancer, our study indicated that bisphosphonates could reduce the risk of colorectal cancer by 11%, which was similar with other five meta-analyses,15–19 but Deng et al.26 and Oh et al.25 suggested that the association between bisphosphonates and the risk of colorectal cancer was not statistically significant. It is well known that bisphosphonates are commonly used for osteoporosis because they have lower bone mineral density (BMD). Previous studies have shown that BMD is associated with cancer, which means that if not adjusted, it might be a confounding factor that masks the protective effect of bisphosphonates.73,74 For other types of cancers, the results are basically similar to previous studies, and the number of related studies is few.26,75
Types and duration of bisphosphonates and confounders
Experimental studies have suggested that nitrogen-containing bisphosphonates have potential antitumour effects. They could reduce the viability of tumour cells by binding to the kinase domain of the human epidermal growth factor receptor 1/2 (HER1/2), and resulting in an overall reduction in global downstream signalling that was driven by overexpression of the HER family,76,77 such as lung,78 breast79 and colorectal cancer.80 We only observed that nitrogen-containing bisphosphonates could reduce 30% risk of endometrial cancer and 6% risk of breast cancer. However, there was no significant association between the use of nitrogen-containing bisphosphonates and risk of colorectal and lung cancer in our study. Simultaneously, we found that non-nitrogen-containing bisphosphonates might be associated with an increased risk of liver and pancreas cancer, which might be due to the greater toxicity on liver and pancreas. Clodronate (non-nitrogen-containing bisphosphonate) might cause evaluation of aminotransferase.81 Moreover, there were rare studies of liver disease developing in patients who use non-nitrogen-containing bisphosphonate.82,83 In addition, from the limited information, we could not find any possible mechanism to explain the association between non-nitrogen-containing bisphosphonates and the risk of pancreas cancer, but we should pay attention to the safety of using this drug. In the future, it might be necessary to further explore the pharmacological mechanisms of non-nitrogen-containing bisphosphonates.
Notably, we did not find that the duration of bisphosphonates had an effect on specific cancers, except breast cancer. For breast cancer, our results were consistent with a meta-analysis by Liu et al.,68 which showed that using bisphosphonates upto at least 1 year (RR = 0.75, 95% CI: 0.66–0.84) seemed to be greater protective on breast cancer than using them for less than 1 year (RR = 0.90, 95% CI: 0.84–0.97). However, Ou et al.23 suggested that bisphosphonate was not associated with risk of breast cancer when the usage time was less than 1 year (RR = 0.93, 95% : 0.86–1.00), but a significant 26% reduction was found upto at least 1 year (RR = 0.74, 95% CI: 0.66–0.83), which was consistent with the study by Newcomb et al.61 Patients with the long-term use of bisphosphonates might have a healthier lifestyle and higher adherence to the drugs, so the benefits observed in the analysis might be overestimated.84 Thus, further studies are needed to consider this potential bias and find the best duration and dose of bisphosphonates.
In this study, the evidence we have on the use of bisphosphonates and risk of cancers is based mainly on observational studies. Many studies are not able to adequately control confounding factors related to cancers. Wright et al.60 demonstrated a small but significantly increased risk of oesophageal cancer in women, not in men. Generally, women prescribe bisphosphonates for prevention and treatment of osteoporosis, while men are more likely to prescribe bisphosphonates for iatrogenic osteoporosis.85,86 Hence, most of the participants in included studies were women, and the number of men was much smaller. To date, there is still a lack of studies on the association between bisphosphonates and risk of cancers in people of different genders. Secondly, some studies have reported that supplemental calcium and vitamin D might have a protective effect on colorectal cancer, which are usually prescribed with bisphosphonates.87,88 Some included studies did not control for calcium and vitamin D when analysing the association between bisphosphonates and the risk of colorectal cancer.52,54,58 In addition, only a few studies adjust the use of statins, hormone replacement therapy (HRT) and family history of cancers, which are also related to cancers.44–46,49
Strengths and limitations
This study has several strengths. First, there are many articles and a large number of participants included in this study, which increases the statistical power of the analysis. Furthermore, we explore the sources of heterogeneity by meta-regression and subgroup analysis. Finally, this systematic review and meta-analysis has an update to the summary articles in this field. However, several limitations of this study must be mentioned. Firstly, our study includes observational studies (cohort studies, case–control studies, nested case–control studies and case cohort studies) that might differ in the design of researches and lack individual information. In addition, evidence from observational studies is weaker than that from randomised controlled trials. Secondly, the meta-analysis of observational studies is susceptible to confounding factors that exist in original studies because most of the included studies use large and anonymous databases.89 Although all included studies attempt to adjust confounding factors, there are still potential confounders that are not considered, which might affect our results. Thirdly, we are unable to perform subgroup analysis of precise sites of oesophageal and gastric cancer, because the original studies rarely report the results in this aspect. Finally, although we have explored several sources of heterogeneity with meta-regression model and subgroup analysis, it still cannot fully explain the heterogeneity in the studies.
Conclusions
In conclusion, the results of this meta-analysis suggest that the use of bisphosphonates is associated with a decreased risk of colorectal, breast and endometrial cancer, but not significantly associated with the risk of oesophageal and other types of cancers. Furthermore, we find that nitrogen-containing bisphosphonates appear to have more antitumour effects, but non-nitrogen- containing bisphosphonates might be associated with an increased risk of liver and pancreas cancer. In addition, the use of bisphosphonates for at least 1 year has a greater protective effect on breast cancer than their use for less than 1 year. We recommend that further large prospective cohort studies are needed to explore the causal association between bisphosphonates and cancers, and more studies of potential mechanism are required. After a careful benefit-and-risk assessment, whether nitrogen-containing bisphosphonates are expected to be used in populations at a high risk for the malignancies needs further discussion.
Supplementary information
Acknowledgements
Not applicable.
Author contributions
Study concept and design: Y.P.L., C.X.J. and Y.Y.L.; data collection and collation: Y.Y.L, S.J.L. and S.C.; statistical analysis: Y.Y.L., L.J.G. and Y.X.Z; writing—original draft: Y.Y.L.; writing—review and editing: all authors; study supervision: Y.P.L., C.X.J., L.J.G. and Y.X.Z. All authors contributed to data analysis, drafting or revision of this paper, and approved the final version. The corresponding authors prove that all listed authors meet the authorship criteria, and that no other eligible authors have been omitted.
Ethics approval and consent to participate
All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
Consent to publish
Not applicable.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yu-Peng Liu, Cun-Xian Jia
Contributor Information
Yu-Peng Liu, Email: liuyupeng@wmu.edu.cn.
Cun-Xian Jia, Email: jiacunxian@sdu.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-01043-9.
References
- 1.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. (Lond.) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.van der Pluijm G, Vloedgraven H, van Beek E, van der Wee-Pals L, Lowik C, Papapoulos S. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J. Clin. Invest. 1996;98:698–705. doi: 10.1172/JCI118841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sewing L, Steinberg F, Schmidt H, Göke R. The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis. 2008;13:782–789. doi: 10.1007/s10495-008-0211-z. [DOI] [PubMed] [Google Scholar]
- 6.Tsubaki M, Itoh T, Satou T, Imano M, Komai M, Ogawa N, et al. Nitrogen-containing bisphosphonates induce apoptosis of hematopoietic tumor cells via inhibition of Ras signaling pathways and Bim-mediated activation of the intrinsic apoptotic pathway. Biochem Pharm. 2013;85:163–172. doi: 10.1016/j.bcp.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Tang XD, Zhang QZ, Shi SH, Yen Y, Li XY, Zhang YF, et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1 alpha/VEGF signaling pathways in human breast cancer cells. Int J. Cancer. 2010;126:90–103. doi: 10.1002/ijc.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reusser NM, Dalton HJ, Pradeep S, Gonzalez-Villasana V, Jennings NB, Vasquez HG, et al. Clodronate inhibits tumor angiogenesis in mouse models of ovarian cancer. Cancer Biol. Ther. 2014;15:1061–1067. doi: 10.4161/cbt.29184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guise TA. Antitumor effects of bisphosphonates: promising preclinical evidence. Cancer Treat. Rev. 2008;34:S19–S24. doi: 10.1016/j.ctrv.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N. Engl. J. Med. 2009;360:89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 11.Vestergaard P. Occurrence of gastrointestinal cancer in users of bisphosphonates and other antiresorptive drugs against osteoporosis. Calcif. Tissue Int. 2011;89:434–441. doi: 10.1007/s00223-011-9539-4. [DOI] [PubMed] [Google Scholar]
- 12.Jung SY, Sohn HS, Park EJ, Suh HS, Park JW, Kwon JW. Oral bisphosphonates and upper gastrointestinal cancer risks in Asians with osteoporosis: a nested case-control study using National Retrospective Cohort Sample Data from Korea. PLoS ONE. 2016;11:e0150531. doi: 10.1371/journal.pone.0150531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardwell CR, Abnet CC, Veal P, Hughes CM, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of cancer. Int J. Cancer. 2012;131:E717–E725. doi: 10.1002/ijc.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passarelli MN, Newcomb PA, LaCroix AZ, Lane DS, Ho GY, Chlebowski RT. Oral bisphosphonate use and colorectal cancer incidence in the Women’s Health Initiative. J. Bone Min. Res. 2013;28:2043–2048. doi: 10.1002/jbmr.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonovas S, Nikolopoulos G, Bagos P. Bisphosphonate use and risk of colorectal cancer: a systematic review and meta-analysis. Br. J. Clin. Pharm. 2013;76:329–337. doi: 10.1111/bcp.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Gao S, Ni X, Chen F, Liu X, Xie H, et al. Exposure to bisphosphonates and risk of colorectal cancer. Br. J. Clin. Pharm. 2013;76:320–328. doi: 10.1111/bcp.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh S, Singh AG, Murad MH, Limburg PJ. Bisphosphonates are associated with reduced risk of colorectal cancer: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013;11:232–239.e231. doi: 10.1016/j.cgh.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Thosani N, Thosani SN, Kumar S, Nugent Z, Jimenez C, Singh H, et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta-analysis. J. Clin. Oncol. 2013;31:623–630. doi: 10.1200/JCO.2012.42.9530. [DOI] [PubMed] [Google Scholar]
- 19.Yang G, Hu H, Zeng R, Huang J. Oral bisphosphonates and the risk of colorectal cancer: a meta-analysis. J. Clin. Gastroenterol. 2013;47:741–748. doi: 10.1097/MCG.0b013e31829e446b. [DOI] [PubMed] [Google Scholar]
- 20.Sun K, Liu JM, Sun HX, Lu N, Ning G. Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies. Osteoporos. Int. 2013;24:279–286. doi: 10.1007/s00198-012-2158-8. [DOI] [PubMed] [Google Scholar]
- 21.Wright E, Schofield PT, Molokhia M. Bisphosphonates and evidence for association with esophageal and gastric cancer: a systematic review and meta-analysis. BMJ Open. 2015;5:e007133. doi: 10.1136/bmjopen-2014-007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou YJ, Chiu HF, Wong YH, Yang YH. Bisphosphonate use and the risk of endometrial cancer: a meta-analysis of observational studies. Pharmacoepidemiol. Drug Saf. 2016;25:1107–1115. doi: 10.1002/pds.4075. [DOI] [PubMed] [Google Scholar]
- 23.Ou YJ, Chiu HF, Wong YH, Yang CC, Yang YH. Bisphosphonate use and the risk of breast cancer: a meta-analysis of observational studies. Pharmacoepidemiol. Drug Saf. 2017;26:1286–1295. doi: 10.1002/pds.4302. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XS, Zhang YM, Li B, Fan B, Zhao Y, Yang SJ. Risk reduction of endometrial and ovarian cancer after bisphosphonates use: a meta-analysis. Gynecol. Oncol. 2018;150:509–514. doi: 10.1016/j.ygyno.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: meta-analysis of observational studies. World J. Gastroenterol. 2012;18:5779–5788. doi: 10.3748/wjg.v18.i40.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y, Zhang Z, Jia X, Cheng W, Zhou X, Liu Y, et al. Oral bisphosphonates and incidence of cancers in patients with osteoporosis: a systematic review and meta-analysis. Arch. Osteoporos. 2019;14:1. doi: 10.1007/s11657-018-0552-3. [DOI] [PubMed] [Google Scholar]
- 27.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. the Alendronate Phase III Osteoporosis Treatment Study Group. N. Engl. J. Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 28.Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N. Engl. J. Med. 1990;323:73–79. doi: 10.1056/NEJM199007123230201. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014).
- 31.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 37.Abrahamsen B, Pazianas M, Eiken P, Russell RG, Eastell R. Esophageal and gastric cancer incidence and mortality in alendronate users. J. Bone Min. Res. 2012;27:679–686. doi: 10.1002/jbmr.1481. [DOI] [PubMed] [Google Scholar]
- 38.Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304:657–663. doi: 10.1001/jama.2010.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee WY, Sun LM, Lin MC, Liang JA, Chang SN, Sung FC, et al. A higher dosage of oral alendronate will increase the subsequent cancer risk of osteoporosis patients in Taiwan: a population-based cohort study. PLoS ONE. 2012;7:e53032. doi: 10.1371/journal.pone.0053032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abrahamsen B, Eiken P, Eastell R. More on reports of esophageal cancer with oral bisphosphonate use. N. Engl. J. Med. 2009;360:1789. doi: 10.1056/NEJMc096026. [DOI] [PubMed] [Google Scholar]
- 41.Chlebowski RT, Chen Z, Cauley JA, Anderson G, Rodabough RJ, McTiernan A, et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J. Clin. Oncol. 2010;28:3582–3590. doi: 10.1200/JCO.2010.28.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao MH, Chen S, Freudenheim JL, Cauley JA, Johnson KC, Mai X, et al. Oral bisphosphonate use and lung cancer incidence among postmenopausal women. Ann. Oncol. 2018;29:1476–1485. doi: 10.1093/annonc/mdy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newcomb PA, Passarelli MN, Phipps AI, Anderson GL, Wactawski-Wende J, Ho GY, et al. Oral bisphosphonate use and risk of postmenopausal endometrial cancer. J. Clin. Oncol. 2015;33:1186–1190. doi: 10.1200/JCO.2014.58.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae YS, Chang J, Park SM. Oral bisphosphonate use and the risk of female breast, ovarian, and cervical cancer: a nationwide population-based cohort study. Arch. Osteoporos. 2019;14:41. doi: 10.1007/s11657-019-0588-z. [DOI] [PubMed] [Google Scholar]
- 45.Khalili H, Huang ES, Ogino S, Fuchs CS, Chan AT. A prospective study of bisphosphonate use and risk of colorectal cancer. J. Clin. Oncol. 2012;30:3229–3233. doi: 10.1200/JCO.2011.39.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alford SH, Rattan R, Buekers TE, Munkarah AR. Protective effect of bisphosphonates on endometrial cancer incidence in data from the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Cancer. 2015;121:441–447. doi: 10.1002/cncr.28952. [DOI] [PubMed] [Google Scholar]
- 47.Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate–Danish National Register Based Cohort Study. Osteoporos. Int. 2012;23:2693–2701. doi: 10.1007/s00198-012-1902-4. [DOI] [PubMed] [Google Scholar]
- 48.Vestergaard P, Fischer L, Mele M, Mosekilde L, Christiansen P. Use of bisphosphonates and risk of breast cancer. Calcif. Tissue Int. 2011;88:255–262. doi: 10.1007/s00223-011-9463-7. [DOI] [PubMed] [Google Scholar]
- 49.Fournier A, Mesrine S, Gelot A, Fagherazzi G, Baglietto L, Clavel-Chapelon F, et al. Use of bisphosphonates and risk of breast cancer in a French cohort of postmenopausal women. J. Clin. Oncol. 2017;35:3230–3239. doi: 10.1200/JCO.2016.71.4337. [DOI] [PubMed] [Google Scholar]
- 50.Chen YM, Chen DY, Chen LK, Tsai YW, Chang LC, Huang WF, et al. Alendronate and risk of esophageal cancer: a nationwide population-based study in Taiwan. J. Am. Geriatr. Soc. 2011;59:2379–2381. doi: 10.1111/j.1532-5415.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- 51.Chung BI, Hellfritzsch M, Ulrichsen SP, Sørensen HT, Ehrenstein V. Bisphosphonate use and risk of renal cell carcinoma: a population-based case-control study. Basic Clin. Pharm. Toxicol. 2018;124:642–646. doi: 10.1111/bcpt.13180. [DOI] [PubMed] [Google Scholar]
- 52.Singh H, Nugent Z, Demers A, Mahmud S, Bernstein C. Exposure to bisphosphonates and risk of colorectal cancer: a population-based nested case-control study. Cancer. 2012;118:1236–1243. doi: 10.1002/cncr.26395. [DOI] [PubMed] [Google Scholar]
- 53.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of common non-gastrointestinal cancers: series of nested case-control studies using two primary-care databases. Br. J. Cancer. 2013;109:795–806. doi: 10.1038/bjc.2013.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to bisphosphonates and risk of gastrointestinal cancers: series of nested case-control studies with QResearch and CPRD data. BMJ. 2013;346:f114. doi: 10.1136/bmj.f114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogtmann E, Corley DA, Almers LM, Cardwell CR, Murray LJ, Abnet CC. Oral bisphosphonate exposure and the risk of upper gastrointestinal cancers. PLoS ONE. 2015;10:e0140180. doi: 10.1371/journal.pone.0140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen DM, Schwartz J, Richardson P, El-Serag HB. Oral bisphosphonate prescriptions and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig. Dis. Sci. 2010;55:3404–3407. doi: 10.1007/s10620-010-1198-1. [DOI] [PubMed] [Google Scholar]
- 57.Busby J, Murchie P, Murray L, Iversen L, Lee AJ, Spence A, et al. The effect of medications which cause inflammation of the gastro-oesophageal tract on cancer risk: a nested case-control study of routine Scottish data. Int J. Cancer. 2017;140:1828–1835. doi: 10.1002/ijc.30612. [DOI] [PubMed] [Google Scholar]
- 58.Vogtmann E, Corley DA, Almers LM, Cardwell CR, Murray LJ, Abnet CC. Oral bisphosphonates and colorectal cancer. Sci. Rep. 2017;7:44177. doi: 10.1038/srep44177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright E, Schofield PT, Seed P, Molokhia M. Bisphosphonates and risk of upper gastrointestinal cancer–a case control study using the General Practice Research Database (GPRD) PLoS ONE. 2012;7:e47616. doi: 10.1371/journal.pone.0047616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br. J. Cancer. 2010;102:799–802. doi: 10.1038/sj.bjc.6605555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rennert G, Rennert HS, Pinchev M, Lavie O. The effect of bisphosphonates on the risk of endometrial and ovarian malignancies. Gynecol. Oncol. 2014;133:309–313. doi: 10.1016/j.ygyno.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Fortuny J, Sima C, Bayuga S, Wilcox H, Pulick K, Faulkner S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol. Biomark. Prev. 2009;18:1448–1456. doi: 10.1158/1055-9965.EPI-08-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J. Clin. Oncol. 2011;29:1146–1150. doi: 10.1200/JCO.2010.33.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J. Clin. Oncol. 2010;28:3577–3581. doi: 10.1200/JCO.2010.28.1113. [DOI] [PubMed] [Google Scholar]
- 66.Chiang CH, Huang CC, Chan WL, Huang PH, Chen TJ, Chung CM, et al. Oral alendronate use and risk of cancer in postmenopausal women with osteoporosis: A nationwide study. J. Bone Min. Res. 2012;27:1951–1958. doi: 10.1002/jbmr.1645. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Zhao S, Chen W, Hu F, Zhu L, Zhang Q, et al. Bisphosphonate use and the risk of breast cancer: a meta-analysis of published literature. Clin. Breast Cancer. 2012;12:276–281. doi: 10.1016/j.clbc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Zhang X, Sun H, Zhao S, Zhang Y, Li D, et al. Bisphosphonates and primary breast cancer risk: an updated systematic review and meta-analysis involving 963,995 women. Clin. Epidemiol. 2019;11:593–603. doi: 10.2147/CLEP.S194056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Journe F, Chaboteaux C, Dumon JC, Leclercq G, Laurent G, Body JJ. Steroid-free medium discloses oestrogenic effects of the bisphosphonate clodronate on breast cancer cells. Br. J. Cancer. 2004;91:1703–1710. doi: 10.1038/sj.bjc.6602181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrici J, Tio M, Eslick GD. Meta-analysis: oral bisphosphonates and the risk of oesophageal cancer. Aliment Pharm. Ther. 2012;36:708–716. doi: 10.1111/apt.12041. [DOI] [PubMed] [Google Scholar]
- 71.Abraham SC, Cruz-Correa M, Lee LA, Yardley JH, Wu TT. Alendronate-associated esophageal injury: pathologic and endoscopic features. Mod. Pathol. 1999;12:1152–1157. [PubMed] [Google Scholar]
- 72.Graham DY, Malaty HM. Alendronate and naproxen are synergistic for development of gastric ulcers. Arch. Intern. Med. 2001;161:107–110. doi: 10.1001/archinte.161.1.107. [DOI] [PubMed] [Google Scholar]
- 73.Lee HF, Wu CE, Lin YS, Hwang JS, Wu CH, Chu PH. Low bone mineral density may be associated with long-term risk of cancer in the middle-aged population: A retrospective observational study from a single center. J. Formos. Med Assoc. 2018;117:339–345. doi: 10.1016/j.jfma.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 74.Nock NL, Patrick-Melin A, Cook M, Thompson C, Kirwan JP, Li L. Higher bone mineral density is associated with a decreased risk of colorectal adenomas. Int. J. Cancer. 2011;129:956–964. doi: 10.1002/ijc.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen LX, Ning GZ, Zhou ZR, Li YL, Zhang D, Wu QL, et al. The carcinogenicity of alendronate in patients with osteoporosis: evidence from cohort studies. PLoS ONE. 2015;10:e0123080. doi: 10.1371/journal.pone.0123080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stachnik A, Yuen T, Iqbal J, Sgobba M, Gupta Y, Lu P, et al. Repurposing of bisphosphonates for the prevention and therapy of nonsmall cell lung and breast cancer. Proc. Natl Acad. Sci. USA. 2014;111:17995–18000. doi: 10.1073/pnas.1421422111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuen T, Stachnik A, Iqbal J, Sgobba M, Gupta Y, Lu P, et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc. Natl Acad. Sci. USA. 2014;111:17989–17994. doi: 10.1073/pnas.1421410111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dacic S. EGFR assays in lung cancer. Adv. Anat. Pathol. 2008;15:241–247. doi: 10.1097/PAP.0b013e31817bf5a9. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez-Conchas GA, Rodriguez-Romo L, Hernandez-Barajas D, Gonzalez-Guerrero JF, Rodriguez-Fernandez IA, Verdines-Perez A, et al. Epidermal growth factor receptor overexpression and outcomes in early breast cancer: A systematic review and a meta-analysis. Cancer Treat. Rev. 2018;62:1–8. doi: 10.1016/j.ctrv.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Krasinskas AM. EGFR Signaling in Colorectal Carcinoma. Pathol. Res Int. 2011;2011:932932. doi: 10.4061/2011/932932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laitinen K, Taube T. Clodronate as a cause of aminotransferase elevation. Osteoporos. Int. 1999;10:120–122. doi: 10.1007/s001980050205. [DOI] [PubMed] [Google Scholar]
- 82.Välimäki MJ, Laitinen K, Patronen A, Puolijoki H, Seppänen J, Pylkkänen L, et al. Prevention of bone loss by clodronate in early postmenopausal women with vertebral osteopenia: a dose-finding study. Osteoporos. Int. 2002;13:937–947. doi: 10.1007/s001980200131. [DOI] [PubMed] [Google Scholar]
- 83.Lewiecki EM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs. 2011;71:791–814. doi: 10.2165/11585470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 84.Eiken P, Vestergaard P. Oral bisphosphonates and colon cancer: an update. Ther. Adv. Musculoskelet. Dis. 2015;7:160–168. doi: 10.1177/1759720X15582144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minisola S, Cipriani C, Scillitani A, Pepe J. More on the use of bisphosphonates in the prevention and treatment of osteoporosis. BMJ. 2015;351:h5868. doi: 10.1136/bmj.h5868. [DOI] [PubMed] [Google Scholar]
- 86.Brown SA, Guise TA. Drug insight: the use of bisphosphonates for the prevention and treatment of osteoporosis in men. Nat. Clin. Pract. Urol. 2007;4:310–320. doi: 10.1038/ncpuro0816. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Giovannucci E. Calcium, vitamin D and colorectal cancer chemoprevention. Best. Pr. Res Clin. Gastroenterol. 2011;25:485–494. doi: 10.1016/j.bpg.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Heine-Bröring RC, Winkels RM, Renkema JMS, Kragt L, van Orten-Luiten A-CB, Tigchelaar EF, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J. Cancer. 2015;136:2388–2401. doi: 10.1002/ijc.29277. [DOI] [PubMed] [Google Scholar]
- 89.Smith GD, Egger M. Meta-analyses of observational data should be done with due care. BMJ. 1999;318:56. doi: 10.1136/bmj.318.7175.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.