Abstract
Bioinspired smart materials represent a tremendously growing research field and the obtainment of new building blocks is at the molecular basis of this technology progress. In this work, colloidal materials have been prepared in few steps starting from ribonucleosides. Nucleobase morpholino β-amino acids are the chimera key intermediates allowing Phe–Phe dipeptides’ functionalization with adenine and thymine. The obtained compounds self-aggregate showing enhanced photoluminescent features, such as deep blue fluorescence and phosphorescence emissions.
Subject terms: Materials chemistry, Organic chemistry, Supramolecular chemistry
Introduction
The development of humankind society has often been measured by the types of material that were used during the ages, from Stone, Iron, Bronze to Steel and, in the second part of the twentieth century, Silicon. In the last two decades, a new evolution of man-made materials has been started, this time powered by nanotechnology. Nanomaterials are thus expected having a strong impact on societal change owing to their wide applications ranging from clean energy to biomedicine1–3. This influence on society is paving the basis to the Nanomaterials Age. Consequently, the development of new, biocompatible nanomaterials characterized by enhanced properties and features is a growing, stimulating research field.
Natural biomacromolecules, as proteins and nucleic acids, can create a wide range of nano-assemblies. Amino acids and nucleotides are at the molecular level of this high complexity and, in the recent years, many studies have been focusing on the development of bio-inspired building blocks that self-assemble into preferred architectures4–10. One successful example is the dipeptide diphenylalanine can build up different morphologies depending on the environment conditions and functionalization11,12. The driving force of diphenylalanine self-assembly is the π–π stacking between the aryl groups that are then enforced by hydrogen bonds stabilizing the final architectures13–17. More in general, amino acid and peptide-based supramolecular structures are normally formed through H-bonding, van der Waals force and π–π interactions. These peptide systems are very stable and can withstand high temperature and exposure to chemicals15,16. In addition, they possess high mechanical stability17. Nucleotide-based architectures are formed due to Watson–Crick interactions allowing specific molecular recognition and base-pairing complementarity. The combination of peptides and nucleic acids could be thus very useful for the design of novel self-organized materials with enhanced properties and features. Nevertheless, nucleopeptides18, i.e. peptides containing both nucleobases and amino acids, are still underexploited in the development of functional materials. Few examples are reported in the literature, such as environment polarity driven self-assembly of helical Aib nucleofoldamers19 and diphenylalanine nucleopeptides hydrogels20. In both examples, the nucleobases are linked to the C- and/or N-terminus of the peptides. A different strategy could be the synthesis of peptides containing nucleo amino acids, i.e. amino acids bearing a nucleobase on the side chain. During the years, several linear α21,22 and β11,12 nucleo amino acids23 have been developed and used for the preparation of tubular or sheet-like aggregates. In this work, we present a new class of β amino acids 1 containing a morpholino ring and a nucleobase. These nucleo amino acids were prepared in enantiopure form from ribonucleosides 2, through nucleo morpholino alcohols 3, as the key intermediates (Scheme 1).
Scheme 1.
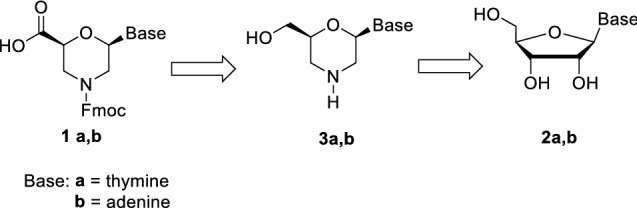
Retrosynthetic pathway to nucleobase morpholino β-amino acids.
Nucleo amino acids 1 were then used for the preparation of ultra-short peptides containing the dipeptide Phe–Phe that showed self-aggregation propensity in water and polar solvents, leading to the obtainment of sub-micrometric spherical morphologies. Finally, given the formally deep-UV active features of the Phe–Phe residue24,25, steady state and time resolved photoluminescence (PL) studies have been performed on the synthesized ultra-short peptides revealing their deep blue fluorescence and an unprecedent phosphorescence.
The synthesis of alcohol intermediates 3 was reported in 1993 by Summerton in a patent26 and consisted in a “one-pot” oxidative ring-opening and reductive amination of the ribose sugar leading to the obtainment of the morpholino ring (Scheme 2). Several modifications of this procedure have been reported in the last years27–30. Here, we started from unprotected ribonucleosides, using the Summerton’s procedure with different work up conditions. Briefly, the sugar ring is converted into the corresponding morpholino through a “one-pot” oxidation/amination reaction using NaIO4 (1.1 eq) and (NH4)2B4O7 (1.15 eq) in MeOH. The crude intermediates A were directly treated with NaCNBH3 (1.7 eq) in MeOH affording compounds 3. The reaction was quenched with HCl and the products 3a and 3b were isolated as hydrochloride salts by crystallization from polar solvents (3a: MeOH, 60%; 3b: ACN, 70%).
Scheme 2.
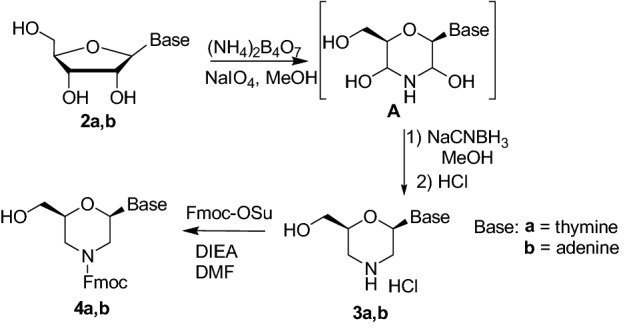
Synthesis of morpholino alcohols 4.
We then investigated the oxidation reaction of the hydroxyl group affording to the β-amino acid function. Firstly, the protection of the endocyclic nitrogen was required to avoid side reactions on the free amine. The Fmoc protecting group was selected, in view of using N-protected compounds 1 for the preparation of nucleopeptide materials. Fmoc group is indeed known to favor self-assembly through π–π stacking31. The reaction was carried out directly on the crude 3a,b obtained in the previous reaction (Scheme 2) simply adding FmocOSu (1 eq) and DIEA (1–3 eq) in DMF. The target compounds 4a and 4b were thus obtained in 85% and 55% yield, respectively. Several oxidants were then tested on the compounds 4a and 4b (Table S1 in Supplementary Information). For compound 4a, having thymine as nucleobase, the combination BIAB/TEMPO32 was found the most effective, affording compound 1a with 90% yield (Scheme 3). On the other hand, in the same conditions, compounds 4b scarcely reacted and the Fmoc deprotection occurred by prolonging reaction times. Acid 1b (70%) was thus obtained by Jones oxidation with CrO3 (Scheme 3).
Scheme 3.
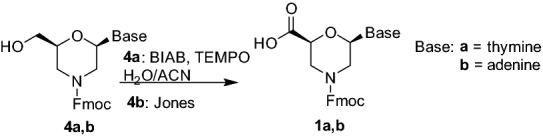
Synthesis of nucleobase morpholino β-amino acids 1.
N-Fmoc protected thymine and adenine nucleo amino acids 1a-b were then used for the preparation of ultra-short nucelopeptides containing diphenylalanine. The coupling with the dipeptide H2N–Phe–Phe–OMe 5 was performed using HOBt/HBTU (1.1 eq each), as condensing agents, and DIEA (4 eq), as the base, leading to compounds 6a,b in 80% yield (Scheme 4).
Scheme 4.
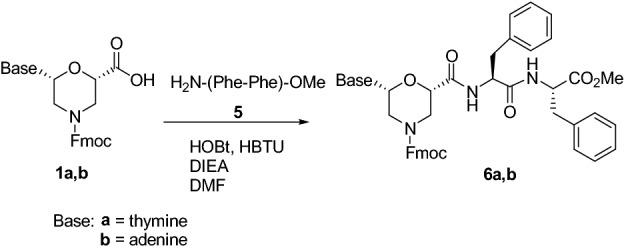
Synthesis of tripeptides 6a,b.
The self-assembly behavior of both compounds 6a,b was studied using the solvent displacement method. A solution of compound 6a,b in hexafluoroisopropanol (HFIP, 100 mg/mL) was diluted with different solvents (distilled water, EtOH, 50% EtOH, MeOH, isopropanol, chloroform) to a final concentration of 2 mg/mL. The formation of sub-micrometric aggregates was observed by drop-casting on silicon wafers for recording SEM after 30 min and after 24 h (see Supplementary information). In water, both compounds 6a and 6b exhibited spherical aggregates (Fig. 1) whose size ranged from a few tens of nanometers to less than a micron. In chloroform, isopropanol and HFIP no aggregates were observed.
Figure 1.
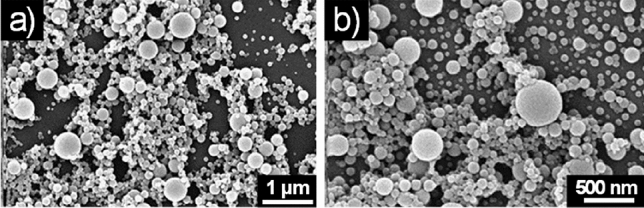
SEM micrographs of the self-assembled structures formed by 6a (a) and 6b (b) in pure water.
In EtOH and MeOH, 6a and 6b had a different behavior. Compound 6a exhibited indeed spherical macro-aggregates in 50% EtOH (Fig. S1 in Supplementary information), while 6b self-aggregates in ordered spherical structures both in EtOH and MeOH (Fig. 2).
Figure 2.
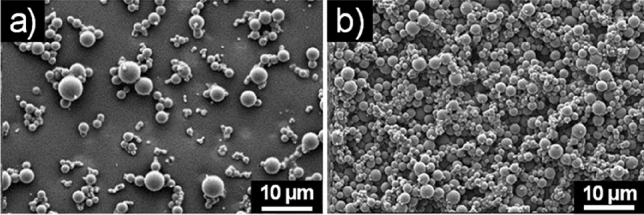
SEM micrographs of the self-assembled structures formed by 6b (a) in EtOH and (b) MeOH.
Considering the promising results obtained in HFIP/ H2O, we performed DLS analysis on 6a and 6b at different HFIP/H2O ratios (see Supplementary information). Our results suggested that the quantity of water is fundamental for determining the formation of aggregates, their size and and their distribution. At the lowest water content (HFIP/H2O, 70:30) the formation of nanocolloidal clusters of 5–6 nm was observed together with larger agglomerates. An enhancement in the size of the aggregates was obtained increasing the water amount, although the distribution was not optimal. When the HFIP content was only the 2% the 6a and 6b suspensions showed only one sharp peak centered at ca. 200 nm, indicating the formation of a monodispersed colloidal suspension (see Table 1 and Supplementary information). In the case of 6a, an increase of the size of the agglomerates was observed by measurements repeated after 24 h and 48 h. A negative ζ-potential was detected for both 6a and 6b, suggesting a protonated state of the nucleobases with a consequent formation of a tight ion pair due to the presence of acidic HFIP.
Table 1.
Hydrodynamic diameter by DLS and ζ-potential of 6a and 6b aggregates.
| H2O/HFIP | 6a | 6b |
|---|---|---|
| 98:2 (t=0) | 190 ± 60 nm | 190 ± 91 nm |
| 98:2 (t=24h) | 342 ± 120 nm | 220 ± 100 nm |
| 98:2 (t=48h) | 342 ± 150 nm | 220 ± 100 nm |
| ζ-potential | − 47 mV | − 16 mV |
The stability of 6a and 6b water aggregates was examined at different pH and upon heating at 120 °C. Both aggregates were not stable at high temperature and at acidic pH. At basic pH (pH = 10), a change of the morphology was observed (Fig. 3).
Figure 3.
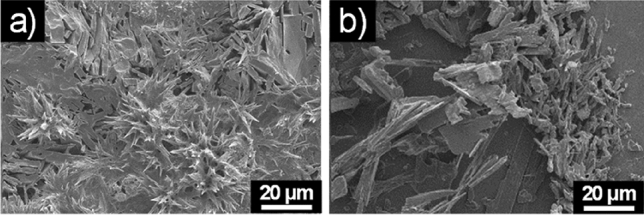
SEM micrographs of the self-assembled structures formed at basic pH by (a) 6a and (b) 6b.
To understand the molecular conformation of the self-assembled structure of 6a and 6b, FT-IR experiments were performed (Fig. 4). In both cases, the minima at 1688 cm−1 in the amide-I region and 1540 cm−1 in the amide-II region suggested the presence of β-sheet conformations33. We thus hypothesized that the closer of sheets along the two axes results in the formation of the spherical structure34. The other minima in the spectrum (1652 cm−1 for 6a and 1660 cm−1 for 6b) are ascribable to adenine (A) and thymine (T) residues, respectively (see Fig. S2 in Supplementary Information).
Figure 4.
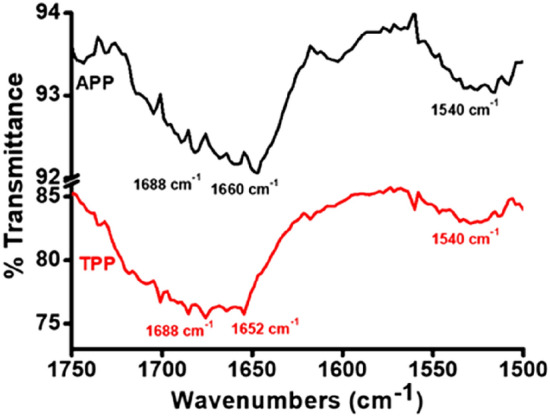
FT-IR spectra of the amide I and amide II regions of 6a (red) and 6b (black).
Photophysical characterizations were performed on diluted solutions (1–2 × 10–5 M) of the tripeptides 6a and 6b (see Supplementary Information for details) and on self-assembled materials in water, obtained from HFIP-water (98:2 v/v) at the concentration of 5 × 10−5 M (Fig. 5). Photophysical characterization in the same conditions were performed on 1a and 1b (see Figs. S7, S8, Table S2 in Supplementary Information). The normalized absorption spectra of the tripeptides 6a and 6b acetonitrile (ACN) solutions (Fig. S3) looked very similar and are characterized by a strong deep UV absorption below 230 nm, a broad band between 240–280 nm and two slightly resolved vibrionic transitions between 280-300 nm (more evident in the excitation spectra, see Figs. S3 and S4 in Supplementary Information). In addition, both compounds 6a,b showed deep blue room temperature photoluminescence in solution (PL, normalized data reported in Fig. 5 gray lines with square drawings and Fig. S4) with a maximum at 303 nm and a barely resolved shoulder at lower energy. The corresponding fluorescence quantum yield (QY) was 0.12 for 6a and 0.13 for 6b; the lifetimes were 5.0 and 5.5 ns respectively. The emissions and the photophysical parameters are similar to the ones measured on the Fmoc protected 1a and 1b (see Fig. S8 and Table S2). In Fig. 5, the PL emissions of the colloids obtained from HFIP-water mixture at 50 µM concentration are superimposed. Compound 6a showed an overall QY of 0.018 and biexponential decay of 1.41(63%) and 6.06 (37%) ns at 330 nm. Its emission was composed by a dominant high energy band around 308 nm (similar to the emission in ACN solution and only slightly red-shifted) followed by a week visible emission tailing up to 500 nm. 6b, with an overall QY of 0.03, displayed a high energy emission at 306 nm characterized by a biexponential decay of 1.08(57%) and 7.07(43%) ns at 330 nm, and a broad unstructured band between 400–600 nm with maximum at 440 nm and lifetime of 2.82(27%) and 13.1(73%) ns. Whereas the former is similar to the molecular emission in diluted solution, the latter could be ascribed to the fluorescence emission linked to the restricted intramolecular rotation of the backbone as previously reported in the literature for the Phe–Phe system25.
Figure 5.
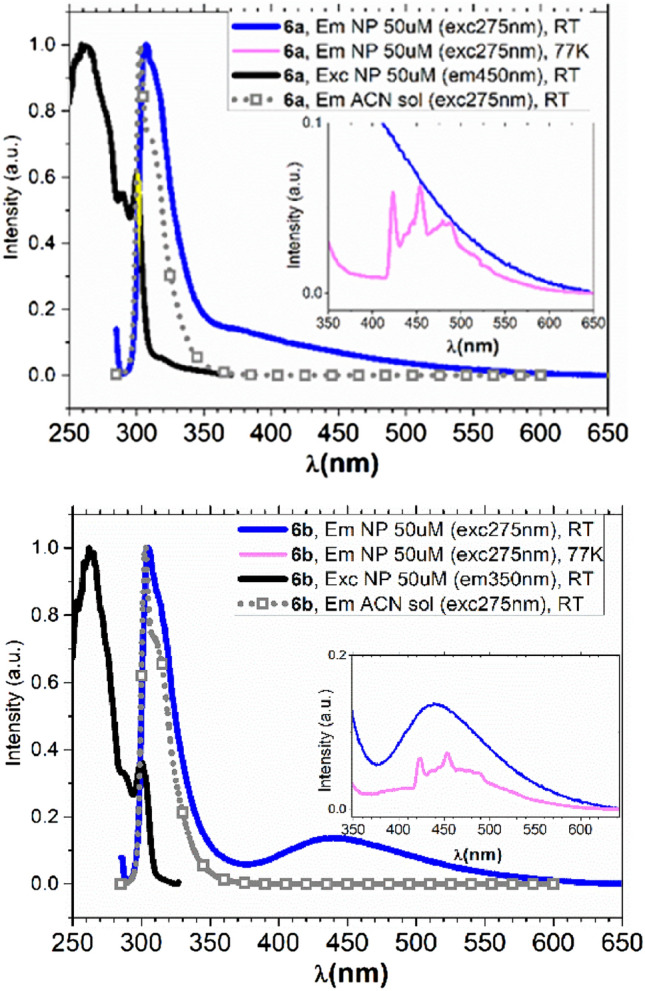
Emission and excitation spectra of 6a (top panel) and 6b (bottom panel) as diluted solution in ACN and as self-assembled NP at both RT and 77 K glass matrix.
Upon cooling the colloidal suspensions of compounds 6a,b at 77 K (pink curve in Fig. 5 insets) a new, previously undescribed, emission appeared in the visible range between 420 and 550 nm and characterized by structurally resolved features. These emissions had a long radiative lifetime of 4.2 and 4.1 s for 6a and 6b, respectively, thus indicating a process originated from a triplet state. To understand the origin of the phosphorescence emission, PL studies were performed on the isolated building blocks to generate compounds 6 (i.e. H2N- Phe–Phe -OMe 5 and the Fmoc protected 1). As evident from Fig. S5 (SI) a structured emission above 400 nm is only observed from the nucleobase thus allowing the attribution of the phosphorescence observed in the tripeptide systems to the adenine or thymine unit35.
In conclusion, a new class of β amino acids containing a morpholino ring and a nucleobase has been developed starting from ribonucleosides. Their synthesis takes advantage from a “one-pot” oxidative ribose ring-opening and reductive amination, followed by the oxidation of the primary alcohol of the sugar. The so obtained β amino acids have been used for the functionalization of Phe–Phe dipeptide leading to sub-micrometric aggregates possessing photoluminescent features of both fluorescence and phosphorescence type. Thus, it was proved that the here presented tripeptides possess the photoluminescent properties given by the β-AA and the self-assembly behaviour due to the presence of Phe–Phe. They hence represent promising tools for the development of bioinspired functional materials with applications not only in the biotechnology field but also in non-biological optoelectronic ones.
Supplementary information
Author contributions
R.B., E.E. and F.V. synthesized the compounds. A.B. performed the luminescence experiments. A.S., S.Y., and M.R. performed self-assembly studies, IR and microscopy experiments. D.M performed the DLS studies. M.L.G. revised the text. S.P. conceived the research, interpreted the exp data and wrote the text. All authors reviewed the manuscript. The manuscript was written through contributions of all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76297-7.
References
- 1.Chuangang H, Jia Q, Ying X, Shenlong Z, Hao C, Liming D. Carbon nanomaterials for energy and biorelated catalysis: recent advances and looking forward. ACS Cent. Sci. 2019;5:389–408. doi: 10.1021/acscentsci.8b00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei S, Zhan Z, Yunlu D, Xiaoyuan C. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 2019;48:3771–3810. doi: 10.1039/C8CS00896E. [DOI] [PubMed] [Google Scholar]
- 3.Pelaz B, et al. Diverse applications of nanomedicine. ACS Nano. 2017;11:2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locarno S, Argentiere S, Ruffoni A, Maggioni D, Soave R, Bucci R, Erba E, Lenardi C, Gelmi ML, Clerici F. Self-assembled hydrophobic Ala-Aib peptide encapsulating curcumin: a convenient system for water insoluble drugs. RSC Adv. 2020;10:9964–9975. doi: 10.1039/C9RA10981A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren J, Wang Y, Yao Y, Wang Y, Fei X, Qi P, Lin S, Kaplan DL, Buehler MJ, Ling S. Biological material interfaces as inspiration for mechanical and optical material designs. Chem. Rev. 2019;119:12279–12336. doi: 10.1021/acs.chemrev.9b00416. [DOI] [PubMed] [Google Scholar]
- 6.Pu F, Ren J, Qu X. Chem. Soc. Rev. 2018;47:1285–1306. doi: 10.1039/C7CS00673J. [DOI] [PubMed] [Google Scholar]
- 7.Bucci R, Das P, Iannuzzi F, Feligioni M, Gandolfi R, Gelmi ML, Reches M, Pellegrino S. Self-assembly of an amphipathic ααβ-tripeptide into cationic spherical particles for intracellular delivery. Org. Biomol. Chem. 2017;15:6773–6779. doi: 10.1039/C7OB01693J. [DOI] [PubMed] [Google Scholar]
- 8.Clerici F, Erba E, Gelmi ML, Pellegrino S. Non-standard amino acids and peptides: From self-assembly to nanomaterials. Tet. Lett. 2016;57:5540–5550. doi: 10.1016/j.tetlet.2016.11.022. [DOI] [Google Scholar]
- 9.Ruffoni A, Cavanna MV, Argentiere S, Locarno S, Pellegrino S, Gelmi ML, Clerici F. Aqueous self-assembly of short hydrophobic peptides containing norbornene amino acid into supramolecular structures with spherical shape. RSC Adv. 2016;10:90754–90759. doi: 10.1039/C6RA17116H. [DOI] [Google Scholar]
- 10.Bonetti A, Pellegrino S, Das P, Yuran S, Bucci R, Ferri N, Meneghetti F, Castellano C, Reches M, Gelmi ML. Dipeptide nanotubes containing unnatural fluorine-substituted β2,3-diarylamino acid and l-alanine as candidates for biomedical applications. Org. Lett. 2015;17:4468–4471. doi: 10.1021/acs.orglett.5b02132. [DOI] [PubMed] [Google Scholar]
- 11.Marchesan S, Vargiu AV, Styan KE. The Phe-Phe motif for peptide self-assembly in nanomedicine. Molecules. 2015;20:19775–19788. doi: 10.3390/molecules201119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan X, Zhu P, Li J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010;39:1877–1890. doi: 10.1039/b915765b. [DOI] [PubMed] [Google Scholar]
- 13.Reches M, Gazit E. 2Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300:625–627. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]
- 14.Guo C, Luo Y, Zhou R, Wei G. Probing the self-assembly mechanism of diphenylalanine-based peptide nanovesicles and nanotubes. ACS Nano. 2012;6:3907–3918. doi: 10.1021/nn300015g. [DOI] [PubMed] [Google Scholar]
- 15.Tao K, Tang Y, Rencus-Lazar S, Yao Y, Xue B, Gilead S, Wei G, Gazit E. Bioinspired supramolecular packing enables high thermo-sustainability. Angew. Chem. Int. Ed. 2020;59:19037–19041. doi: 10.1002/anie.202008702. [DOI] [PubMed] [Google Scholar]
- 16.Adler-Abramovich L, Reches M, Sedman VL, Allen S, Tendler JB, Gazit E. Thermal and chemical stability of diphenylalanine peptide nanotubes: implications for nanotechnological applications. Langmuir. 2006;22:1313–1320. doi: 10.1021/la052409d. [DOI] [PubMed] [Google Scholar]
- 17.Kol N, Adler-Abramovich L, Barlam D, Shneck RZ, Gazit E, Rousso I. Self-assembled peptide nanotubes are uniquely rigid bioinspired supramolecular structures. Nano Lett. 2005;5:1343–1346. doi: 10.1021/nl0505896. [DOI] [PubMed] [Google Scholar]
- 18.Roviello GN, Benedetti E, Pedone C, Bucci EM. Nucleobase-containing peptides: an overview of their characteristic features and applications. Amino Acids. 2010;39:45–57. doi: 10.1007/s00726-010-0567-6. [DOI] [PubMed] [Google Scholar]
- 19.Marafon G, Menegazzo I, De Zotti M, Crisma M, Toniolo C, Moretto A. Tuning morphological architectures generated through living supramolecular assembly of a helical foldamer end-capped with two complementary nucleobases. Soft Matter. 2017;13:4231–4240. doi: 10.1039/C7SM00764G. [DOI] [PubMed] [Google Scholar]
- 20.Datta D, Tiwar O, Ganesh KN. New archetypes in self-assembled Phe-Phe motif induced nanostructures from nucleoside conjugated-diphenylalanines. Nanoscale. 2018;10:3212–3224. doi: 10.1039/C7NR08436F. [DOI] [PubMed] [Google Scholar]
- 21.Geotti-Bianchini P, Crisma M, Peggion C, Bianco A, Formaggio F. Conformationally controlled, thymine-based α-nucleopeptides. Chem. Comm. 2009;1:3178–3180. doi: 10.1039/b822789f. [DOI] [PubMed] [Google Scholar]
- 22.Roviello GN, Musumeci D. Synthetic approaches to nucleopeptides containing all four nucleobases, and nucleic acid-binding studies on a mixed-sequence nucleo-oligolysine. RSC Adv. 2016;6:63578–63585. doi: 10.1039/C6RA08765E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava R, Kumar Ray A, Diederichsen U. Higher aggregation of β-peptide networks controlled by nucleobase pairing. Eur. J. Org. Chem. 2009;1:4793–4800. doi: 10.1002/ejoc.200900511. [DOI] [Google Scholar]
- 24.Zhixing G, Ming M, Yunsong D, Huanga S. New J. Chem. 2016;40:1970. doi: 10.1039/C5NJ03184B. [DOI] [Google Scholar]
- 25.Zhixing G, Hao X. Photoluminescence of diphenylalanine peptide nano/microstructures: from mechanisms to applications. Macromol. Rapid Commun. 2017;38:1700370. doi: 10.1002/marc.201700370. [DOI] [PubMed] [Google Scholar]
- 26.Summerton, J.E., Weller, D.D. (1993) U.S. Patent No. 518544413.
- 27.Tarasenko YV, Abramova TV, Mamatuk VI, Silnikov VN. Effective synthesis of fluorescently labeled morpholino nucleoside triphosphate derivatives. Nucleosides Nucleotides Nucleic Acids. 2016;35:32–42. doi: 10.1080/15257770.2015.1114125. [DOI] [PubMed] [Google Scholar]
- 28.Abramova TV, Belov SY, Tarasenko YV, Silnikov VN. Solid-phase-supported synthesis of morpholinoglycine oligonucleotide mimics. J. Org. Chem. 2014;10:1151–1158. doi: 10.3762/bjoc.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattanayak S, Paul S, Nandi B, Sinha S. Improved protocol for the synthesis of flexibly protected morpholino monomers from unprotected ribonucleosides. Nucleosides Nucleotides Nucleic Acids. 2012;31:763–782. doi: 10.1080/15257770.2012.724491. [DOI] [PubMed] [Google Scholar]
- 30.Zhang N, Tan C, Cai P, Jiang Y, Zhang P, Zhao Y. Synthesis and properties of morpholino chimeric oligonucleotides. Tet. Lett. 2008;49:3570–3573. doi: 10.1016/j.tetlet.2008.04.035. [DOI] [Google Scholar]
- 31.Tao K, Levin A, Adler-Abramovich L, Gazit E. Fmoc-modified amino acids and short peptides: simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016;45:3935–3953. doi: 10.1039/C5CS00889A. [DOI] [PubMed] [Google Scholar]
- 32.Bucci R, Contini A, Clerici F, Pellegrino S, Gelmi ML. From glucose to enantiopure morpholino β-amino acid: a new tool for stabilizing γ-turns in peptides. Org. Chem. Front. 2019;6:972–982. doi: 10.1039/C8QO01116H. [DOI] [Google Scholar]
- 33.Ghadiri MR, Granja JR, Milligan RA, McRee RA, Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366:324–327. doi: 10.1038/366324a0. [DOI] [PubMed] [Google Scholar]
- 34.Reches M, Gazit E. Self-assembled peptide nanotubes are uniquely rigid bioinspired supramolecular structures. Nano Lett. 2004;4:581–585. doi: 10.1021/nl035159z. [DOI] [PubMed] [Google Scholar]
- 35.Nikitin T, Kopyl S, Shur VY, Kopelevich YV, Kholkin AL. Low-temperature photoluminescence in self-assembled diphenylalanine microtubes. Phys. Lett. Sect A Gen. Atom. Solid State Phys. 2016;380:1658–1662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


