Abstract
The habenula is a phylogenetically conserved epithalamic structure, which conveys negative information via inhibition of mesolimbic dopamine neurons. We have previously shown the expression of kisspeptin (Kiss1) in the habenula and its role in the modulation of fear responses in the zebrafish. In this study, to investigate whether habenular Kiss1 regulates fear responses via dopamine neurons in the zebrafish, Kiss1 peptides were intracranially administered close to the habenula, and the expression of dopamine-related genes (th1, th2 and dat) were examined in the brain using real-time PCR and dopamine levels using LC–MS/MS. th1 mRNA levels and dopamine levels were significantly increased in the telencephalon 24-h and 30-min after Kiss1 administration, respectively. In fish administered with Kiss1, expression of neural activity marker gene, npas4a and kiss1 gene were significantly decreased in the ventral habenula. Application of neural tracer into the median raphe, site of habenular Kiss1 neural terminal projections showed tracer-labelled projections in the medial forebrain bundle towards the telencephalon where dopamine neurons reside. These results suggest that Kiss1 negatively regulates its own neuronal activity in the ventral habenula via autocrine action. This, in turn affects neurons of the median raphe via interneurons, which project to the telencephalic dopaminergic neurons.
Subject terms: Neuroscience, Neural circuits, Social behaviour
Introduction
The habenula is an evolutionarily conserved epithalamic structure in vertebrates. In teleosts, the habenula is divided into the dorsal (dHb) and the ventral habenula (vHb)1, which send efferent outputs to different targets2. In the zebrafish (Danio rerio), the dHb can be further divided into the left and the right subnuclei that project and terminate in the ventral and the dorsal interpeduncular nuclei (IPN), respectively1,2. On the other hand, neurons of the vHb project to the ventro-anterior corner of the median raphe (MR), a subregion of the MR through the fasciculus retroflexus to the superior raphe3,4. In the zebrafish, the role of dHb-IPN pathway has been implicated in the control of aversive responses such as fear and social conflict5–7. However, a recent optogenetic study revealed that stimulation of vHb neurons evokes place avoidance behaviour in zebrafish8. We have previously demonstrated the co-expression of kisspeptin (Kiss1), a neuropeptide and its cognate G-protein coupled receptor-54 (GPR54 = Kiss1R) in the vHb of zebrafish9. Central administration of Kiss1 peptides significantly suppress kiss1 gene expression, suggesting an autocrine regulation of the Kiss1 gene10. Further, Kiss1 has been shown to exhibit neuromodulatory role in odorant (alarm substance)-induced fear-like response11,12, and a more recent study showed impairment of aversion-based learning in kiss1-mutant fish13.
In mammals, the habenula conveys anti-reward and aversive information via inhibition of mesolimbic dopamine neurons14,15. Anatomically, neurons in the lateral habenula (LHb) of rats send efferent projections that terminate on the mesolimbic dopamine neurons. Stimulation of the LHb16 or exposure to aversive stimuli17 inhibits the activity of dopamine-containing neurons in the midbrain. In addition, optogenetic activation of the habenula-dopaminergic pathway promotes active, passive and conditioned behavioural avoidance in rodents15. In addition to rodents, the role of dopamine signalling in aversive response is also conserved in fish18,19. In the tilapia (cichlid fish) dopaminergic neurons have been suggested to mediate fear responses to novel environments20. Although, the above studies in vertebrates suggest a potential link between the habenula-dopamine system and aversive response, more studies are still needed to confirm these pathways of aversive response.
The present study was performed to elucidate possible interaction between the habenular Kiss1 and the dopamine system, by examining the effect of Kiss1 peptides on the expression of dopamine-related genes: tyrosine hydroxylase (th1 and th2) and dopamine transporter (dat/slc6a3) using real-time PCR, and dopamine levels using LC–MS/MS in the brain of adult zebrafish. To identify the dopaminergic neuronal population responsive to Kiss1 administration, we examined the expression of neural activity-dependent marker, neuronal PAS domain-containing protein 4 a (npas4a) gene21. Finally, to examine the potential association between habenular Kiss1 neurons and dopaminergic neuronal population, we applied neural tracer in the MR, site of Kiss1 terminal projections.
Results
Effect of Kiss1 on dopamine-related gene expression
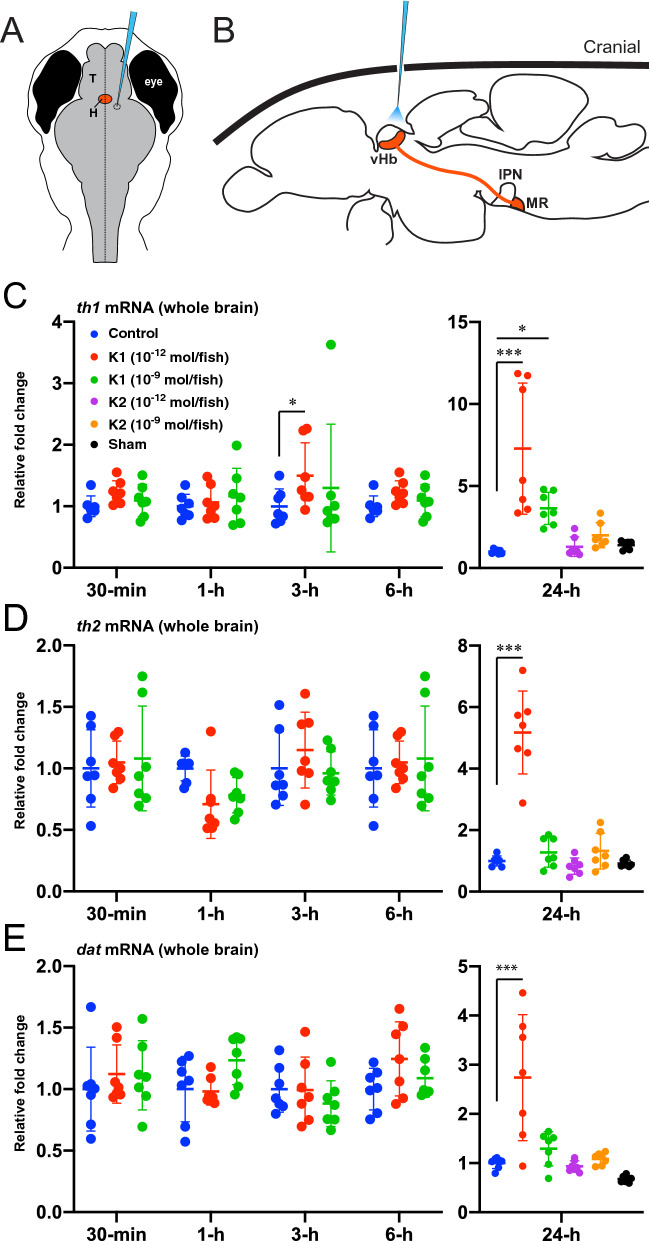
Central administration of Kiss1 peptides at dose of 10–12 mol/fish significantly (P < 0.05 or P < 0.0001) upregulated expression levels for th1, th2 and dat mRNAs in the whole brain of fishes only 24-h after administration (Fig. 1C–E) but not 30-min, 1-h, 3-h or 6-h after administration as compared with those of control fishes, except for small decrease in th1 mRNA levels in the group treated with 10–12 mol/fish of Kiss1 at 3-h post administration (Fig. 1C). th1 expression was also increased at 24-h when the fish were treated with Kiss1 10–9 mol/fish (P < 0.05) (Fig. 1C). Administration with a Kiss1 paralogue, Kiss2 peptides (10–12 and 10–9 mol/fish) and sham-treatment had no effect on any of dopamine-related genes at 24-h post administration.
Figure 1.
Effect of Kiss1 treatment on expression of dopamine-related genes. (A,B) Schematic drawings of horizontal (A) and sagittal (B) views of a zebrafish brain illustrating intracranial administration of Kiss1 solution. An incision is made into the skull over the anterior part of the left optic tectum using a sterilized barbed-end needle (A). Through the incision made, Kiss1 solution was administered nearby Kiss1 neurons in the ventral habenula (vHb) using a heat-pulled glass capillary micropipette (B). T telencephalon, H habenula, IPN interpeduncular nucleus, MR median raphe. Adopted from Ogawa et al. and Lim et al.11,70. (C–E) Expression levels of th1 (C), th2 (D) and dat (E) mRNAs were measured in the whole brain (n = 7) at 30-min, 1-h, 3-h, 6-h, and 24-h following the intracranial administration with either vehicle (control; distilled water) or Kiss1 (K1) or its paralogous peptide, Kiss2 (K2) at doses of 10–12 or 10–9 mol/ fish. Data are representative of mRNA expression in relative to eef1a1l1 housekeeping gene and presented mean ± SEM as fold-change. *P < 0.05; ***P < 0.0001 versus control group using 2-way ANOVA followed by Dunnett’s multiple comparison test.
Regional effect of Kiss1 on dopamine-related gene expression
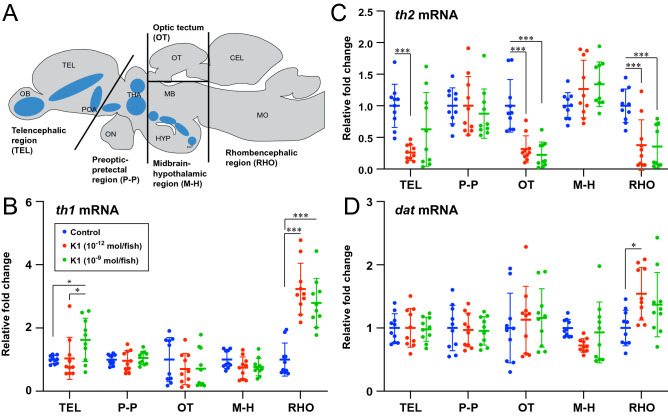
To examine the regional effect of Kiss1 administration on dopamine system, expression levels of dopamine-related genes were examined in five macro-dissected brain regions (Fig. 2A). Kiss1 administration differentially altered the expression levels of th1 and/or th2 mRNAs in the telencephalic, optic tecum and rhombencephalic regions but there was no change in the preoptic-pretectal and midbrain-hypothalamic regions (Fig. 2B–D).
Figure 2.
Effect of Kiss1 on the expression of dopamine-related genes in different brain regions. (A) Schematic sagittal view of a zebrafish brain illustrating the macro-dissected brain regions that were subjected to analyses of gene-expression levels. Blue-shaded regions indicate the distribution of dopaminergic cell populations. (B–D) Relative expression (fold-change) of th1 (B), th2 (C) and dat (D) mRNAs (n = 10 fish/group) in macro-dissected brain regions 24-h after central administration of either vehicle (control; distilled water) or Kiss1 (K1) with doses of 10–12 or 10–9 mol/ fish. Data presented as mean ± SD. *P < 0.05; ***P < 0.0001 using two-way ANOVA, Tukey's multiple comparisons test.
In the telencephalic region, th1 mRNA levels were significantly (P < 0.05) higher in fish treated with Kiss1 at the dose of 10–9 mol/fish as compared with control and Kiss1 at the dose of 10–12 mol/fish (Fig. 2B), and th2 mRNA levels were significantly (P < 0.0001) lower in fish treated with Kiss1 at the dose of 10–12 mol/fish as compared to controls (Fig. 2C). In the optic tectum, there was no effect of Kiss1 administration on th1 mRNA levels, while th2 mRNA levels were significantly (P < 0.0001) lower in fish treated with Kiss1 at the doses of 10–12 and 10–9 mol/fish as compared to controls (Fig. 2C). In the rhombencephalic region, th1 mRNA levels were significantly (P < 0.0001) higher in fish treated with Kiss1 at the doses of 10–12 and 10–9 mol/fish as compared to controls (Fig. 2B) and th2 mRNA levels were significantly (P < 0.0001) lower in the fish treated with Kiss1 at the doses of 10–12 and 10–9 mol/fish as compared to controls (Fig. 2C). There was no change in dat mRNA levels throughout the brain regions, except in the rhombencephalic region, where dat mRNA levels were significantly (P < 0.05) higher in fish treated with Kiss1 at the dose of 10–12 mol/fish as compared to controls (Fig. 2D).
Effect of Kiss1 on dopamine levels in macro-dissected brain
Dopamine (DA) levels were measured in three macro-dissected brain regions following Kiss1 administration (Fig. 3A). All groups including Kiss1-treated and controls treated with saline, absolute DA levels fluctuated time-dependently in all three brain regions (Fig. 3B–D). In the telencephalic and preoptic-pretectal regions, basal levels of DA seen at 30-min and 1-h post saline administration were decreased at 3-h (telencephalon: P < 0.0001 vs 30-min and 1-h; preoptic area, P < 0.001 vs 30-min, P < 0.05 vs 1-h) but returned to similar levels at 6-h post saline administration (telencephalon: P < 0.0001; preoptic are: P < 0.001 vs 3-h). On the other hand, in the midbrain-hypothalamic region, DA levels continued to increase from 3-h until 6-h post saline administration (P < 0.0001 vs 30-min and 1-h; P < 0.001 vs 3-h).
Figure 3.
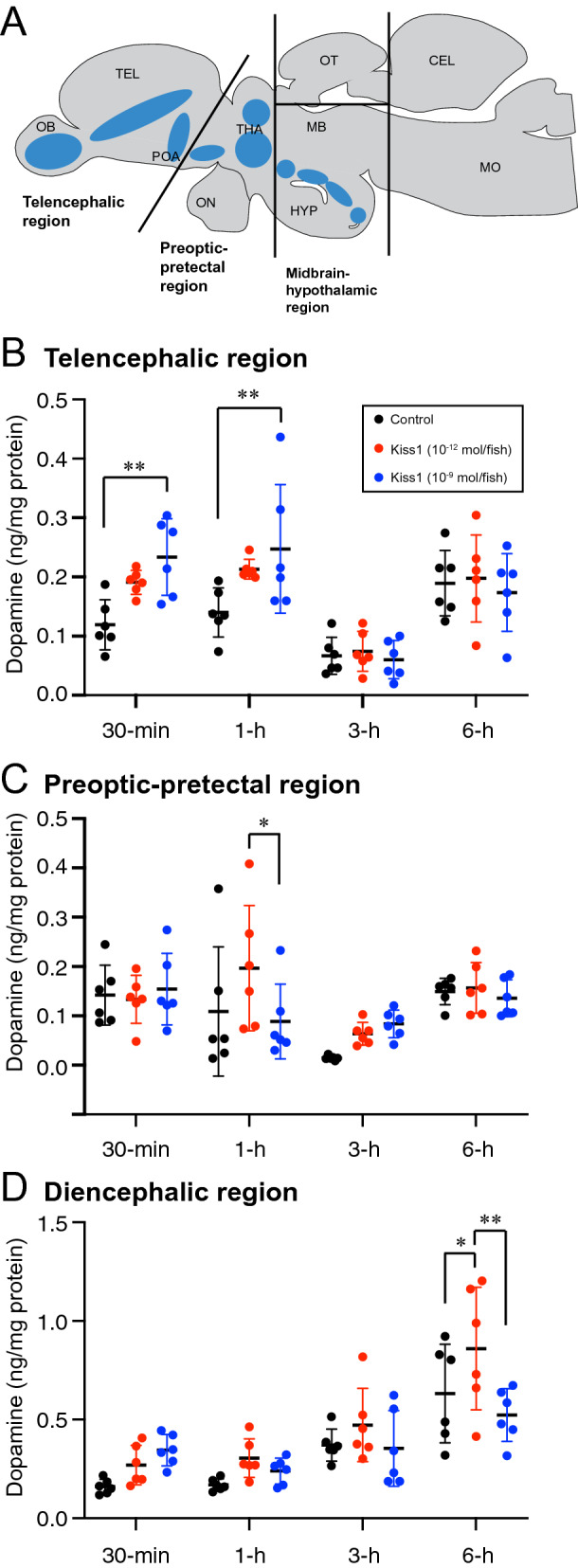
Effect of Kiss1 on dopamine levels in different brain regions. (A) Schematic sagittal view of a zebrafish brain illustrating the macro-dissected brain regions that were subjected to analyses of dopamine content levels and the distribution of dopaminergic cell populations (blue-shaded zones). (B–D) Dopamine content levels (ng per mg protein; n = 6 fish/group) in macro-dissected telencephalon (B), preoptic-pretectal (C) and midbrain-hypothalamic (D) regions, 30-min, 1-h, 3-h and 6-h after central administration of either vehicle (control; distilled water) or Kiss1 with doses of 10–12 or 10–9 mol/ fish. Data presented as mean ± SD. *P < 0.05; **P < 0.01 using 2-way ANOVA followed by Tukey’s multiple comparison test.
Kiss1 administration differentially altered DA levels in time-dependent and brain region-dependent manner (Fig. 3B–D).
Telencephalic region: Administration of Kiss1 at the dose of 10–12 mol/fish significantly increased DA levels 30-min after the administration, which further continued to increase until 1-h post administration (P = 0.0018 and P = 0.0035 for 30-min and 1-h, respectively) (Fig. 3B). There was no difference in DA levels between Kiss1 at the dose of 10–9 mol/fish and control at any time point.
Preoptic-pretectal region: There was no effect of Kiss1 administration on DA levels at 30-min, 3-h and 6-h post administration, except at 1-h post administration, where DA levels were significantly higher in fish treated with Kiss1 at the doses of 10–12 mol/fish as compared to fish treated with 10–9 mol/fish of Kiss1 (Fig. 3C).
Midbrain-hypothalamic region: Unlike the telencephalic or preoptic-pretectal regions, DA levels were significantly increased only 6-h after administration of Kiss1 at the dose of 10–12 mol/fish (Fig. 3D).
Effect of Kiss1 administration on the habenula activity
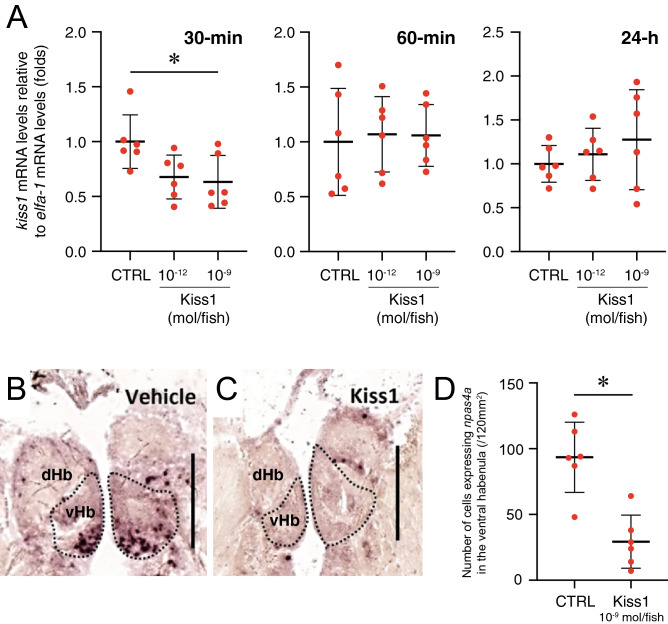
To confirm the effect of Kiss1 administration on the habenula activity, kiss1 gene expression was measured in the brain of fish at 30-min, 1-h and 24-h after Kiss1 administration. In fish treated with Kiss1 with dose of 10–9 mol/ fish, kiss1 mRNA levels in 30-min post administration were significantly decreased as compared to vehicle controls (P = 0.0263, Fig. 4A). There was no effect of Kiss1 on kiss1 mRNA expression in 1-h and 24-h post administration. We also examined effect of Kiss1 on neural activity in the habenula using a neural activity marker gene, npas4a expression. In the fish treated with Kiss1 (10–9 mol/ fish), number of cells expressing npas4a mRNA was significantly lower in the ventral habenula as compared to vehicle treated controls (P = 0.0008, Fig. 4B–D).
Figure 4.
Effect of Kiss1 on the habenula activity. (A) Central administration of Kiss1 (10–9 mol/fish) significantly suppressed expression of kiss1 mRNA levels (against the eef1a1l1 in fold-change ± SD, n = 6 fish/ group) 30-min, but not 1-h and 24-h after administration. (B,C) Expression of npas4a in the vHb 30-min after the administration of vehicle (control, B) or Kiss1 (10–9 mol/fish, C). Scale bars 100 µm. (D) Number of cells expressing npas4a in the habenula were significantly lower as compared to control fish. *P < 0.05 vs. controls using one-way ANOVA followed by Dunnett’s multiple comparison test (kiss1) or unpaired t-test (npas4a).
Neuronal activity in dopaminergic neurons followed by Kiss1 administration
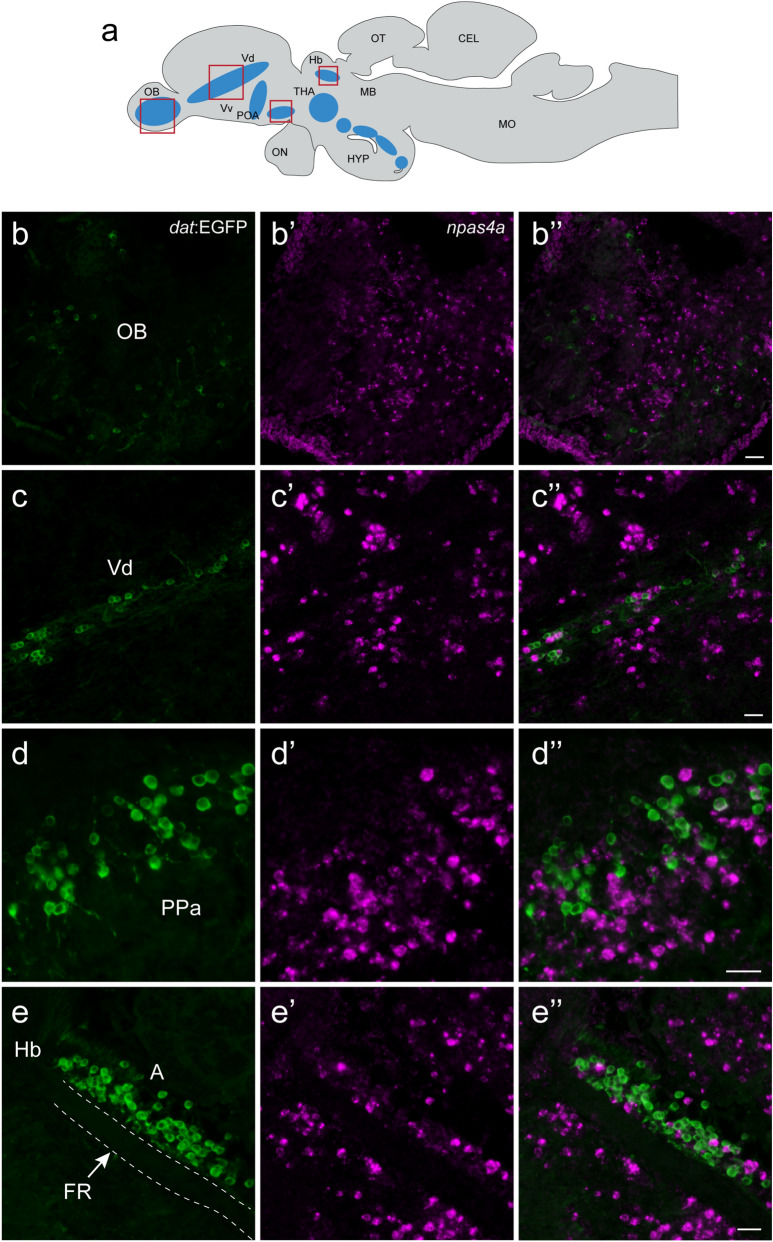
To confirm whether and which dopaminergic neuronal population (Fig. 5a) is activated by Kiss1 administration, we examined the effect of Kiss1 administration on the expression of activity-dependent neural marker, npas4a in dopaminergic neurons using the transgenic Tg(dat:EGFP) zebrafish at 30-min post administration (Fig. 5b–e). Fluorescent in situ hybridization showed expression of npas4a mRNA in several brain regions including the brain regions containing dopaminergic neurons (Fig. 5b'–e'). However, there was no expression of npas4a in EGFP-labelled dopaminergic neurons in any brain regions including the olfactory bulb (Fig. 5b"), ventral telencephalon (Fig. 5c"), preoptic region (Fig. 5d"), and the periventricular pretectal nucleus (Fig. 5e") in both control (data not shown) and Kiss1-treated fish (Fig. 5b"−e"). Similarly, no expression of npas4a gene was observed in other dopaminergic cell populations (data not shown).
Figure 5.
Expression of npas4a gene in the brain of transgenic dat:EGFP fish followed by 30-min post Kiss1 administration. (a) Schematic sagittal view of a zebrafish brain illustrating the distribution of dopaminergic cell population (blue-shaded zones) in the brain of zebrafish. Boxes with red indicate the boundaries of the photomicrographic illustrations in below (b–e). (b–e) EGFP (green)-labelled dopaminergic neurons in the olfactory bulb (OB, b); dorsal nucleus of ventral telencephalic area (Vd, c); anterior part of the parvocellular preoptic nucleus (PPa, d) and in the anterior thalamic nucleus (A, e) above the fasciculus retroflexus (FR). Although cells expressing npas4a mRNA (magenta) were seen in several brain regions (b'–e'), there was no expression of npas4a in dopaminergic cells (b"−e"). Scale bars 20 µm.
Neural tracing from the median raphe
To reveal the potential link between habenular Kiss1 neurons and dopaminergic neurons, a thin-strip of nylon membrane coated with neural tracer was selectively applied to the MR where habenula Kiss1 neuronal terminals are located in transgenic Tg(kiss1:mCherry) zebrafish (Figs. 6a–l and 7A). As negative control, we used a brain without tracer implantation (Fig. 6a–f) and a brain in which tracer was applied to the IPN region (Fig. 7A’). In the brain implanted with a tracer-coated strip in the MR (Fig. 6g–i), tracer-labelled neural tracts were seen in the fasciculus retroflexus towards the habenula (Fig. 6j–l). In addition, observation of series of the brain sections (Fig. 7B) revealed the presence of tracer-labelled nerve fibers in the medullary/brain stem regions, mesencephalic regions including the optic tectum and tegmentum, and in the optic chiasma (Fig. 7c–e). Further, distinct neural tracts were labelled in the diencephalon, which are extended towards the telencephalon. This tract is known as the medial forebrain bundle (MFB, Fig. 7c,f). In the brain implanted with a tracer into the IPN (Fig. 7A’), tracer-labelled nerve fibers were seen in the mesencephalic and rhombencephalic regions including the optic tectum, tegmentum, and cerebellum, and in the medullary regions (Fig. 7c’–f’) including the griseum centrale (GC, Fig. 7e’).
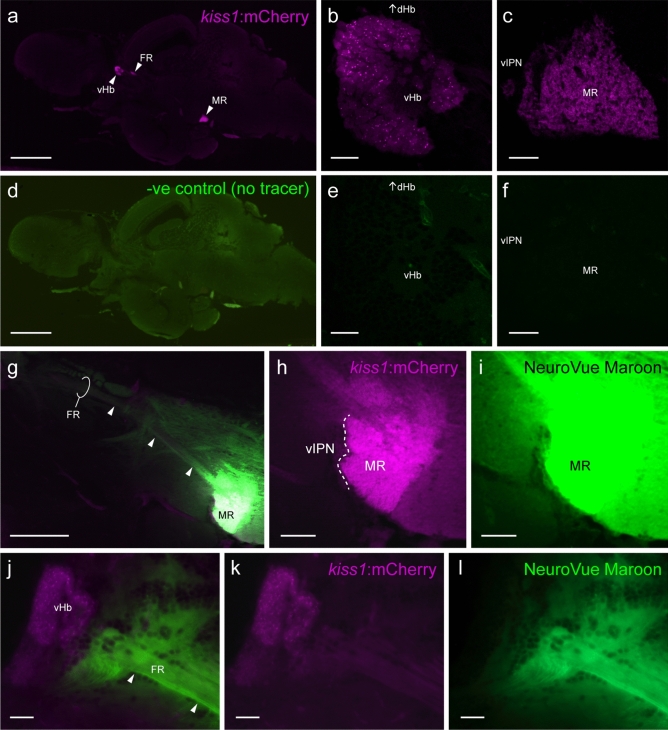
Figure 6.
Neural tracer application into the median raphe. (a–f) Mid-sagittal visualization of mCherry (magenta)-labelled Kiss1 efferent projection from the ventral habenula (vHb, b) through the fasciculus retroflexus (FR) towards the median raphe (MR, c) in the brain of Tg(kiss1:mCherry) zebrafish without neural tracer implantation (d–f). (g–i) A thin-strip of the nylon membrane-coated with NeuroVue tracer (NeuroVue Maroon, green) was selectively inserted into the MR (h,i). (j–l) Photomicrograph of mid-sagittal visualization of tracer-labelled neural tracks (arrow heads) from the MR (g) towards the vHb (j). Scale bars (a,d) 500 μm; (b,c,e,f,j–l) 20 μm; (g) 200 μm; (h,i) 50 μm.
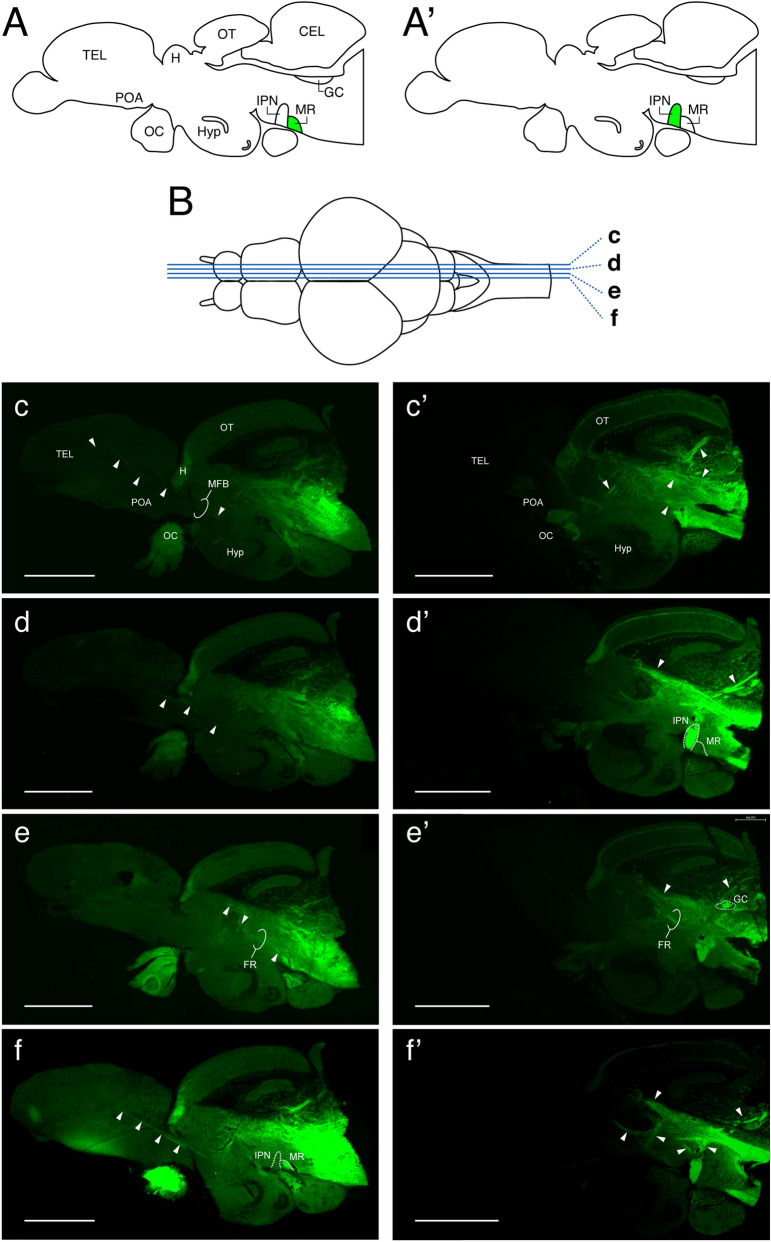
Figure 7.
Tracer-labelled neuronal projection from the median raphe. (A,A’) Schematic sagittal view of the zebrafish brain illustrating the application sites of the neural tracer: the median raphe (MR, A) and interpeduncular nucleus (IPN, A’). (B) A schematic horizontal view of the zebrafish brain illustrating levels at which sagittal sections (c–f and c’–f’) were taken. (c–f) Photomicrograph of mid-sagittal visualization of tracer (NeuroVue Maroon, green)-labelled neural fibers/tracks (arrow heads) from the MR. Tracer-labelled fibers/tracks were seen in the mesencephalon including the fasciculus retroflexus (FR, e), and in the rhombencephalon (e–f). Tracer-labelled tracks were seen from the diencephalon towards the medio-dorsal region of the telencephalon which is the medial forebrain bundle (MFB, e,f). In addition, some fibers were also seen in the cerebellum (e,f). (c’–f’) In the brain where the tracer was applied to the IPN (e’–f’), tracer-labelled fibers/tracks were mainly seen in the dorsal region of the mesencephalon including the FR, and in the rhombencephalon and medulla spinal regions. In the rhombencephalon, fibers were seen in the griseum centrale (GC, e’). In addition, thick fibers were also seen in the cerebellum (c’–f’). Scale bars 500 μm.
Discussion
The role of LHb as a centre for processing negative stimuli and its-related decision-making through the modulation of midbrain dopaminergic neurons is well established in mammals22. Although the habenula structure and the dopamine system in the central nervous system are well conserved across the vertebrate species, the link between them remains to be identified in non-mammalian vertebrates. We have previously shown the expression of Kiss1 and Kiss1R in the vHb-MR3,9, and their role in fear responses11. In the present study, we elaborate further the possible link between habenular Kiss1 and dopaminergic neurons in the zebrafish.
Administration of Kiss1 at the dose of 10–12 mol/fish elevated the expression of dopamine-related genes 24-h post administration, while Kiss1 at the dose of 10–9 mol/fish effected only th1 expression 3-h post administration in the whole brain. However, at the brain regional levels, expression of dopamine-related genes appeared to be differentially altered in different brain regions and different concentrations of Kiss1 solutions, suggesting that not all dopaminergic neurons are sensitive to Kiss1. th1 gene expression was upregulated only in the telencephalon and the hindbrain, and th2 gene expression was downregulated in the telencephalon, optic tectum and the hindbrain despite of the absence of dopaminergic neurons in some of the brain regions. On the other hand, the dopamine levels increased in the telencephalon, preoptic area and the hypothalamus after Kiss1 administration with peaks at different time points, which is inconsistent with gene expression results. At present, it is unclear how Kiss1 modulates dopamine-related genes and how these gene expression levels are associated with dopamine content levels at different times in different brain regions. Nevertheless, differential effects of Kiss1 on dopamine-related genes and dopamine content levels indicate that Kiss1 may have neuromodulatory effect on dopaminergic neurons. As in mammals, tyrosine hydroxylase encoded by th1 gene is responsible for dopamine production23 and dopamine transporter encoded by dat is responsible for reuptake of extracellular dopamine in fish24. Therefore, upregulation of th1 gene expression and dopamine content by Kiss1 administration in the telencephalon-POA could be functionally correlated, while the upregulation of dopamine levels seen in the hypothalamus at 6-h post-administration could be induced by an indirect effect of Kiss1 administration. Further functional characterization of cellular and physiological properties of each dopaminergic neural populations and their association with Kiss1 is necessary to identify the dopaminergic cell population that are selectively sensitive to Kiss1 action. The significant effect of Kiss1 administration on dopamine-related gene expression only appeared at 24-h after administration, while its effect on dopamine content levels was first observed at 30-min post administration. This time gap between gene expression and dopamine content levels suggest that upregulation of dopamine-related genes is due to the secondary effect of Kiss1 on dopamine content levels. We also noticed significant alterations in dopamine levels in all the brain regions examined in the control group. Since all brain specimens were collected at a fixed time (1300 till 1500) of the day, the alteration of dopamine levels is not likely due to the circadian rhythmicity of dopamine release and turnover25,26. However, it is possible that the injection itself or the associated handling stress may have affected the dopamine content levels as the dopamine system is known to be influenced by stress27,28. On the other hand, in the zebrafish, th2 has been shown to modulate the synthesis of serotonin (5-HT) in vivo and has tryptophan hydroxylase activity in vitro29. In fact, neurons immunoreactive for zebrafish TH2 are highly co-localized with 5-HT in the hypothalamus in the zebrafish30. Therefore, downregulation of th2 expression might have affected 5-HT rather than dopaminergic levels in the telencephalon, optic tectum and hindbrain regions. In addition, because tyrosine hydroxylase is also expressed in noradrenergic neurons31, Kiss1 administration may have also stimulated synthesis and release of norepinephrine.
To date, there has been no study that has shown morphological or physiological interaction between habenular Kiss1 and the dopaminergic system in any species. In addition, functional Kiss1Rs are not expressed in brain regions containing dopaminergic neurons in the zebrafish3. Although an association between hypothalamic kisspeptin and dopamine has been demonstrated in some species32–35, in the present study, administration of the Kiss1 paralogous peptide, Kiss2, which is mainly expressed in the hypothalamus in the zebrafish9, had no effect on the expression of dopamine-related genes. Therefore, the effect of Kiss1 on dopamine levels is likely due to the activation of habenular Kiss1-Kiss1R signalling and not Kiss1-Kiss2R signalling. In the brain of zebrafish, kiss1 and kissr1 mRNA are expressed in the vHb neurons, although a small amount of kissr1 mRNA was also detected in microdissected ventral IPN region9,10. Kiss1R protein is also expressed along Kiss1 neuronal projections derived from the vHb towards presynaptic terminals at the MR3,9. Zebrafish kissr1 (also referred to as kiss1rb) gene has been shown to produce 4 additional alternative splice variants encoding truncated forms of Kiss1R, which are expressed outside of the habenula3,36. However, none of the truncated Kiss1R forms have Kiss1 peptides-binding capabilities36. Since there is no expression of functional Kiss1R outside of vHb-MR pathway (postsynaptic zone of Kiss1 terminals or interneurons within the MR), Kiss1 may act on Kiss1 neurons in a closed-loop autocrine fashion10, which corresponds with the reduced kiss1 expression in the fish administered with Kiss1. We also found a significant reduction of npas4a expression in the vHb following exogenous Kiss1 administration10, indicating that administered Kiss1 peptides would have hyperpolarized vHb neurons13 through Kiss1R. Hence, the inhibition of habenula Kiss1 neural activities and Kiss1 levels are likely to be induced by negative feedback of exogenous Kiss1 on habenula Kiss1 neurons. However, the exact location of action sites of Kiss1 within Kiss1 neurons (cell coma, axon or presynaptic terminals) and how Kiss1 neural terminal at the MR further transmit their neural activities downstream remain unknown.
To determine the neural activity of dopaminergic neurons, we utilised Npas4, as it has recently been recognized as a sensitive and rapid neuronal activity marker for both excitatory and inhibitory (e.g. glutamatergic and GABAergic) neurons in mammals and zebrafish21,37–40. However, we failed to detect co-expression of npas4a in dopaminergic neuronal populations following Kiss1 administration. Although Npas4 expression is selectively induced by neuronal activity, it is however, required when activity-dependent synapses are newly formed21,38. Therefore, although Kiss1 administration stimulates dopaminergic synthesis, but it may not have induced any synaptic formation in dopaminergic neurons. In addition, in rats, basal levels (without any stimuli) of Fos-related antigen expression is seen only in 10–20% of hypothalamic dopaminergic neurons33, and similarly in the zebra finches, basal levels of expression of Fos is seen only in 0.1–2% of preoptic/hypothalamic dopaminergic neurons41. Therefore, absence of npas4a in dopaminergic neurons could also be due to low levels of expression of neural activity markers in these neurons.
Although dopamine levels in all three macro-dissected brain regions increased following Kiss1 administration, they showed different pattern of responses (time and amplitude) against Kiss1 administration. The increment of th1 mRNA and dopamine levels in the telencephalic regions at 30-min and 1-h post administration of Kiss1 was the most apparent, while effect of Kiss1 on dopamine levels in the diencephalic region was only seen at 6-h post administration. In mammals, LHb neurons mainly innervate the tail of the ventral tegmental area (VTA, also known as rostromedial tegmental nucleus), which project heavily to midbrain dopamine neurons through the GABAergic pathway42,43. In zebrafish, dopaminergic neurons in the periventricular nucleus of posterior tuberculum in the diencephalic cluster are considered a homologue of mammalian midbrain dopaminergic population44,45. However, there is neither a direct innervation by Kiss1 fibers nor Kiss1R expression in the diencephalic regions3,46. In addition, there was no prominent and rapid effect of Kiss1 on dopamine levels in the diencephalic regions, suggesting that the telencephalic dopaminergic clusters could be the primary action target for habenular Kiss1 neurons. In the present study, our tracer experiment showed putative connections from the MR region to the forebrain as proposed previously8. Tracer-labelled nerve fibres were also seen in the mesencephalic and rhombencephalic regions. However, similar distribution of tracer-labelled fibres, except for those in the telencephalon, were also observed when the tracer was implanted into the IPN region, an adjacent region to the MR. Hence, it can be speculated that the fibres labelled in the mesencephalon and rhombencephalon are primarily derived from the IPN due to leakage of the tracer from the MR, but the fibres/tracts labelled in the diencephalon towards the telencephalon are derived from the MR. The tract linking the diencephalon and the telencephalon is known as the medial or lateral forebrain bundle (MFB/LFB), that starts in the telencephalon and extends into two distinct tracts; lateral to the dorsal preglomerular area and medial to the posterior tuberal nucleus in the fish brain47. Direct connections between the MR and MFB/LFB have not been shown in the brain of teleosts. However, in rats, the MR-forebrain tract lies in the ventromedial aspect of the MFB and projects to medial forebrain areas48. Similarly, in a cartilaginous fish (Platyrhinoidis triseriata), retrograde neural tracer injection into the forebrain bundle showed tracer-labelled cells in the superior raphe49. These histological analyses indicate possible link between the MR and the telencephalon via the forebrain bundle. Habenular Kiss1 neurons send projections and make putative terminals in proximity of GABAergic and serotonergic neurons in the MR of zebrafish3,50. Therefore, it can be postulated that Kiss1 neurons may act on telencephalic dopaminergic clusters via interneurons located in the MR51.
Although the physiological role of Kiss1-Kiss1R signalling in habenula neurons remains unclear, expression of Kiss1R (= GPR54) in the habenula has been identified in several fish species and also in mammals52,53. In mammals and some non-mammalian species, Kiss1 primarily acts as modulator of reproductive functions via regulation of the hypothalamus-pituitary axis54. In mammals, Kiss1 suppresses the activity of the tuberoinfundibular dopaminergic neurons, which has been implicated in prolactin secretion33,34. Recently, the potential involvement of Kiss1-Kiss1R signalling in cognitive functions has been reported. In mice, Kiss1 peptide (kisspeptin-13) enhances memory including passive avoidance memory consolidation and also mitigates memory impairment induced by Amyloid-β55,56, suggesting possible link between Kiss1 and dopaminergic signalling. However, neither direct association of Kiss1 with nor expression of Kiss1R in midbrain dopaminergic neurons have been reported. Recent accumulated evidences have implicated the role of habenula in spatial and aversive memory processing57–59. Further, it is well known that midbrain dopamine system is necessary for associative learning and temporal stability of memories60,61. Thus, it can be postulated that Kiss1-Kiss1R signalling could influence cognitive functions via indirect action through the habenula-VTA pathway in mammals.
In summary, the present study demonstrates stimulatory effect of centrally administered Kiss1 peptides on dopamine levels in the telencephalic regions. In fish administered with Kiss1, neural activity and kiss1 gene expression were significantly decreased, suggesting autocrine and negative feedback regulation of Kiss1 neurons. Since the habenular Kiss1 neurons form terminals in the MR11,46 and our tracer study showed potential link between the MR and the telencephalon via the MFB, we speculate that habenular Kiss1 negatively regulates forebrain dopamine neurons through interneurons in the MR (Fig. 8).
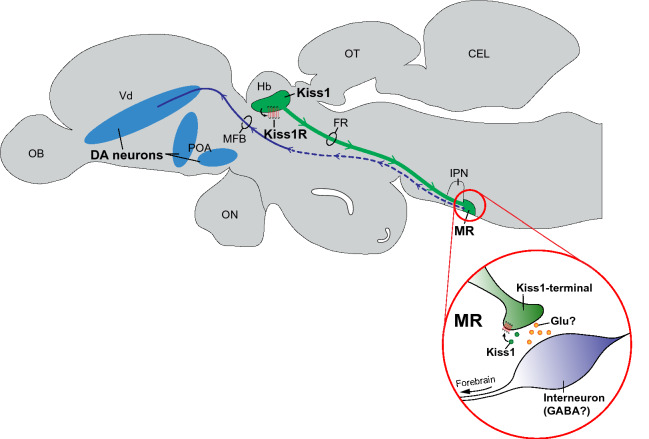
Figure 8.
Schematic drawing illustrating putative connection from the habenula Kiss1 towards telencephalic dopaminergic neurons in the brain of zebrafish. Habenula Kiss1 neurons send projection and terminate at the median raphe (MR) through the fasciculus retroflexus (FR). Habenula Kiss1 neuronal activity is controlled by autocrine feedback of Kiss1 via co-expressing Kiss1R. At the MR, inhibition of Kiss1 neurons may influence on postsynaptic glutamatergic (Glu) transmission on interneurons such as GABAergic neurons (in red circle). Neurons receive Kiss1 inputs within the MR further send projections towards the telencephalon via the medial forebrain bundle (MFB) and possibly act on the telencephalic dopaminergic (DA) cells. OB olfactory bulb, Vd ventral telencephalon, POA preoptic area, ON optic nerve, Hb habenula, OT optic tectum, CEL cerebellum, IPN interpeduncular nucleus.
Materials and methods
Animals
Sexually mature (> 6 months old) male, AB wild-type zebrafish (Danio rerio) were obtained from the zebrafish facility at the Institute of Molecular and Cell Biology, Singapore. Transgenic, Tg(dat:EGFP) zebrafish line, in which the enhanced green fluorescent protein (EGFP) is expressed in dopaminergic neurons under the control of cis-regulatory elements of the dopamine transporter (dat) gene62 was generously provided by Professor Marc Ekker from University Ottawa, Canada. Tg(kiss1:mCherry) zebrafish line, in which the mCherry is expressed in habenular Kiss1 neurons under the control of kiss1 gene promoter46, was generously provided by Professor Wei Hu, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China. Fish were maintained in freshwater aquaria at 26 ± 0.5 °C with controlled natural photo regimen (14/10 h; light/dark) at the BRIMS (Brain Research Institute) fish facility, Monash University Malaysia. Fish were fed Tetramine flaks (Tetra, Germany) twice daily. All fish were acclimatized for two weeks before experiments.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guidelines to promote the wellbeing of animals used for scientific purposes: The assessment and alleviation of pain and distress in research animals (2008) by the National Health and Medical Research Council of Australia (https://www.nhmrc.gov.au/guidelines-publications/ea18). Fish were anesthetised by using either 0.01% benzocaine exposure or rapid-chilling, which have been acceptable forms of anaesthesia and euthanasia in zebrafish63. All experimental protocols were approved by the Animal Ethics Committee of Monash University (Approval nos. MARP/2013/095 and MARP/2017/022).
Intracranial administration of Kiss1 peptides and sample collection
Central administration of Kiss1 peptides was performed as described previously in Kizil and Brand (2011) and Ogawa et al. (2012)10,64. For whole brain gene expression assay, AB fish were anesthetized by immersion in 0.01% benzocaine (Sigma, USA) solution and intracranially administered with 1 μL of either kisspeptin1-15 (Kiss1; pyroglut-NVAYYNLNSFGLRY-NH2; Open Biosystems) or kisspeptin2-10 (Kiss2; FNYNPFGLRF-NH2; BioGenes) with two different doses (10–12 and 10–9 mol/fish in distilled water). For injection, the cranial bone close to the habenula above the left side of the anterior part of the optic tectum was incised using a sterilized barbed-end needle (BD Precision Glide 30G × 0.5″, BD Medical, New Jersey) (Fig. 1A), and through this incision,
Kiss1 solution was administered using a heat-pulled glass capillary micropipette attached with a microinjector (IM-9B, Narishige, Tokyo, Japan) (Fig. 1B). For control, the same volume of distilled water was administered. Fish in sham group received the puncture procedure without any administration.
Real-time PCR for dopamine-related genes and kiss1 gene
Expression of dopamine-related genes: th1, th2 and dat, and kiss1 mRNA levels were measured in whole and macro-dissected brains using real-time PCR. For whole brain, following intracranial injection, fish were euthanized by rapid-cooling in ice cold water 30-min, 1-h, 3-h, 6-h or 24-h after administration (n = 7/group), while for the macro-dissected samples, the brains were dissected 24-h after administration (n = 10/group). For the fish treated with sham and Kiss2, the brains were collected 24-h after administration. All sample collections were conducted consistently between 13:00–14:00 for every experimental protocol. For regional gene expression assay, the whole brain was further macro-dissected into five different brain regions: telencephalic region, preoptic-pretectum region, optic tectum, midbrain-hypothalamic region, rhombencephalic region (Fig. 2A) under a stereoscopic microscope. Whole or macro-dissected brain samples were homogenised in TRIzol reagent (Life Technologies), and total RNA were isolated and dissolved in 10 μL of RNase-free water. The total RNA was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcriptase kit (Applied Biosystems) in a 20 μL reaction mixture according to the manufacturer’s instruction. Real-time PCR were performed using the Applied Biosystems StepOne (PE Applied Biosystems, CA, USA) in a total volume of 10 μl reactions contained 1 × POWER SYBR Green PCR Master Mix (PE Applied Biosystems, CA, USA), 0.2 μM each gene-specific forward and reverse primers (Table 1) and 1 µl cDNA. The reaction was programmed for 50 °C for 2-min, 95 °C for 10-min and 40 cycles of 95 °C for 15-s and 60 °C for 1-min, followed by a dissociation stage. The threshold cycle (Ct) of each gene of interest was obtained and normalised against mRNA expression levels of eukaryotic translation elongation factor 1 alpha 1, like 1 (eef1a1l1). The data were then analysed according to 2-ΔΔCt relative gene expression quantification.
Table 1.
Sequence of primers used for real-time PCR.
| Gene | GenBank accession no | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| th1 | BC163630.1 | GCACCTGTCGGATGTTAGCA | ATTTGACCTCCTTTGTGGTTTTG |
| th2 | NM_001001829.1 | AAGAGCAAGAGAAGCAGTGGAGA | TGAAGATGTCGGTGTCGGAG |
| dat (slc6a3) | NM_131755.1 | CATTTACCCAGAAGCCATTGC | GCGTGTCGATTCCTAAAGTCA |
| kiss1 | AB245404 | ACAAGCTCCATACCTGCAAGTG | AATACTGAAAATGCCCAGAGGG |
| eef1a1l1 | NM_131263.1 | ACCCTCCTCTTGGTCGCTTT | CCGATTTTCTTCTCAACGCTCTT |
Dopamine quantification
Dopamine levels in macro-dissected brain samples were determined using liquid chromatogram (LC)-double mass spectrometry (MS/MS) (LC–MS/MS) following the procedures previously developed by Kim et al. (2016)65 with some modifications. AB fish were first administered with Kiss1 at the doses of 10–12 and 10–9 mol/fish or saline (n = 6 /group), and the brains were collected at 30-min, 1-h, 3-h, and 6-h after Kiss1 administration. The dissected brain was further macro-dissected into three different brain regions (Fig. 3A) based on the classification of dopaminergic cell clusters demonstrated by Paula et al. (2010)44 under a stereoscopic microscope. Telencephalic complex consists of the olfactory bulb, subpallium, and anterior preoptic area; Preoptic-pretectal complex consists of those in the preoptic area, pretectum, and ventral thalamus; Diencephalic complex consisted of the hypothalamic area, caudal hypothalamic region and the midbrain. Details of chemicals used, sample preparation procedures and condition of LC–MS/MS are described below:
Chemicals
All solvents were of high-performance LC grade. Acetonitrile, formic acid and dopamine were obtained from Sigma Aldrich (St. Louis, MO, USA). Working solutions were prepared in ultrapure water provided by a Milli-Q system (Millipore, Bedford, MA, USA). Each analytical stock solution (1 mg/ mL) was prepared in 0.111 M ascorbic acid in a 1:1 mixture solution to prevent oxidation and stored at 80 °C. A working internal standard solution (buffer A), 20 pg/μL of isoproterenol (Nacalai, Japan) was prepared in buffer B (50% acetonitrile,0.1% formic acid, and 0.111 M ascorbic acid) immediately before analysis. Stock of 10 mg/mL dopamine standard were diluted in buffer A, followed by calibration curve in which dopamine stock was diluted in buffer A according to corresponding concentration (0.025, 0.25, 1.25, 1.5, 2.0, 2.5, 4.0 pg/μL).
Sample preparation
The macro-dissected brain was homogenized in 80 µL of buffer B supplemented with 20 pg/µL of the internal standard solution using micropestle for 60s. For the investigation of selectivity, matrix effects and sensitivity, authentic homogenized filtered samples were used. The sample preparation was processed briefly in an ice bath to prevent the possible degradation of the analytes. Five microliter of the samples were injected into the LC–MS/MS system. Quality control was done by protein quantification against bovine serum albumin using BioRad Protein Assay dye (Cat#: 5000006, BioRad) according to the manufacturer’s instructions. Dopamine levels in the brain tissue homogenate were normalized by total protein content levels. The assay was read on Labsystems multiskan 352 plate reader (Thermo Fisher Scientific) at a wavelength of 595 nm.
LC analysis
LC–MS analysis was conducted using a 1240 infinity LC system and 6460 triple quadrupole MS/MS (Agilent Technologies, Santa Clara, CA, USA) coupled with a 1240 infinity extra binary pump and degasser (Agilent Technologies) used for operating an additional column. Data were processed using the MassHunter software (B. 04.00, Agilent Technologies). The LC–MS analysis setting was determined based on Kim et al. (2016)65. The mobile phase consisted of 0.1% formic acid in 5 mM ammonium formate and 0.1% formic acid in acetonitrile for the entire analysis and the flow rate for both the loading pump and the analytical pump was 300 mL/min. The temperature of the auto- sampler was set to 4 °C. The sample was loaded onto separation column SB C18 (2.1 mm—250 mm, 3 mm Agilent) on valve position 1. Gradient conditions 0–1.0 min, 5% B; 1.0–1.8 min, 5–90% B; 3.5 min, 90. Column was maintained at 45 °C.
MS/MS condition
The MS/MS system was operated using polarity-switching electrospray ionization (ESI). The optimal conditions for the analysis were determined based on Kim et al. (2016)65 as follows: capillary voltage, 4.5 kV; nebulization pressure, 35 psi; temperature of drying gas, 350 °C; drying gas flow, 10 L/min; sheath gas temperature, 250 °C; sheath gas flow, 5 L/min; and nozzle voltage, 0.5 kV. The time segment with the start time of 2.5 min was applied to prevent flowing interference into the mass spectrometer. The electron multiplier voltage for the run time of 2.5–4.4 min was set to 40 V in the negative ESI. Multiple reaction monitoring transitions were chosen for dopamine and internal standards.
In situ hybridization of npas4a
Effect of Kiss1 treatment on neural activities in the habenula and dopaminergic neurons were examined by expression of neural activity marker, npas4a expression, which were examined in the brain of AB-wild type or Tg(dat:EGFP) fish, respectively. The whole brain (n = 6 fish/group) was dissected 30-min after the Kiss1 administration and fixed in buffered 4% paraformaldehyde for overnight at 4 °C and then expression of npas4a were examined by in situ hybridization. For npas4a mRNA expression in the habenula, coronal sections (10 µm) (n = 6 for control and Kiss-treated) of wild-type AB male were hybridized with DIG-labelled npas4a riboprobes (737nt, GenBank accession number: NM_001045321) for 16 h at 55 °C. DIG-labelled napas4a mRNA was detected with either anti-DIG-AP or anti-DIG-POD antibody (Roche) followed by chromogenic development with NBT/BCIP or amplification using TSA Plus Cyanine 3 System (Perkin Elmer/AKOYA Biosciences), respectively. After TSA amplification of npas4a mRNA signals, EGFP signals were further enhanced with a rabbit anti-GFP antibody (1:500 dilution, Millipore Cat# AB3080, RRID:AB_91337) followed by incubation with Alexa Fluor 488-labeled anti-rabbit IgG (1:500 dilution, Thermo Fisher Scientific).
Neural tracer application into the median raphe
To examine the possible target of the Kiss1-terminus region in the median raphe (MR), a lipophilic tracer, NeuroVue Jade dye (green emitting dye; excitation maximum, 478 nm; emission maximum, 508 nm)-coated filter (Polysciences, Inc., Warrington, PA) was applied to the MR region in the brain of Tg(kiss1:mCherry) fish (n = 3). A nylon filter coated with dye was cut in a small pieces in triangular thin-strips (approx. 0.1 mm width × 1 m length) using an agitate scissors under a stereoscopic microscope, which allows to apply the tracer into the target brain region more precisely with less leakage of tracer from the target area66. As negative controls for tracer applications, the brain without tracer implantation (n = 1) and the brain with tracer application into the IPN region (n = 3) were prepared.
The brain of Tg(kiss1:mCherry) fish was dissected and fixed in buffered 4% paraformaldehyde for overnight at 4 °C. The whole brain was cut into half longitudinally using a surgical knife and a dye-coated thin-strip was inserted to the mCherry-labelled MR region via sharp forceps under a florescence stereoscopic microscope (Nikon, SMZ1500). After dye application, the brain specimen were placed in buffered 4% paraformaldehyde and incubated at 37 °C for 3-days. For observation, the brain specimen was cryoprotected in 20% sucrose and embedded in Tissue Tek OCT compound (Sakura Finetechnical, Tokyo, Japan). The sagittal brain sections (15 μm thickness) were cut using a cryostat and were thaw-mounted onto 3-aminopropylsilane-coated glass slides.
Microscopy
Acquisition of the microscopy images were carried out as described previously67. Images for DIG-labelled npas4a-expressing cells were captured with a Zeiss MIRAX slide scanning system (Carl Zeiss, Germany) using the Mirax Viewer Image Software (3DTech, Budapest, Hungary). Fluorescent images were captured separately with a fluorescence microscope (ECLIPS 90i; Nikon, Tokyo, Japan) that was attached to a digital cooled CCD camera (DMX-1200C, Nikon) with appropriate excitation filters for Cyanine 3, Alexa Fluor 488, NeuroVue Jade and mCherry, and computer software (NIS Elements D3.2; Nikon) was used to superimpose the two images. The red channel was then converted to magenta, and brightness and contrast adjustments were made in Adobe Photoshop CC (Adobe, San Jose, CA, USA).
Data and statistical analysis
All data are presented as means ± SEM, and statistical analyses were performed using independent t-test to observe any statistical significance between controls and experimental groups. Statistical differences among experimental groups were analyzed using one-way or two-way ANOVA, followed by a Tukey’s multiple comparison or a Dunnett’s multiple comparison post-hoc test (Prism 8, GraphPad Software, CA). Differences were considered statistically significant at P < 0.05. Sample size were calculated using G*Power 3.1 (https://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/)68,69. Animals were randomly drawn from the tank immediately before testing, and the order with which phenotypes were tested was randomized via generation of random numbers using the randomization tool in https://www.randomization.com/. No exclusion criteria were pre-determined.
Acknowledgements
We thank Monash University Malaysia for the Graduate Merit Research Scholarship to N.M.A.S. This work is supported by the Malaysian Ministry of Higher Education, FRGS/1/2014/ST03/MUSM/02/1 (to S.O.) and FRGS/1/2016/STG03/MUSM/01/1 (to I.S.P.). This study was conducted with a technical support provided by the Bioimaging Infrastructure Platform and the Drug Discovery Platform, Monash University Malaysia.
Author contributions
S.O. and I.S.P., conceptualization; N.M.A.S., investigation; N.M.A.S. and S.O., formal analysis, and N.M.A.S., writing—original draft preparation; S.O. and I.S.P. writing—review and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aizawa H. Habenula and the asymmetric development of the vertebrate brain. Anat. Sci. Int. 2013;88:1–9. doi: 10.1007/s12565-012-0158-6. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa H, Amo R, Okamoto H. Phylogeny and ontogeny of the habenular structure. Front. Neurosci. 2011;5:1. doi: 10.3389/fnins.2011.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan FM, Ogawa S, Parhar IS. Neuronal connectivity between habenular glutamate-kisspeptin1 co-expressing neurons and the raphe 5-HT system. J. Neurochem. 2015;135:814–829. doi: 10.1111/jnc.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amo R, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J. Neurosci. 2010;30:1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agetsuma M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, et al. The habenula prevents helpless behavior in larval zebrafish. Curr. Biol. 2010;20:2211–2216. doi: 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Chou M-Y, et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science. 2016;352:87–90. doi: 10.1126/science.aac9508. [DOI] [PubMed] [Google Scholar]
- 8.Amo R, et al. The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron. 2014;84:1034–1048. doi: 10.1016/j.neuron.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Kitahashi T, Ogawa S, Parhar IS. Cloning and expression of kiss2 in the zebrafish and medaka. Endocrinology. 2009;150:821–831. doi: 10.1210/en.2008-0940. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa S, Ng KW, Ramadasan PN, Nathan FM, Parhar IS. Habenular Kiss1 neurons modulate the serotonergic system in the brain of zebrafish. Endocrinology. 2012;153:2398–2407. doi: 10.1210/en.2012-1062. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Nathan FM, Parhar IS. Habenular kisspeptin modulates fear in the zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3841–3846. doi: 10.1073/pnas.1314184111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan FM, Ogawa S, Parhar IS. Kisspeptin1 modulates odorant-evoked fear response via two serotonin receptor subtypes (5-HT1A and 5-HT2) in zebrafish. J. Neurochem. 2015;133:870–878. doi: 10.1111/jnc.13105. [DOI] [PubMed] [Google Scholar]
- 13.Lupton, C. et al. Loss of the habenula intrinsic neuromodulator kisspeptin1 affects learning in larval zebrafish. eNeuro4, ENEURO.0326–0316.2017. 10.1523/ENEURO.0326-16.2017 (2017). [DOI] [PMC free article] [PubMed]
- 14.Wang D, et al. Learning shapes the aversion and reward responses of lateral habenula neurons. Elife. 2017;6:e23045. doi: 10.7554/eLife.23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamatakis AM, et al. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J. Neurosci. 2016;36:302–311. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J. Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 18.Naderi M, Jamwal A, Chivers DP, Niyogi S. Modulatory effects of dopamine receptors on associative learning performance in zebrafish (Danio rerio) Behav. Brain Res. 2016;303:109–119. doi: 10.1016/j.bbr.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Chandroo KP, Duncan IJH, Moccia RD. Can fish suffer?: perspectives on sentience, pain, fear and stress. Appl. Anim. Behav. Sci. 2004;86:225–250. doi: 10.1016/j.applanim.2004.02.004. [DOI] [Google Scholar]
- 20.Mok E-M, Munro A. Effects of dopaminergic drugs on locomotor activity in teleost fish of the genus Oreochromis (Cichlidae): involvement of the telencephalon. Physiol. Behav. 1998;64:227–234. doi: 10.1016/S0031-9384(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 23.Formella I, et al. Transient knockdown of tyrosine hydroxylase during development has persistent effects on behaviour in adult zebrafish (Danio rerio) PLoS ONE. 2012;7:e42482. doi: 10.1371/journal.pone.0042482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kacprzak V, et al. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol. Biochem. Behav. 2017;157:1–8. doi: 10.1016/j.pbb.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaldy H, et al. Circadian rhythms of dopamine and dihydroxyphenyl acetic acid in the mouse striatum: effects of pinealectomy and of melatonin treatment. Neuroendocrinology. 2002;75:201–208. doi: 10.1159/000048238. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, et al. Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention deficiency and hyperactive behavior. J. Neurosci. 2015;35:2572–2587. doi: 10.1523/jneurosci.2551-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neckameyer WS, Weinstein JS. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress. 2005;8:117–131. doi: 10.1080/10253890500147381. [DOI] [PubMed] [Google Scholar]
- 28.Feenstra MG. Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Prog. Brain Res. 2000;126:133–163. doi: 10.1016/S0079-6123(00)26012-3. [DOI] [PubMed] [Google Scholar]
- 29.Ren G, Li S, Zhong H, Lin S. Zebrafish tyrosine hydroxylase 2 gene encodes tryptophan hydroxylase. J. Biol. Chem. 2013;288:22451–22459. doi: 10.1074/jbc.M113.485227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenova SA, Chen YC, Zhao X, Rauvala H, Panula P. The tyrosine hydroxylase 2 (TH2) system in zebrafish brain and stress activation of hypothalamic cells. Histochem. Cell Biol. 2014;142:619–633. doi: 10.1007/s00418-014-1240-z. [DOI] [PubMed] [Google Scholar]
- 31.Pickel VM, Joh TH, Field PM, Becker CG, Reis DJ. Cellular localization of tyrosine hydroxylase by immunohistochemistry. J. Histochem. Cytochem. 1975;23:1–12. doi: 10.1177/23.1.234988. [DOI] [PubMed] [Google Scholar]
- 32.Escobar S, et al. Expression of kisspeptins in the brain and pituitary of the european sea bass (Dicentrarchus labrax) J. Comp. Neurol. 2013;521:933–948. doi: 10.1002/cne.23211. [DOI] [PubMed] [Google Scholar]
- 33.Szawka RE, et al. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology. 2010;151:3247–3257. doi: 10.1210/en.2009-1414. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro AB, et al. Kisspeptin regulates tuberoinfundibular dopaminergic neurones and prolactin secretion in an oestradiol-dependent manner in male and female rats. J. Neuroendocrinol. 2015;27:88–99. doi: 10.1111/jne.12242. [DOI] [PubMed] [Google Scholar]
- 35.Sawai N, Iijima N, Takumi K, Matsumoto K, Ozawa H. Immunofluorescent histochemical and ultrastructural studies on the innervation of kisspeptin/neurokinin B neurons to tuberoinfundibular dopaminergic neurons in the arcuate nucleus of rats. Neurosci. Res. 2012;74:10–16. doi: 10.1016/j.neures.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Onuma TA, Duan C. Duplicated Kiss1 receptor genes in zebrafish: distinct gene expression patterns, different ligand selectivity, and a novel nuclear isoform with transactivating activity. FASEB J. 2012;26:2941–2950. doi: 10.1096/fj.11-201095. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel I, et al. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell. 2014;157:1216–1229. doi: 10.1016/j.cell.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Lin Y. Npas4: linking neuronal activity to memory. Trends Neurosci. 2016;39:264–275. doi: 10.1016/j.tins.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klarić T, Lardelli M, Key B, Koblar S, Lewis M. Activity-dependent expression of neuronal PAS domain-containing protein 4 (npas4a) in the developing zebrafish brain. Front. Neuroanat. 2014;8:1. doi: 10.3389/fnana.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teles, M. C., Cardoso, S. D. & Oliveira, R. F. Social plasticity relies on different neuroplasticity mechanisms across the brain social decision-making network in zebrafish. Front. Behav. Neurosci.. 10.3389/fnbeh.2016.00016 (2016). [DOI] [PMC free article] [PubMed]
- 41.Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman A, et al. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology. 2011;60:381–387. doi: 10.1016/j.neuropharm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur. J. Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panula P, et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Parker MO, Brock AJ, Walton RT, Brennan CH. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front. Neural Circuits. 2013;7:1. doi: 10.3389/fncir.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y, et al. The distribution of kisspeptin (Kiss)1- and Kiss2-positive neurones and their connections with gonadotrophin-releasing hormone-3 neurones in the zebrafish brain. J. Neuroendocrinol. 2015;27:198–211. doi: 10.1111/jne.12251. [DOI] [PubMed] [Google Scholar]
- 47.Wullimann, M. F., Rupp, B. & Reichert, H. Neuroanatomy of the zebrafish brain: a topologocal atlas. (Birkhauser, 1996).
- 48.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 49.Fiebig E, Bleckmann H. Cell groups afferent to the telencephalon in a cartilaginous fish (Platyrhinoidis triseriata) A WGA-HRP study. Neurosci. Lett. 1989;105:57–62. doi: 10.1016/0304-3940(89)90011-6. [DOI] [PubMed] [Google Scholar]
- 50.Lillesaar C, Stigloher C, Tannhauser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. J. Comp. Neurol. 2009;512:158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- 51.Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogawa S, Parhar I. Biological significance of kisspeptin-KissR1 signaling in the habenula of teleost species. Front. Endocrinol. 2018;9:222. doi: 10.3389/fendo.2018.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee DK, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/S0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 54.Tena-Sempere M. Kisspeptin signaling in the brain: recent developments and future challenges. Mol. Cell Endocrinol. 2010;314:164–169. doi: 10.1016/j.mce.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Jiang JH, et al. Kisspeptin-13 enhances memory and mitigates memory impairment induced by Aβ1–42 in mice novel object and object location recognition tasks. Neurobiol. Learn. Mem. 2015;123:187–195. doi: 10.1016/j.nlm.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Telegdy G, Adamik Á. The action of kisspeptin-13 on passive avoidance learning in mice: involvement of transmitters. Behav. Brain Res. 2013;243:300–305. doi: 10.1016/j.bbr.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Tomaiuolo M, Gonzalez C, Medina JH, Piriz J. Lateral habenula determines long-term storage of aversive memories. Front. Behav. Neurosci. 2014;8:170. doi: 10.3389/fnbeh.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goutagny R, et al. Interactions between the lateral habenula and the hippocampus: implication for spatial memory processes. Neuropsychopharmacology. 2013;38:2418–2426. doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathis V, Cosquer B, Avallone M, Cassel J-C, Lecourtier L. Excitatory transmission to the lateral habenula is critical for encoding and retrieval of spatial memory. Neuropsychopharmacology. 2015;40:2843–2851. doi: 10.1038/npp.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- 61.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Xi Y, et al. Transgenic zebrafish expressing green fluorescent protein in dopaminergic neurons of the ventral diencephalon. Dev. Dyn. 2011;240:2539–2547. doi: 10.1002/dvdy.22742. [DOI] [PubMed] [Google Scholar]
- 63.Thurman CE, Rasmussen S, Prestia KA. Effect of 3 euthanasia methods on serum yield and serum cortisol concentration in zebrafish (Danio rerio) J. Am. Assoc. Lab. Anim. Sci. 2019;58:823–828. doi: 10.30802/AALAS-JAALAS-18-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kizil C, Brand M. Cerebroventricular microinjection (CVMI) into adult zebrafish brain is an efficient misexpression method for forebrain ventricular cells. PLoS ONE. 2011;6:e27395. doi: 10.1371/journal.pone.0027395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim M, Lee J-G, Yang CH, Lee S. Silica stationary phase-based on-line sample enrichment coupled with LC-MS/MS for the quantification of dopamine, serotonin and their metabolites in rat brain microdialysates. Anal. Chim. Acta. 2016;923:55–65. doi: 10.1016/j.aca.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Heilingoetter CL, Jensen MB. Histological methods for ex vivo axon tracing: a systematic review. Neurol. Res. 2016;38:561–569. doi: 10.1080/01616412.2016.1153820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogawa S, et al. Distribution of LPXRFa, a gonadotropin-inhibitory hormone ortholog peptide, and LPXRFa receptor in the brain and pituitary of the tilapia. J. Comp. Neurol. 2016 doi: 10.1002/cne.23990. [DOI] [PubMed] [Google Scholar]
- 68.Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 69.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G* Power 31: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 70.Lim FT, Ogawa S, Parhar IS. Spred-2 expression is associated with neural repair of injured adult zebrafish brain. J. Chem. Neuroanat. 2016;77:176–186. doi: 10.1016/j.jchemneu.2016.07.005. [DOI] [PubMed] [Google Scholar]