Abstract
Introduction
Tropical diseases are public health problems affecting hundreds of millions of people globally. However, the development of adequate, affordable, and accessible treatments is mostly neglected, resulting in significant morbidity and mortality that could otherwise be averted. Leishmaniasis is one of the neglected tropical diseases caused by the obligate intracellular protozoan Leishmania parasite and transmitted by the bite of infected phlebotomine sandflies. No systematic review and meta-analysis has been done to identify the prevalence and risk factors of leishmaniasis to the authors’ knowledge. Therefore, the objective was to determine the prevalence and risk factors of human leishmaniasis in Ethiopia.
Methods
Eleven studies conducted in all regions of Ethiopia, which were fully accessible, written in any language, and original articles done on prevalence and risk factors of leishmaniasis, were included. STATA™ version 11.1 was used for statistical analysis. Chi-square, I2, and p values were assessed to check heterogeneity. A random effects model with heterogeneity taken from an inverse-variance model was employed to estimate the pooled effect. Subgroup meta-analysis was computed to reduce random variations among each article’s point prevalence, and Egger and funnel plots were used to check for publication bias.
Results
The highest proportion of human leishmaniasis was reported from a study done in Amhara region (39.1%), and the lowest was reported from a survey done in Tigray (2.3%). The overall pooled prevalence of leishmaniasis was 9.13% (95% CI 5–13.27). Subgroup analysis by region revealed moderate heterogeneity (I2 = 51.8%) in studies conducted in the Southern Nations Nationalities and Peoples Region (SNNPR). The presence of hyraxes and being male were associated with an increased risk of human leishmaniasis.
Conclusion
The prevalence of leishmaniasis in Ethiopia remains high (9.13%), with significant risk factors being male and the presence of hyraxes within a 300-m radius of the sleeping area.
Keywords: Ethiopia, Infectious disease, Leishmaniasis, Neglected tropical diseases, Prevalence
Key Summary Points
| Globally, neglected tropical diseases affect hundreds of millions and place even more at risk, and nearly 40 million people are suffering from stigma secondary to one such disease, cutaneous leishmaniasis. |
| World Health Organization/Tropical Disease Research program focuses on the development of new diagnostics, vaccines, and drugs to combat these complex conditions. However, tropical diseases are globally dispersed, making it difficult for the treatment to be available and affordable for those most in need, who are often socioeconomically and politically marginalized (like Ethiopia). |
| Leishmaniasis impacts public health, socioeconomics, and the nation’s economy and growth. There is lack of systematic review and meta-analysis on prevalence and risk factors of human leishmaniasis at country level. This direction further indicated a need to assess the prevalence and risk factors of human leishmaniasis. |
| Such a gap in knowledge further complicates the full understanding and evidentiary base needed to combat this disease. Therefore, this study determined the pooled prevalence and risk factors of human leishmaniasis in Ethiopia. |
| The prevalence of leishmaniasis remains high in Ethiopia (9.13%), and gender (male) and the presence of hyraxes within a 300-m radius of the sleeping area were significant risk factors. Vaccine development, methods of diagnosis, and treatment that can be affordable for developing countries should be given priority. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13055780
Introduction
Globally, an estimated of 58,000 new cases of visceral leishmaniasis and 220,000 new cases of cutaneous leishmaniasis occur every year [1]. Nearly 40 million people are suffering from stigma due to inactive cutaneous leishmaniasis scars [2]. Neglected tropical diseases are a significant public health problem in Ethiopia, which is experiencing a considerable burden compared to other sub-Saharan African countries [3]. Leishmaniasis affects both humans and animals [4, 5]. The prevalence of animal leishmaniasis in Ethiopia was 11% (95% CI 5–16%) [6]. Neglected tropical diseases resulted in 3593 deaths in 2015 in Ethiopia [7]. Within Ethiopia, there are many neglected tropical diseases that result in the loss of quality of life and loss of lives. One such disease, leishmaniasis, a protozoan disease, is caused by over 20 different Leishmania spp., which are transmitted by female phlebotomine sandflies to humans [8]. The presentation of leishmaniasis can be subclinical, localized, or disseminated with its symptomatic stage manifesting as either acute or chronic [9]. There are three major types of the disease: visceral, cutaneous, and mucocutaneous [10], with consequences ranging from skin lesions to death [11]. East Africa is the second-largest visceral leishmaniasis focus after the Indian subcontinent. East Africa contributes 30,000–40,000 new cases per year to the global burden, with the largest proportion of new leishmaniasis cases occurring in Ethiopia, South Sudan, and Sudan [12, 13]. Visceral leishmaniasis is characterized by anemia, prolonged fever, weight loss, and hepatosplenomegaly. If left untreated, it is fatal in 95% of cases [14, 15].
Globally, tropical diseases affect hundreds of millions and place even more at risk [8, 16]. The World Health Organization/Tropical Disease Research (WHO/TDR) program focuses on the development of new diagnostics, vaccines, and drugs to combat these complex conditions. However, tropical diseases are globally dispersed, making it difficult for the treatment to be available and affordable for those most in need, who are often socioeconomically and politically marginalized. Furthermore, drug development is mostly neglected because of the relatively small populations affected, coupled with users' inability to pay for such drugs [16, 17].
Leishmaniasis impacts public health, socioeconomics, and the nation’s economy and growth [18]. Leishmaniasis causes high mortality, morbidity, affects the quality of life, causes mental illness, stigma, and psychosocial morbidity among those affected [17, 19]. Assefa did a systematic review and meta-analysis on leishmaniasis [6]. But it was focused on the prevalence of leishmaniasis among humans and animals. This direction further indicated a need to assess the prevalence and risk factors of human leishmaniasis as there is a lack of systematic review and meta-analysis on prevalence and risk factors of human leishmaniasis at the country level [1]. Such a gap in knowledge further complicates the full understanding and evidentiary base needed to combat this disease. Therefore, this study determined the pooled prevalence and risk factors of human leishmaniasis in Ethiopia.
Methods
Study Area and Design
This systematic review and meta-analysis considered original studies, including cross-sectional and retrospective cohort studies, conducted in all Ethiopian regions on prevalence and factors associated with leishmaniasis. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Eligibility Criteria and Search Strategy
Inclusion Criteria
Fully accessible (either through online, library, or author access at no cost) published articles concerned with leishmaniasis prevalence and risk factors, peer-reviewed, published in any language, and including Ethiopian populations.
Exclusion Criteria
We excluded systematic reviews, meta-analysis, commentaries, case–control (denominators are already predetermined), case series, case reports, studies done in animals, and inaccessible articles (unable to contact the primary author after three attempts to retrieve the full text of the papers). Following the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) guidelines [20], a comprehensive computer search in PubMed, Google Scholar, Cochrane Library, Science Direct, African Journals Online, and JURN on prevalence and risk factors of leishmaniasis was performed. The search was done with geographical location restricted to Ethiopia. Related articles and references were identified from some included articles and were also searched. Boolean operators (“OR” or “AND”) were used in combination or separately to search the terms. Peer-reviewed articles published from November 1993 until December 2019, which were fully accessible, were included. Medical Subject Headings (MeSH) terms (((magnitude) OR leishmaniasis) OR associated factors) OR Ethiopia, (((prevalence) OR leishmaniasis) OR risk factors) OR Ethiopia) were initially used. The last search was done using (((prevalence) AND leishmaniasis) AND risk factors) AND Ethiopia.
Study Selection and Quality Assessment
Three individuals (MH, KG, and JN) searched the electronic databases using similar MeSH terms to identify possible eligible articles on the basis of the title and abstracts imported to Endnote version 7 (Thomson Reuters, NY, USA). Duplicate reports were removed. Title and abstract reviews were performed. Articles with adequate data concerning the inclusion criteria were saved for full-text assessment. The Newcastle–Ottawa Scale [21] adapted for cross-sectional study quality assessment was used to assess the quality of each article. Each article was scored by three reviewers independently. In the event of disagreements, the reviewers reached a consensus or voted to determine the disposition of the article. Articles with a quality assessment score of greater than 6/10 were considered high quality and were included in this review.
Data Extraction
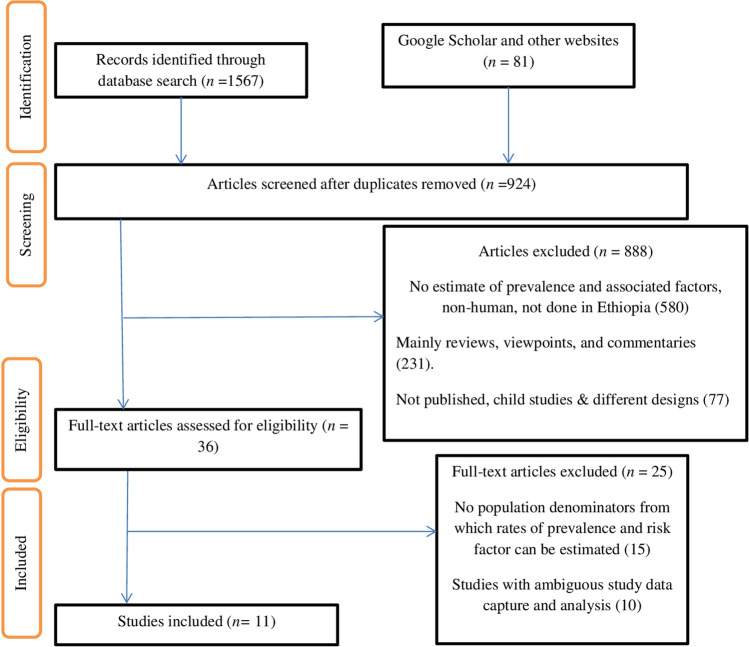
Three individual reviewers (MH, KG, and JN) independently conducted the data extraction using a standardized data extraction checklist. The checklist included the name of the first author, prevalence, standard error, type of test, year of publication, a region of the study, study setting, study population, gender of participants, age, study design, sample size, type of leishmaniasis, and other variables. If authors disagreed on whether to include or exclude an article, a majority decision was made (Fig. 1).
Fig. 1.
PRISMA flowchart showing the number of articles identified in the systematic review and meta-analysis on the prevalence of human leishmaniasis and associated factors
Data Analysis
STATA™ version 11.1 statistical software was used for analysis. The standard error for each original study was calculated using the binomial distribution formula. Chi-square, I2, and p values were assessed to check heterogeneity. Though the pooled I2 shows no heterogeneity, we queried differences in assumptions amongst the individual studies. Therefore, a random effects model with heterogeneity taken from an inverse-variance model (DerSimonian and Laird, 1986) was employed to estimate the pooled effect. Subgroup meta-analysis was computed on the basis of the type of study design, the region where the research was conducted, and the type of leishmaniasis to reduce random variations among the point prevalence of each article. Egger and funnel plot tests were done to check for publication bias. First author, year of publication, point prevalence, and 95% confidence interval were presented using forest plots. Each identified potential determinant factor was checked regarding the effect on the prevalence of human leishmaniasis. The size of each box on the forest plot indicates the weight of each article, and the cross lines refer to a 95% confidence interval.
Results
Descriptive and Meta-Analysis
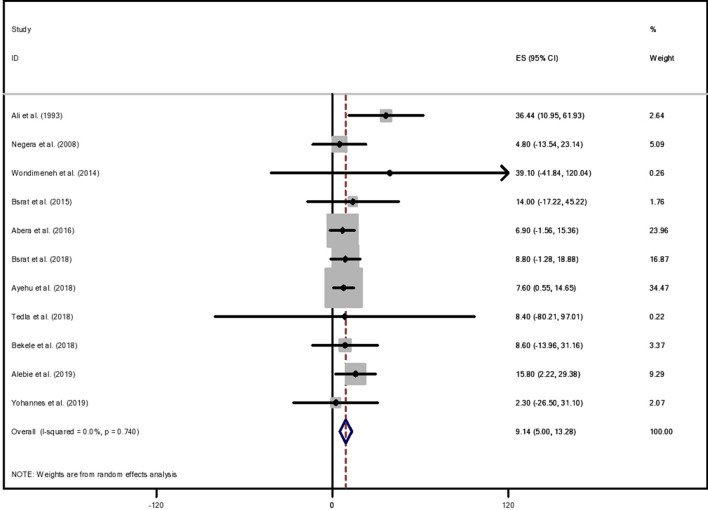
The years of publication of included articles ranged from November 1993 to September 2019. Of the total articles that fulfilled the eligibility criteria, 11 were selected for systematic review and meta-analysis, ten of which were community-based. The total sample size included from all the eligible articles was 50,883. Over one-third (36.36%) of the studies were conducted in the Tigray region. The highest proportion of leishmaniasis was reported from a study done in Amhara region (39.1%), followed by SNNPR (36.4%), and the lowest prevalence was reported from a study done in Tigray region (2.3%) [22–24] (Table 1). Of the 11 studies, nine studies were cross-sectional [22, 24–31], and the rest [23, 32] were retrospective studies. Included studies were done in five of the nine regions of the country. Although the value of I2 = 0.0%, differences in the assumptions of individual articles were expected; hence, a random effects model was employed using sample size and positive participants to determine the country-level pooled prevalence. The overall pooled prevalence of leishmaniasis was 9.13% (95% CI 5–13.27) with heterogeneity chi-squared = 6.83, p = 0.741, df = 10, and I2 = 0.0% (Fig. 2).
Table 1.
Descriptive summary of the 11 studies done on prevalence and risk factors of human leishmaniasis in Ethiopia
| Authors | Year of publication | Sample size | Type of test | Region of the study | Study setting | Prevalence (95% CI) |
|---|---|---|---|---|---|---|
| Ali et al. | 1993 | 730 | rK39 | SNNPR | Community | 36.4 (10.91–61.89) |
| Negera et al. | 2008 | 1907 | rK39 | SNNPR | Community | 4.8 (− 13.54 to 23.14 |
| Wondimeneh et al. | 2014 | 7161 | DAT | Amhara | Hospital | 39.1 (− 41.84 to 120.04) |
| Bsrat et al. | 2015 | 2106 | rK39 | Tigray | Community | 14.0 (− 17.22 to 45.22) |
| Abera et al. | 2016 | 289 | rK39 | Somali or Benshangul | Community | 6.9 (− 1.56 to 15.36) |
| Bsrat et al. | 2018 | 329 | rK39 | Tigray | Community | 8.8 (− 1.28 to 18.88) |
| Tedla et al. | 2018 | 26,511 | rK39 | Tigray | Hospital | 8.4 (− 80.21 to 97.01) |
| Ayehu et al. | 2018 | 185 | rK39 | Amhara | Community | 7.6 (0.55–14.65) |
| Bekele et al. | 2018 | 1682 | rK39 | SNNPR | Community | 8.6 (− 13.96 to 31.16) |
| Alebie et al. | 2019 | 361 | rK39 | Somali or Benshangul | Community | 15.8 (2.22–29.38) |
| Yohannes et al. | 2019 | 9622 | Clinical | Tigray | Community | 2.3 (− 26.50 to 31.10) |
Fig. 2.
Forest plot of pooled prevalence of human leishmaniasis in Ethiopia
Subgroup Meta-Analysis
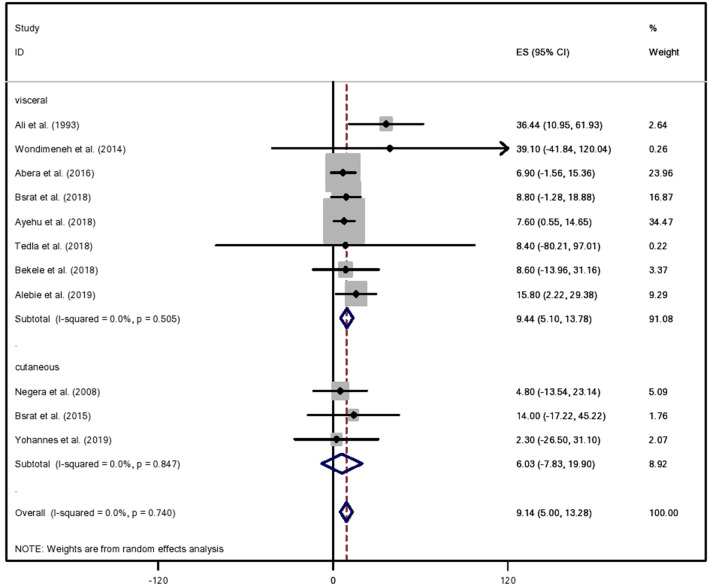
Subgroup meta-analysis was done on the basis of the region of the study, the type of leishmaniasis, and type of study design employed. Subgroup analysis by region (SNNPR, Tigray, Amhara, Somali, and Benshangul Gumuz) revealed moderate heterogeneity (I2 = 51.8%) in studies conducted in SNNPR. No heterogeneity was observed regarding study designs, and subtotal random proportion of the study designs showed that 9.13% (95% CI 4.91–13.21) was attributable to cross-sectional study design (Table 2). Regarding the type of leishmaniasis, the pooling subtotal under a random effects model implies that 9.44% (95% CI 5.1–13.78) of the type of leishmaniasis was visceral (kala-azar), and the subtotal prevalence for cutaneous leishmaniasis was 6.03% (95% CI 7.83–19.9) (Fig. 3).
Table 2.
Prevalence of human leishmaniasis estimates by subgroup analysis in the region and study design
| Region/study design | Authors | RR | 95% CI | Weight | Heterogeneity statistic | df | p value | I-squared | Tau-squared |
|---|---|---|---|---|---|---|---|---|---|
| SNNPR | Ali et al. (1993) | 36.4 | 10.9–61.9 | 2.6 | 4.2 | 3 | 0.13 | 51.8% | 134.7 |
| Negera et al. (2008) | 4.8 | − 13.5 to 23.1 | 5.1 | ||||||
| Bekele et al. (2018) | 8.6 | − 14 to 31.2 | 3.4 | ||||||
| Pooled effect size | 15.1 | − 3.2 to 33.3 | 11.1 | ||||||
| Tigray | Bsrat et al. (2015) | 14 | − 17.2 to 45.2 | 1.8 | 0.3 | 3 | 0.96 | 0.0% | 0.00 |
| Bsrat et al. (2018) | 8.8 | − 1.3 to 18.9 | 16.9 | ||||||
| Tedla et al. (2018) | 8.4 | − 80.2 to 97 | 0.2 | ||||||
| Yohannes et al. (2019) | 2.3 | − 26.5 to 31.1 | 2.1 | ||||||
| Pooled effect size | 8.6 | − 0.5 to 17.6 | 20.9 | ||||||
| Amhara | Wondimeneh et al. (2018) | 39.1 | − 41.8 to 120 | 0.3 | 0.6 | 1 | 0.45 | 0.0% | 0.00 |
| Ayehu et al. (2018) | 7.6 | 0.5–14.7 | 34.6 | ||||||
| Pooled effect size | 7.8 | 0.8–14.9 | 34.7 | ||||||
| Somali or Benshangul Gumuz | Abera et al. (2016) | 6.9 | − 1.6 to 15.4 | 24 | 1.2 | 1 | 0.28 | 15.9% | 6.30 |
| Alebie et al. (2019) | 15.8 | 2.2–29.4 | 9.3 | ||||||
| Pooled effect size | 9.7 | 1.6–17.8 | 33.3 | ||||||
| Overall pooled effect | 9.13 | 5–13.3 | 100 | 6.8 | 10 | 0.74 | 0.0% | 0.00 | |
| Cross-sectional | Ali et al. (1993) | 36.4 | 10.9–61.9 | 2.6 | 6.3 | 8 | 0.6 | 0.0% | 0.00 |
| Negera et al. (2008) | 4.8 | − 13.5 to 23.1 | 5.1 | ||||||
| Bsrat et al. (2015) | 14 | − 17.2 to 45.2 | 1.8 | ||||||
| Abera et al. (2016) | 6.9 | − 1.6 to 15.4 | 24 | ||||||
| Bekele et al. (2018) | 8.6 | − 14 to 31.2 | 3.4 | ||||||
| Bsrat et al. (2018) | 8.8 | − 1.3 to 18.9 | 16.9 | ||||||
| Ayehu et al. (2018) | 7.6 | 0.5–14.7 | 34.6 | ||||||
| Alebie et al. (2019) | 15.8 | 2.2–29.4 | 9.3 | ||||||
| Yohannes et al. (2019) | 2.3 | − 26.5 to 31.1 | 16.9 | ||||||
| Pooled effect size | 9.1 | 4.9–13.2 | 99.5 | ||||||
| Retrospective cross-sectional | Wondimeneh et al. (2018) | 39.1 | − 41.8 to 120 | 0.3 | 0.25 | 1 | 0.6 | 0.0% | 0.00 |
| Tedla et al. (2018) | 8.4 | − 80.2 to 97 | 0.2 | ||||||
| Pooled effect size | 25.1 | − 34.6 to 85 | 0.5 | ||||||
| Overall pooled effect | 9.13 | 5–13.3 | 100 |
Fig. 3.
Subgroup meta-analysis by type of human leishmaniasis in Ethiopia
Factors Associated with Human Leishmaniasis
A total of 23 variables (Table 3) were examined in this review for their effect on human leishmaniasis prevalence. Thirteen of the variables (i.e., the residence of daily laborers, knowledge about the mode of transmission, dog ownership, educational status, presence of termite mounds, presence of bed nets, resettlement status, the residence of participants, sleeping under a Ballantine tree, type of house, season, origin, and presence of cave within a 300-m radius) were mentioned in single articles as risk factors. Therefore, the pooled effect was not calculated for such variables. From the remaining ten variables tested for their effect on the prevalence of human leishmaniasis, the presence of hyraxes within a 300-m radius of sleep area and gender were significantly associated with the prevalence of human leishmaniasis in the final systematic review and meta-analysis. Pooling from two studies [24, 30] showed that the presence of hyraxes within a 300-m radius of the sleeping area was associated with the prevalence of human leishmaniasis (OR 2.96; 95% CI 1.67–5.245). Pooling from ten studies [22–30, 32] showed that female individuals were less likely to be affected by leishmaniasis compared to their male counterparts (OR 0.6; 95% CI 0.46–0.82). No other study variables showed an association with the prevalence of human leishmaniasis (Table 4).
Table 3.
Select risk factors for human leishmaniasis in Ethiopia
| Sociodemographic | Residence/accommodation | Sleeping related | Environmental | Health behaviors |
|---|---|---|---|---|
| Sex | Residence of daily laborers | Indoor/outdoor | Acacia | Bed Nets |
| Age |
House materials (mud, stone, wood/mud) |
Domestic animals sleeping nearby | Termite mounds | Knowledge of transmission modes |
| Origin (high/lowland) | Resettlement status | Sleeping under Ballantine tree | Dog ownership | Travel history to endemic areas |
| Occupation (farmer/not farmer) | Cave within 300-m radius | Black and cracked soil | ||
| Residence (rural/urban) | Gorge within 300-m radius | Season (dry/wet) | ||
| Education (formal/no formal) | Hyraxes within 300-m radius |
Table 4.
Effect of selected risk factors for human leishmaniasis in Ethiopia
| Variables | Authors | RR | 95% CI | % Weight | Heterogeneity chi-squared | df | p value | I-squared | Tau-squared |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Ali et al. (1993) | 0.9 | 0.8–1.1 | 17.4 | 70.0 | 9 | 0.00 | 7.1% | 0.12 |
| Wondimeneh et al. (2014) | 0.9 | 0.9–0.98 | 18 | ||||||
| Bsrat et al. (2015) | 0.8 | 0.6–1.1 | 14.9 | ||||||
| Abera et al. (2016) | 0.2 | 0.06–0.4 | 4.3 | ||||||
| Bekele et al. (2018) | 0.2 | 0.1–0.4 | 7.9 | ||||||
| Bsrat et al. (2018) | 0.1 | 0.0–0.3 | 3.4 | ||||||
| Tedla et al. (2018) | 0.0 | 0.01–0.1 | 4.7 | ||||||
| Ayehu et al. (2018) | 2.2 | 0.2–29.4 | 1.2 | ||||||
| Alebie et al. (2019) | 1.2 | 0.8–2 | 12 | ||||||
| Yohannes et al. (2019) | 0.0 | 0.7–1.1 | 15.8 | ||||||
| Pooled RR | 0.6 | 0.5–0.8 | 100 | ||||||
| Presence of hyraxes | Bsrat et al. (2015) | 2.3 | 1.7–3 | 54.6 | 4.8 | 1 | 0.03 | 79% | 0.13 |
| Yohannes et al. (2019) | 4.1 | 2.6–6.4 | 45.4 | ||||||
| Pooled RR | 3 | 1.7–5.2 | 100 | ||||||
| Age | Ali et al. (1993) | 0.5 | 0.4–0.6 | 15 | 114.6 | 7 | 0.00 | 93.9& | 0.38 |
| Negera et al. (2008) | 1.5 | 0.9–2.5 | 13 | ||||||
| Wondimeneh et al. (2014) | 1.5 | 1.4–1.6 | 15.5 | ||||||
| Abera et al. (2016) | 0.7 | 0.3–1.8 | 10 | ||||||
| Bsrat et al. (2018) | 2 | 1–4.1 | 11.4 | ||||||
| Bekele et al. (2018) | 2.9 | 0.9–9.5 | 8 | ||||||
| Alebie et al. (2019) | 1.5 | 0.9–2.6 | 12.8 | ||||||
| Yohannes et al. (2019) | 2.9 | 2–4.3 | 14.2 | ||||||
| Pooled RR | 1.4 | 0.9–2.3 | 100 | ||||||
| Sleeping area | Bsrat et al. (2018) | 4.3 | 1.8–10.3 | 10.7 | 2.4 | 3 | 0.49 | 0.0% | 0.00 |
| Ayehu et al. (2018) | 2.1 | 0.7–6.4 | 6.4 | ||||||
| Alebie et al. (2019) | 2.1 | 1.2–3.5 | 29 | ||||||
| Yohannes et al. (2019) | 2.1 | 1.43.1 | 53.9 | ||||||
| Pooled RR | 2.2 | 1.7–3 | 100 | ||||||
| Domestic animals near sleeping areas | Ayehu et al. (2018) | 2.8 | 1–7.6 | 27 | 3.9 | 2 | 0.14 | 48.8% | 0.18 |
| Alebie et al. (2019) | 5.3 | 1.7–16.5 | 23.4 | ||||||
| Yohannes et al. (2019) | 1.6 | 0.9–2.7 | 49.6 | ||||||
| Pooled RR | 2.5 | 1.3–4.9 | 100 | ||||||
| Travel history | Abera et al. (2016) | 1.8 | 0.7–4.5 | 58 | 0.4 | 1 | 0.52 | 0.0% | 0.00 |
| Ayehu et al. (2018) | 2.9 | 1–8.3 | 42 | ||||||
| Pooled RR | 2.2 | 1.1–4.4 | 100 | ||||||
| Presence of acacia near the sleeping area | Bsrat et al. (2018) | 1.8 | 0.8–3.9 | 49.4 | 7.2 | 1 | 0.01 | 86.1 | 0.91 |
| Alebie et al. (2019) | 7.6 | 3.7–15.7 | 50.6 | ||||||
| Pooled RR | 3.7 | 0.9–15.5 | 100 | ||||||
| Presence of black soil | Bsrat et al. (2018) | 1.7 | 0.7–3.9 | 58.4 | 0.6 | 1 | 0.43 | 0.0% | 0.00 |
| Ayehu et al. (2018) | 2.9 | 1.1–7.7 | 41.7 | ||||||
| Pooled RR | 2.1 | 1.1–4 | 100 | ||||||
| Occupation | Abera et al (2016) | 1.1 | 0.3–3.6 | 10.9 | 0.1 | 2 | 0.95 | 0.0% | 0.00 |
| Bsrat et al. (2018) | 1.2 | 0.7–2.5 | 27.7 | ||||||
| Alebie et al. (2019) | 1.0 | 0.6–1.7 | 61.5 | ||||||
| Pooled RR | 1.1 | 0.7–1.6 | 100 | ||||||
| Presence of gorge | Bsrat et al. (2015) | 1.4 | 1.1–1.7 | 51.7 | 15.9 | 1 | 0.00 | 93.7 | 0.44 |
| Yohannes et al. (2019) | 3.6 | 2.4–5.4 | 48.4 | ||||||
| Pooled RR | 2.2 | 0.9–5.6 | 100 |
Publication Bias and Small Study Effect Assessment
Egger and funnel plot tests were done to determine publication bias (Fig. 4), yielding no evidence of bias with a p value of 0.19 (95% CI − 0.356 to 1.548).
Fig. 4.
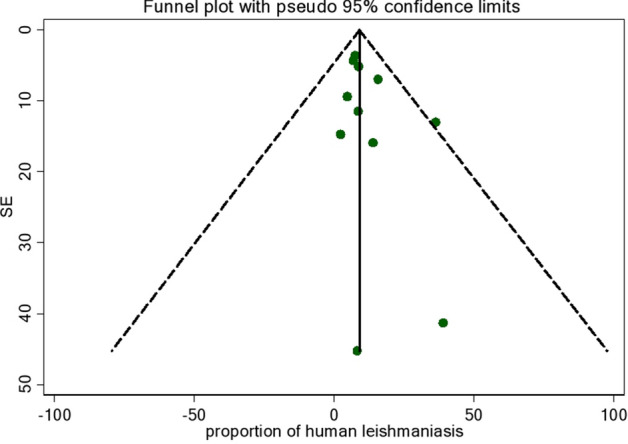
Funnel plot showing a risk of bias on leishmaniasis prevalence and standard error in Ethiopia
Discussion
Despite the prevalence of neglected tropical diseases in Ethiopia, funding to control and investigate geographic distribution is limited [3]. Risk assessment at the country level is poor in Ethiopia and the non-specific symptoms of leishmaniasis have made it difficult to treat. Many of the available methods of diagnosis are not appropriate in Ethiopia [10]. Therefore, this study focused on prevalence and risk factors of human leishmaniasis at the country level.
Human leishmaniasis was present, highly prevalent, and reported in more than half of the regions of the country. These findings are supported by previous studies suggesting that the Horn of Africa bears the largest burden of leishmaniasis with northern regions of Ethiopia around the borders of Sudan most affected [33, 34]. This pattern might reflect the unavailability of a vaccine, inadequate vector control, and insufficient access to new drugs [9]. Others suggest that this trend might be due to migration and conflict [34]; lack of advancement in diagnostics and treatment of the disease; or unaffordability of the new advanced technologies (i.e., vaccine, treatment, and diagnostics) to eradicate leishmaniasis [35].
Leishmaniasis was more prevalent in Amhara region followed by SNNPR, showing low reported prevalence in Tigray. Visceral leishmaniasis was the most prevalent type across all regions. This study showed that the risk of having leishmaniasis is increased among male individuals, which mirrors a previous study in Sudan [36]. This finding could potentially reflect that male individuals are more likely to be in agricultural fields and move during the night. This study showed that the presence of hyraxes within a 300-m radius of the sleeping area increased the risk of acquiring the disease. Previous studies revealed similar findings [12, 37] with an explanation that hyraxes are natural reservoirs of leishmaniasis [3]. In some studies, the presence of domestic animals around sleeping areas was ambiguous for risk factors for the prevalence of leishmaniasis [36, 38], although this finding was less evident in another study [37].
In contrast, the current study revealed that the presence of domestic animals does not affect the prevalence of leishmaniasis. This systematic review and meta-analysis showed that the remaining variables (Table 4) did not affect leishmaniasis prevalence. These findings are in disagreement with previous studies that suggest sleeping outdoors [34, 36, 38], age [36], presence of a gorge within a 300-m radius of the sleeping area [24, 30], presence of acacia [33, 36], and travel history to leishmaniasis endemic areas [27] were significantly associated with human leishmaniasis.
Conclusion
The prevalence of leishmaniasis remains high in Ethiopia (9.13%), and gender (male) and the presence of hyraxes within a 300-m radius of the sleeping area were significant risk factors. Even though there is a reduction in the magnitude of leishmaniasis and there are advances in diagnosis and treatments [16, 17], this disease remains challenging to assess and manage in developing countries like Ethiopia. Substantial gaps and inconsistencies were seen between the findings of the individual articles and systematic review and meta-analysis, clearly indicating the need for more intensive and national studies on this condition. Leishmaniasis was one of the WHO’s targets for elimination and control by 2020. However, our findings highlight that leishmaniasis is still a prevalent and neglected disease, contributing its share to the global burden of neglected tropical diseases (NTD). Since the current medications are decades old and full of limitations [35], new vaccines, methods of diagnosis, and treatment for its eradication should be given priority, and prophylaxes should also be considered to prevent relapse. Researchers around the world might also consider their investment in human leishmaniasis, especially in innovation for new treatments and diagnostics that can be affordable for developing countries. Epidemiological investigations of possible risk factors for human leishmaniasis, clinical trials for evidence-based practices, investigations of treatment failure, and investigation on the geographical distribution of hyraxes are recommended. Mass screening and follow-ups of interventions are also recommended.
Acknowledgements
The authors thank Adigrat University and Berhane Fisha for coordinating and supporting the development and preparation of the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
MH idea conception, proposal preparation, data analysis, wrote the paper, presented the paper, and drafted the manuscript. PP, KG, JN, EA, TH, YA, GG, HH, DG, and AG cooperated in the development of the proposal, presentation of the proposal, and data analysis and critically reviewed the manuscript. All authors accepted the final manuscript and took part in the evaluation and review of the manuscript.
Disclosures
Mekonnen Haftom, Pammla Petrucka, Kbrom Gemechu, Jemila Nesro, Embay Amare, Tsegu Hailu, Yohannes Ashebir, Gdiom Gebreheat, Haftea Hagos, Destaalem Gebremedhin and Alem Gebremariam declare that they have no competing interests.
Compliance with Ethics Guideline
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.
References
- 1.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7(5):e35671. [DOI] [PMC free article] [PubMed]
- 2.Bailey F, Mondragon-Shem K, Hotez P, et al. A new perspective on cutaneous leishmaniasis—implications for global prevalence and burden of disease estimates. PLoS Negl Trop Dis. 2017;11(8):e0005739. doi: 10.1371/journal.pntd.0005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deribe K, Meribo K, Gebre T, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. 2012;5(1):240. doi: 10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palatnik-de-Sousa CB, Day MJ. One Health: the global challenge of epidemic and endemic leishmaniasis. Parasit Vectors. 2011;4(1):1–10. doi: 10.1186/1756-3305-4-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esch KJ, Petersen CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev. 2013;26(1):58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assefa A. Leishmaniasis in Ethiopia: a systematic review and meta-analysis of prevalence in animals and humans. Heliyon. 2018;4(8):e00723. doi: 10.1016/j.heliyon.2018.e00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deribew A, Kebede B, Tessema GA, et al. Mortality and disability-adjusted life-years (dalys) for common neglected tropical diseases in Ethiopia, 1990–2015: evidence from the global burden of disease study 2015. Ethiop Med J. 2017;55(1):3. [PMC free article] [PubMed] [Google Scholar]
- 8.Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 9.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366(9496):1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 10.Kühne V, Rezaei Z, Pitzinger P, Büscher P. Systematic review on antigens for serodiagnosis of visceral leishmaniasis, with a focus on East Africa. PLoS Negl Trop Dis. 2019;13(8):e0007658. doi: 10.1371/journal.pntd.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Den Boer M, Argaw D, Jannin J, Alvar J. Leishmaniasis impact and treatment access. Clin Microbiol Infect. 2011;17(10):1471–1477. doi: 10.1111/j.1469-0691.2011.03635.x. [DOI] [PubMed] [Google Scholar]
- 12.Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357(10):1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 13.Tsegaw T, Gadisa E, Seid A, et al. Identification of environmental parameters and risk mapping of visceral leishmaniasis in Ethiopia by using geographical information systems and a statistical approach. Geospatial Health. 2013;7(2):299–308. doi: 10.4081/gh.2013.88. [DOI] [PubMed] [Google Scholar]
- 14.Hailu A, Gramiccia M, Kager P. Visceral leishmaniasis in Aba-Roba, south-western Ethiopia: prevalence and incidence of active and subclinical infections. Ann Trop Med Parasitol. 2009;103(8):659–670. doi: 10.1179/000349809X12554106963555. [DOI] [PubMed] [Google Scholar]
- 15.Khalil E, Zijlstra EE, Kager PA, El Hassan AM. Epidemiology and clinical manifestations of Leishmania donovani infection in two villages in an endemic area in eastern Sudan. Trop Med Int Health. 2002;7(1):35–44. doi: 10.1046/j.1365-3156.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- 16.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2(12):701. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 17.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359(9324):2188–2194. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- 18.Gadisa E, Tsegaw T, Abera A, et al. Eco-epidemiology of visceral leishmaniasis in Ethiopia. Parasit Vectors. 2015;8(1):381. doi: 10.1186/s13071-015-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pires M, Wright B, Kaye PM, et al. The impact of leishmaniasis on mental health and psychosocial well-being: a systematic review. PLoS One. 2019;14(10):e0223313. [DOI] [PMC free article] [PubMed]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali A, Ashford R. Visceral leishmaniasis in Ethiopia. I. Cross-sectional leishmanin skin test in an endemic locality. Ann Trop Med Parasitol. 1993;87(2):157–161. doi: 10.1080/00034983.1993.11812749. [DOI] [PubMed] [Google Scholar]
- 23.Wondimeneh Y, Takele Y, Atnafu A, Ferede G, Muluye D. Trend analysis of visceral leishmaniasis at Addis Zemen health center, Northwest Ethiopia. BioMed Res Int. 2014;2014:2014:545393. 10.1155/2014/545393. [DOI] [PMC free article] [PubMed]
- 24.Yohannes M, Abebe Z, Boelee E. Prevalence and environmental determinants of cutaneous leishmaniasis in rural communities in Tigray, northern Ethiopia. PLoS Negl Trop Dis. 2019;13(9):e0007722. doi: 10.1371/journal.pntd.0007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abera A, Tasew G, Tsegaw T, et al. Visceral leishmaniasis in Benishangul-Gumuz regional state, Western Ethiopia: reemerging or emerging? Am J Trop Med Hygiene. 2016;95(1):104–108. doi: 10.4269/ajtmh.15-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alebie G, Worku A, Yohannes S, Urga B, Hailu A, Tadesse D. Epidemiology of visceral leishmaniasis in Shebelle Zone of Somali Region, eastern Ethiopia. Parasit Vectors. 2019;12(1):209. doi: 10.1186/s13071-019-3452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayehu A, Aschale Y, Lemma W, et al. Seroprevalence of asymptomatic leishmania donovani among laborers and associated risk factors in agricultural camps of West Armachiho District, Northwest Ethiopia: a cross-sectional study. J Parasitol Res. 20182018:5751743. 10.1155/2018/5751743 [DOI] [PMC free article] [PubMed]
- 28.Bekele F, Belay T, Zeynudin A, Hailu A. Visceral leishmaniasis in selected communities of Hamar and Banna-Tsamai districts in Lower Omo Valley, South West Ethiopia: Sero-epidemological and Leishmanin Skin Test Surveys. PloS One. 2018;13(5):e0197430. 10.1371/journal.pone.0197430 [DOI] [PMC free article] [PubMed]
- 29.Bsrat A, Berhe M, Gadissa E, et al. Serological investigation of visceral Leishmania infection in human and its associated risk factors in Welkait District, Western Tigray, Ethiopia. Parasite Epidemiol Control. 2018;3(1):13–20. doi: 10.1016/j.parepi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bsrat A, Berhe N, Balkew M, et al. Epidemiological study of cutaneous leishmaniasis in Saesie Tsaeda-emba district, eastern Tigray, northern Ethiopia. Parasit Vectors. 2015;8(1):149. doi: 10.1186/s13071-015-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negera E, Gadisa E, Yamuah L, et al. Outbreak of cutaneous leishmaniasis in Silti woreda, Ethiopia: risk factor assessment and causative agent identification. Trans R Soc Trop Med Hyg. 2008;102(9):883–890. doi: 10.1016/j.trstmh.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Gebremichael TD, Bariagabr FH, Abreha HH. Incidence and trends of leishmaniasis and its risk factors in Humera, Western Tigray. J Parasitol Res. 2018;2018:8463097. [DOI] [PMC free article] [PubMed]
- 33.Argaw D, Mulugeta A, Herrero M, et al. Risk factors for visceral leishmaniasis among residents and migrants in Kafta-Humera, Ethiopia. PLoS Negl Trop Dis. 2013;7(11):e2543. [DOI] [PMC free article] [PubMed]
- 34.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3(8):e412. [DOI] [PMC free article] [PubMed]
- 35.van Griensven J, Gadisa E, Aseffa A, Hailu A, Beshah AB, Diro E. Treatment of cutaneous leishmaniasis caused by Leishmania aethiopica: a systematic review. PLoS Negl Trop Dis. 2016;10(3):e0004495. [DOI] [PMC free article] [PubMed]
- 36.Nackers F, Mueller YK, Salih N, et al. Determinants of visceral leishmaniasis: a case-control study in Gedaref State, Sudan. PLoS Negl Trop Dis. 2015;9(11):e0004187. [DOI] [PMC free article] [PubMed]
- 37.van Henten S, Adriaensen W, Fikre H, et al. Cutaneous leishmaniasis due to leishmania aethiopica. EClinicalMedicine. 2018;6:69–81. doi: 10.1016/j.eclinm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yared S, Deribe K, Gebreselassie A, et al. Risk factors of visceral leishmaniasis: a case control study in north-western Ethiopia. Parasit Vectors. 2014;7(1):470. doi: 10.1186/s13071-014-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.