Abstract
The purified oxindole alkaloids, isomitraphylline and mitraphylline from Uncaria perrottetii, revealed their ability to break amyloid aggregates in vitro suggesting their therapeutic potentials in Alzheimer’s disease (AD). Thioflavin-T assay for assessing amyloid-beta (Aβ) aggregation of these alkaloids exhibited inhibitions at 60.321% ± 2.61 (50 μM) for isomitraphylline and 43.17% ± 3.48 (50 μM) for mitraphylline. Neuroprotective effects were elaborated against Aβ-induced SH-SY5Y cells at 20 μM and 10 μM for isomitraphylline, and 20 μM for mitraphylline. In addition, both alkaloids attenuated and protected the H2O2-induced SH-SY5Y cell cytotoxicity at 20 μM. The intracellular ROS levels of SH-SY5Y cells from H2O2-induced oxidative stress were reduced at 20 μM and 10 μM, and the mitochondrial membrane potentials of Aβ-induced SH-SY5Y cells were protected at 20 μM. The overall results suggested the potentials of both alkaloids to target certain pathological biomarkers of AD and could be further investigated as therapeutic or preventive drug leads against AD.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02535-4) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Oxindole alkaloids, SH-SY5Y cells, Uncaria
Introduction
Alzheimer’s disease (AD) is a dominant neurological disorder characterized by cognitive impairments and synaptic dysfunctions affecting mostly the elderlies (Bagyinzsky et al. 2017). Similar with other neurological diseases, the progression of AD is correlated to mitochondrial dysfunction, oxidative stress, protein misfolding, abnormal amyloid-beta (Aβ) deposition, alterations in calcium homeostasis, and inflammation (Taylor et al. 2016). It is estimated that about 82 million people in 2030 and 152 million in 2050 worldwide will be affected by AD (Emmerzaal et al. 2015). Currently, there are only five drugs approved by the US FDA to minimize the progression of symptoms related to AD. These are natural product-based compounds, which include the acetylcholinesterase inhibitors, galantamine, revastigmine, donepezil, and tacrine, and the NMDA receptor antagonist memantine (Guo et al. 2016; Huang et al. 2019). These drugs only provide symptomatic relief of AD without significant effectiveness in the disease progression and prevention. Hence, the challenges of finding an alternative treatment or therapy from natural products are warranted. Due to their diverse structures and pharmacological activities, natural products and their derivatives continuously play an important role in the discovery and drug development as potential avenues of finding therapeutic agents in neurodegenerative diseases (Angeloni and Vauzour 2019).
The genus Uncaria Schreb. (Rubiaceae) comprised of 34 species and distributed among tropical areas in Africa, Southeast Asia, and South America (Ridsdale 1978). In the Philippines, ten species were reported, including the two endemic U. nervosa Elmer and U. perrottetii (A. Rich.) Merr. Several compounds were elaborated from phytochemical studies of Uncaria species containing alkaloids, flavonoids, phenylpropanoids, quinovic acid glycosides, and triterpenoids, in which the alkaloids were identified predominantly as bioactive constituents (Heitzman et al. 2005). Pharmacological investigations revealed antiviral, anti-inflammatory, antioxidant, cytotoxicity, immune-stimulant, hypotensive, mutagenicity, and antibacterial properties (Heitzman et al. 2005). The potent in vivo inhibitory activity of the extracts of U. rhynchophylla against AD and Parkinson’s disease were previously reported (Shim et al. 2009; Xian et al. 2011). As part of our research discovery in finding neuroprotective agents from nature and their potential mechanisms, two isomeric oxindole alkaloids, isomitraphyline and mitraphylline, were purified from the Philippine endemic U. perrottetii and their protection and/or cytotoxicity in oxidative stress-induced human neuroblastoma SH-SY5Y cells were investigated. The potential neuroprotective effects of isomitraphylline and mitraphylline against amyloid aggregation were not investigated previously.
Materials and methods
General considerations
The extraction of U. perrottetii leaves and the isolation and identification of the alkaloids from the U. perrottetii crude base extract have been previously reported (Olivar et al. 2018). Isomitraphylline (45 mg) and mitraphylline (38 mg) were the predominant alkaloids isolated from the crude base extract based on the afforded weights. The NMR spectra of the alkaloids are presented in the Online Resource Material. Aβ1-42 (Aggresure™) was dissolved at 250 μg/mL in sterile PBS.
Thioflavin T (ThT) assay
The inhibition of Aβ1-42 aggregation was evaluated as previously described (Tan et al. 2019; Xia et al. 2019). Briefly, Aβ dissolved in PBS was incubated with or without the alkaloids or phenol red (positive control) at 37 °C for 24 h in a 384-well plate. ThT solution was added and incubated for another 15 min. Fluorescence signal (Ex 450 nm; Em 510 nm) was measured using a PerkinElmer Victor-3® multi-plate reader.
Cell culture
Human neuroblastoma SH-SY5Y cells (ATCC CRL-2266) were maintained in DMEM supplemented with 10% FBS, 1% kanamycin, and 1% penicillin at 37 °C and 5% CO2 and passaged twice per week. Experiments were performed at 80–90% confluence.
Cell Cytotoxicity
Cell viability measurements were performed using the ATP luminescence assay as described previously (Tan et al. 2019, 2020a). SH-SY5Y cells at 2 × 104 cells/well density were subcultured in 96-well plate and incubated for 24 h. After incubation, cells were treated with the alkaloids for 24 h. The media were removed, cells were washed with PBS, fresh media were added, and incubated for another 30 min. Then, CellTiter-Glo® luminescent reagent was added and the luminescence was read on a multi-plate reader. Data were analyzed and the % cell viability was expressed relative to the control.
Neuroprotective activity assay
Determination of the neuroprotective activity in Aβ or H2O2-induced SH-SY5Y cytotoxicity was performed as previously described (Gonzalez-Sarrias et al. 2017; Yu et al. 2014a) and evaluated by the ATP luminescence assay. Neuroblastoma SH-SY5Y cells were seeded in 96-well plate at 2 × 104 cells/ well and incubated for 24 h. After stabilization, cells were pre-treated with the alkaloids for 6 h before incubation with Aβ1-42 (5 μM) or H2O2 (100 μM). A solvent control (untreated control cells), Aβ or H2O2 alone, and alkaloids alone treatments were also included. After incubation, the % cell viabilities were determined in triplicate experiments.
Measurement of reactive oxygen species (ROS)
ROS measurements were performed using the 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) staining method as previously described (Gonzalez-Sarrias et al. 2016; Peñalver et al. 2020). After 24 h stabilization, SH-SY5Y (2 × 104 cells/wells) cells were pre-treated with the alkaloids for 2 h before incubation with 100 μM H2O2 for another 4 h. After incubation, cells were treated with 25 μM H2DCFDA and incubated for another 2 h in the dark at 37 °C. Fluorescence intensity (Ex 495 nm, Em 520 nm) was measured in a microplate reader. The ROS level was calculated as a percentage of the untreated control cells (100%) in triplicate measurements.
Mitochondrial membrane potential (ΔΨm) assay
Measurement of the ΔΨm was performed using the tetramethylrhodamine, methyl ester (TMRE) staining method as previously described (Alvariño et al. 2019). After 24 h SH-SY5Y (2 × 104 cells/well) acclimatization, cells were pre-treated with the alkaloids for 2 h and then combined with 5 μM Aβ1-42 for 24 h. After treatment, 1 μM TMRE staining solution was added and incubated at 37 °C for 30 min. The fluorescence (Ex 549 nm, Em 575 nm) was read in a microplate reader. The ΔΨm was calculated as a percentage of the untreated control cells (100%) in triplicate measurements.
Statistical analysis
Data are reported as the mean ± SD of at least three experiments. Statistical analysis was determined by one-way ANOVA followed by Tukey’s honestly significant difference (HSD) post-hoc test. Statistical significance was considered at *p < 0.05.
Results and discussion
In the continuous search for potential neuroprotective natural products against AD (Tan et al. 2019, 2020a, b), the alkaloids isomitraphylline and mitraphylline (Fig. 1) from previous purifications from U. perrottetii (Olivar et al. 2018) were investigated. These two alkaloids were abundant in the genus Uncaria. Isomitraphylline was isolated from 18 Uncaria species, and mitraphylline could be isolated from 20 Uncaria species (Olivar et al. 2018). Previous reports on these oxindole alkaloids demonstrated their cytotoxic and antiproliferative effects in various cancer cell lines including their potential as anticancer agents (Bacher et al. 2006; Pilarski et al. 2010; Yu et al. 2015). Furthermore, these alkaloids were the major compounds identified in the extracts of U. tomentosa by UPLC-MS analysis (Azevedo et al. 2019). The crude extract of U. perrottetii was shown to have an immunostimulatory effects in vivo (Nudo and Catap 2011). The study of the biological activity of isolated natural products from the plant extracts would be essential in elucidating their potential cellular and molecular mechanisms.
Fig. 1.
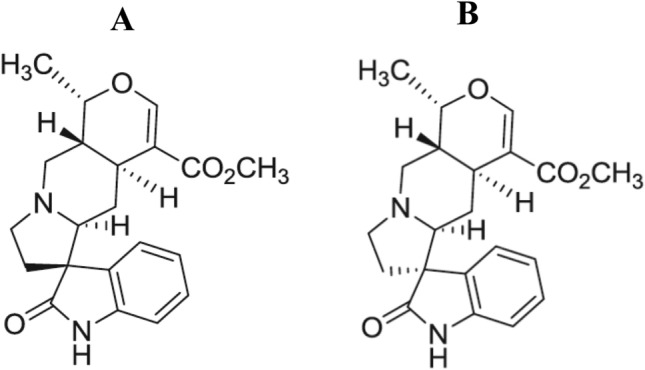
Chemical structure of isomitraphylline (a) and mitraphylline (b)
Thioflavin T (ThT) assay
The abnormal aggregation of Aβ peptides is one of the pathological characteristics of AD. This could lead to the formation of its oligomers, protofibrils, and insoluble plaques which would cause dysfunctions in mitochondrial, resulting in increases of oxidative stresses and neuroinflammations (Jakob-Roetne and Jacobsen 2009). The inhibition of Aβ aggregation of the two alkaloids was determined using the ThT assay. Both alkaloids showed moderate inhibitory effects of 61.32% (± 2.61) and 63.27% (± 3.48) at 50 μM concentrations of isomitraphylline and mitraphylline, respectively. These results indicated a no significant difference (p < 0.05) in comparison to the phenol red as the positive control (Necula et al. 2007; Wu et al. 2006) with 67.31% (± 3.04) inhibition at 50 μM. At 5 μM, isomitraphylline and mitraphylline showed 21.63% (± 5.47) and 19.96% (± 3.85) inhibitions of Aβ aggregation, respectively. The capability of the two alkaloids for breaking Aβ aggregations prompted the investigations of their neuroprotective effects in oxidative stress-induced neuroblastoma SH-SY5Y cells from Aβ1-42 and H2O2, and their underlying mechanisms were evaluated focusing on the ROS productions and mitochondrial membrane potentials.
Effect of the alkaloids on viability of SH-SY5Y cells
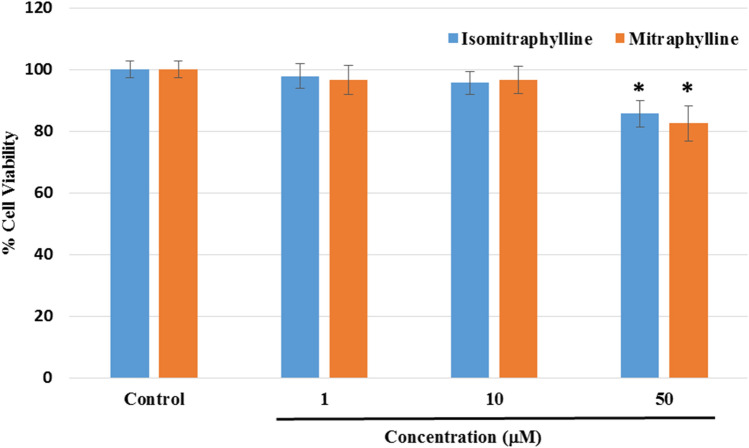
Before the neuroprotective experiments, the cytotoxicity in neuroblastoma SH-SY5Y cells of the alkaloids at 1, 10, and 50 μM concentrations was determined by measuring the cell viability using the ATP luminescence assay. The cells were incubated with the compounds for 24 h. Figure 2 showed a significant difference in the % cell viability using the highest concentration at 50 μM, while the 10 and 1 μM did not exert any significant cytotoxic effect (p < 0.05) in comparison to the control cells. Hence, the succeeding treatments for the neuroprotection assay used 1, 10, and 20 μM concentrations.
Fig. 2.
Effects of the alkaloids on cell viability in neuroblastoma SH-SY5Y cells as determined by the ATP luminescence assay. The results indicate % cell viability vs the control cells (untreated) and reported as mean ± SD of triplicate experiments. The (*) indicates a significant difference with the control cells at p < 0.05
Neuroprotective effects of the alkaloids on the oxidative stress-induced cytotoxicity
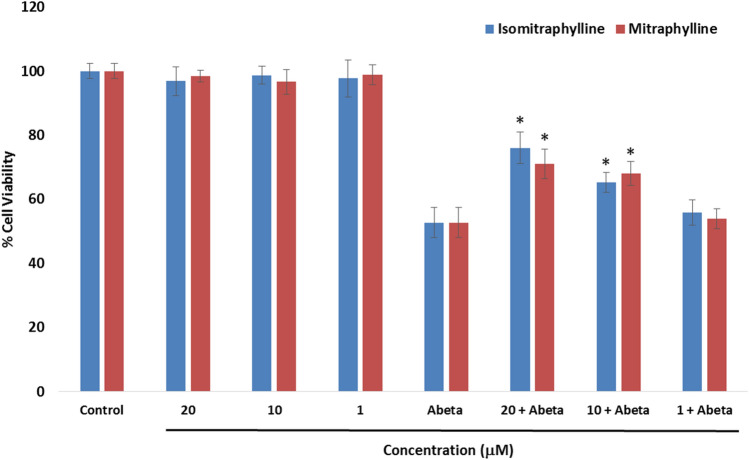
The ability of the alkaloids to protect human neuroblastoma SH-SY5Y cells against oxidative damage stimulated by Aβ1-42 or H2O2 was evaluated by the ATP luminescence assay. In Fig. 3, the alkaloids were tested for their neuroprotective potential against Aβ-induced cell injuries in SH-SY5Y cells. Aβ had been widely employed to generate neuronal cell damage to analyze the protective potential of plant extracts and natural products (An et al. 2019; Okello et al. 2011; Park and Kim, 2002; Yu et al. 2014b). A 5 μM concentration, Aβ revealed 52.71% cell viability. The neuroprotective effects were evaluated by pre-treatment of the cells with the alkaloids for 6 h before incubation with the 5 μM Aβ for 24 h. In the non-Aβ-treated groups, the cells did not exert any antiproliferative activity against the SH-SY5Y cell line. In the Aβ-treated groups, both the alkaloids at 10 μM and 20 μM showed significant protective effects (p < 0.05) in comparison to the Aβ-treated only cells. Isomitraphylline exhibited a cell viability of 65.32% (± 3.06) at 10 μM and 76.02% (± 4.89) at 20 μM. Mitraphylline revealed 68.04% (± 3.79) and 71.06% (± 4.56) cell viabilities at 10 μM and 20 μM, respectively. The 1 μM alkaloid concentration did not show protective effect in comparison to the Aβ-treated group (p < 0.05). These results justified the inhibition of Aβ aggregation of these alkaloids as suggested from the potential screening drug candidates by ThT assay. Furthermore, the neuroprotective effects of mitraphylline also corroborated with previous data demonstrating the significant binding of mitraphylline with the Aβ1-40 protein (Frackowiak et al. 2006).
Fig. 3.
Neuroprotective effects of the alkaloids on Aβ1-42 (Abeta)-induced cytotoxicity using the ATP luminescence assay. Neuroblastoma SH-SY5Y cells were pre-treated with the alkaloids for 6 h, followed by treatment with 5 μM Aβ for 24 h. The results indicate % cell viability vs the control cells and reported as mean ± SD of triplicate experiments. The (*) indicates a significant difference with the Aβ-treated group at p < 0.05
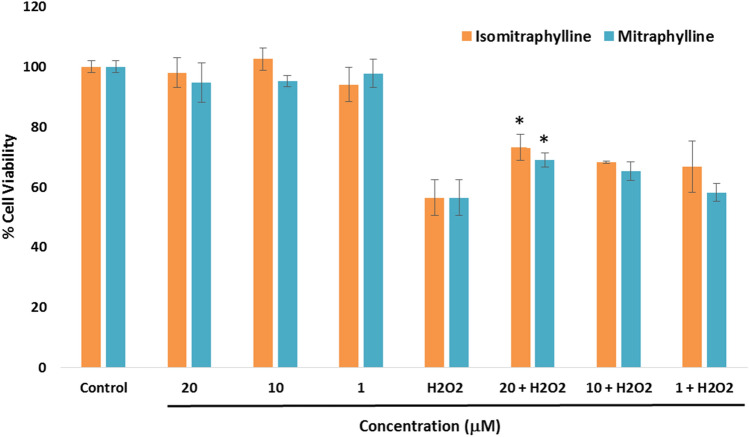
The effects of the alkaloids on the oxidative damage caused by H2O2 were evaluated as shown in Fig. 4. H2O2 treatment (100 μM) of the SH-SY5Y cells decreased the cell viability to 56.45% in comparison to the control group, while the non-H2O2-treated cells showed no significant cell viability difference (p < 0.05). To determine the neuroprotective activity, the cells were pre-treated with various concentrations of the alkaloids for 6 h, followed by subsequent addition of the 100 μM H2O2 for 24 h. Both alkaloids provided significant neuroprotection to the neuroblastoma SH-SY5Y cells against H2O2-induced cytotoxicity at 20 μM with cell viability of 73.14% (± 4.38) for isomitraphylline and 69.03% (± 2.43) for mitraphylline in comparison to the H2O2-treated cells (p < 0.05). Both alkaloids did not show significant protection against H2O2 to SH-SY5Y cells at lower concentrations (1 and 10 μM).
Fig. 4.
Neuroprotective effects of the alkaloids on H2O2-induced SH-SY5Y cell cytotoxicity using the ATP luminescence assay. The cells were pre-treated with the alkaloids for 6 h, before incubation with 100 μM H2O2 for 24 h. The results indicate % cell viability vs the negative control and reported as mean ± SD of triplicate experiments. The (*) indicates a significant difference with the H2O2-treated group at p < 0.05
Effects of the alkaloids on the ROS production and mitochondrial membrane potential
The increase of misfolded proteins contributed to mitochondrial dysfunction and proliferation of ROS releases in neuronal cells (Kumar et al. 2018). Microgial activation also could influence the elevated level of ROS and reactive nitrogen species in AD, resulting in oxidative damaged environment and cell death (Ali et al. 2013). Also, exposure of the neuroblastoma SH-SY5Y cells to various stressors led to superoxide productions (Chen et al. 2013). In fact, the free radicals could attack the membrane phospholipids and damage DNA, causing loss of mitochondrial membrane potentials, mutated proteins, and cell apoptosis (Wang et al. 2010; Yu et al. 2014b; Zeng et al. 2010).
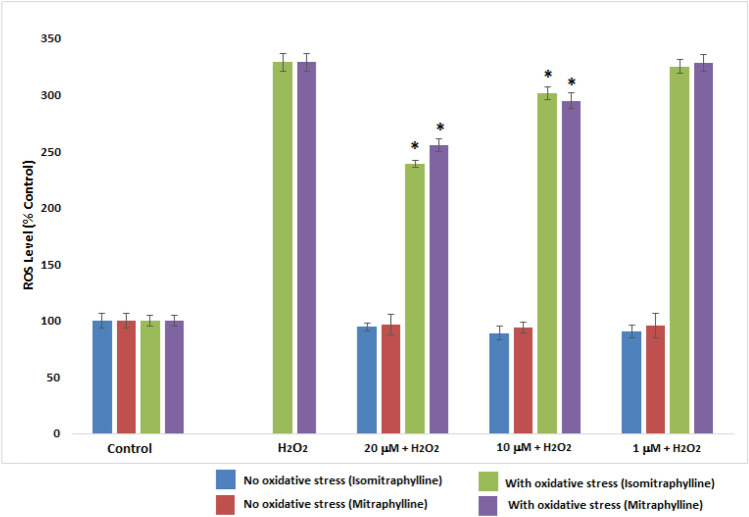
The effect of the alkaloids on the intracellular ROS levels was shown in Fig. 5. SH-SY5Y cells were pre-treated with the alkaloids in their non-cytotoxic concencentrations for 2 h and incubated with 100 μM H2O2 for another 4 h to induce oxidative damage. The ROS levels generated were evaluated using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) staining. Cells treated with H2O2 alone showed an enhanced intracellular ROS levels (329.06%) in comparison to the control cells. Both alkaloids significantly reduced ROS levels at 10 μM and 20 μM concentrations in comparison to the H2O2-treated alone SH-SY5Y cells (p < 0.05). Isomitraphylline demonstrated the decrease ROS levels to 301.78% and 238.92% for 10 and 20 μM concentrations, respectively. Mitraphylline influenced the reduction of ROS levels by 295.09% at 10 μM and 255.54% at 20 μM ROS levels. The 1 μM concentration did not show any significant ROS level reduction in comparison to the H2O2-treated alone SH-SY5Y cells (p < 0.05). The intracellular ROS level of the alkaloids alone was also measured after 6 h of incubation (no oxidative strress). The ability of the alkaloids to reduce the ROS generation in H2O2-treated SH-SY5Y cells validated the neuroprotective activity, as shown in Fig. 5.
Fig. 5.
Effect of the alkaloids on the intracellular ROS level. SH-SY5Y cells were incubated with the alkaloids for 6 h (No oxidative stress). SH-SY5Y cells were pre-treated with the alkaloids for 2 h, and incubated with 100 μM H2O2 for another 4 h (With oxidative stress). ROS generation was measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). Values are the mean ± SD of triplicate experiments and expressed as % of the control. The (*) indicates a significant difference compared to the H2O2-treated group alone at p < 0.05
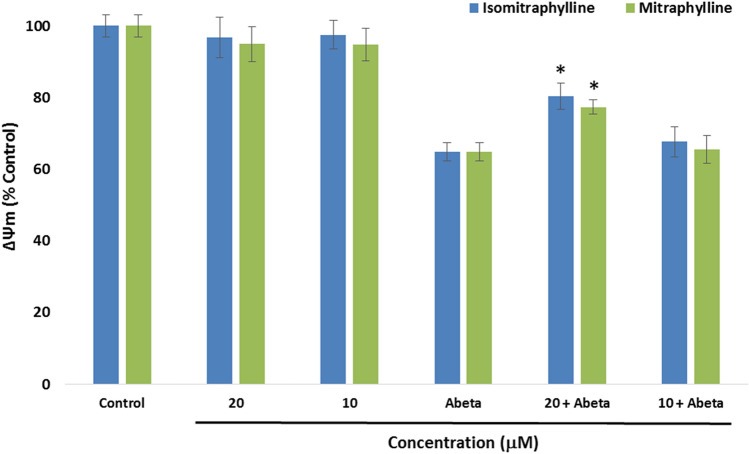
In Fig. 6, the mitochondrial membrane potential (ΔΨm) was measured using 10 μM and 20 μM, as these concentrations could have protective effects against Aβ as indicated in Fig. 3. After alkaloids pre-treatment for 2 h, the cells were incubated with 5 μM Aβ for another 24 h. The ΔΨm was measured using the tetramethylrhodamine, methyl ester (TMRE) stain. Treatment of the SY-SY5Y cells with the Aβ alone showed a significant decrease of ΔΨm in comparison to the control cells (p < 0.05). SH-SY5Y cells untreated with Aβ also gave comparable ΔΨm values to the control cells. However, only the 20 μM alkaloid + Aβ-treated cells presented significant increases in the MMP in comparison to the Aβ-treated alone SH-SY5Y cells. The ΔΨm of the SH-SY5Y cells for the isomitraphylline gave 80.42% (± 3.66), while mitraphylline demonstrated 77.34% (± 2.05). In comparison, the Aβ-treated alone cells showed 64.79% (± 2.56) ΔΨm. These results signify the potential attenuation of the mitochodrial dysfunction by the oxindole alkaloids by reducing ROS generation.
Fig. 6.
Effects of the alkaloids on the mitochondrial membrane potential (ΔΨm). SH-SY5Y cells were pre-treated with the alkaloids for 2 h, and incubated with 5 μM Aβ1-42 (Abeta) for 24 h. ΔΨm was measured using the tetramethylrhodamine, methyl ester (TMRE) stain. Values are the mean ± SD of triplicate experiments and expressed as % of the control. The (*) indicates a significant difference compared to the Abeta-treated group alone at p < 0.05
The structural diversity of small molecules has contributed to the researches in finding potential leads in drug discovery including neurodegenerative diseases. AD is a complex illness revolving in an interconnected genetic, biochemical, and metabolic pathways (Poloni et al. 2021; Reus et al. 2020). Hence, a molecule which targets more than one pathological hallmarks in AD is envisioned as a potential possibility in designing and developing effective drugs against AD. Several review papers have also reported on the ability of natural products with diverse structures as amyloid inhibitors (Rajasekhar et al. 2015; Tewari et al. 2018; Velander et al. 2017). Majority of these natural products are flavonoids and polyphenols such as brazilin, curcumin, resveratrol, tanshinone, and epigallocatechin-3-gallate which also highlighted their anti-oxidant, anti-inflammatory, and metal chelating properties as possible mechanisms as potential multifunctional anti-amyloid compounds against AD (Rajasekhar et al. 2015; Velander et al. 2017).
In this study, isomitraphylline and mitraphylline as potential candidates for further investigations in AD models are reported. Both alkaloids were able to demonstrate inhibition of the Aβ aggregation in the ThT assay. Their protective potential in the Aβ-induced or H2O2-induced neuroblastoma SY-SY5Y cells revealed inhibitions caused by the cytotoxic effects in the SH-SY5Y cells of either Aβ or H2O2. To validate these neuroprotective effects, both alkaloids showed reductions in the intracellular ROS level in H2O2-treated SH-SY5Y cells and mitochondrial dysfunctions in Aβ-induced SH-SY5Y cells. These parameters both associate to oxidative stress which is one of the pathological hallmarks associated with AD (Xia et al. 2019). Oxidative stress causes an increase in the level of reactive oxygen species leading to mitochondrial dysfunction. This eventually leads to neuronal death-causing age-related illnesses including AD.
There is a limited study conducted on the neuroprotective effects of the oxindole alkaloids. This report is the first study on the neuroprotective effects of isomitraphylline and mitraphylline. Noteworthy, several related oxindole alkaloids including corynoxeine, rhynchophylline, isorhynchophylline, and isoorynoxeine, and the yohimbine indole alkaloids geissoschizine methyl ether, hirsuteine, and hirsutine have been investigated on their neuroprotective effects based on glutamate-induced cell death. The alkaloids rhynchophylline, isorhynchophylline, isoorynoxeine, hirsuteine, and hirsutine showed protective effects on glutamate-induced neuronal death in cerebellar granule cells by inhibiting the Ca2+ influx (Shimada et al. 1999). The in vitro neuroprotective effects of these type of alkaloids project their potential as anti-AD drugs. However, in vivo studies are required to fully explore their therapeutic capacity against AD.
Conclusion
Collectively, our findings indicated the neuroprotective effects of the oxindole alkaloids, isomitraphylline and mitraphylline, for their therapeutic potentials against AD. The inhibitions of amyloid-beta (Aβ) aggregation by both alkaloids were demonstrated by the ThT assay, and their neuroprotective effects on Aβ-damaged neuroblastoma SH-SY5Y cells also supported their potentials. Reduction of oxidative stress was manifested from their protective effects on H2O2-induced SH-SY5Y cells. Both alkaloids reduced the ROS levels and mitochondrial dysfunctions. Hence, these alkaloids could be further investigated and developed as potential therapeutic agents for the treatment of AD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was funded by the National Research Foundation of Korea (NRF) Grants awarded by the Korean government (NRF-2020R1A2B5B01002463). The authors also acknowledged Dr. Felicidad Christina Ramirez (UST College of Science) on the help on statistical analysis.
Author contributions
Conceptualization, MAT and SSAA; methodology, MAT; formal analysis, MAT; writing—original draft preparation, MAT; writing—review and editing, MAT and SSAA; project administration, MAT and SSAA; funding acquisition, SSAA All authors have read and agreed to the published version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
The article does not contain any studies involving human participants or animals.
References
- Alvariño R, Alonso E, Lacret R, Oves-Costales D, Genilloud O, Reyes F, Alfonso A, Botana LM. Caniferolide A, a macrolide from Streptomyces caniferus, attenuates neuroinflammation, oxidative stress, amyloid-beta, and tau pathology in vitro. Mol Pharm. 2019;16:1456–1466. doi: 10.1021/acs.molpharmaceut.8b01090. [DOI] [PubMed] [Google Scholar]
- Ali S, Hamed A, Soltan M, Hegazy U, Elgorashi E, El-Garf I, Hussein A. In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of Alzheimer disease. BMC Complement Altern Med. 2013;13:1–10. doi: 10.1186/1472-6882-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JP, Ha TKQ, Kim HW, Ryu B, Kim J, Park J, Lee CH, Oh WK. Eudesmane glycosides from Ambrosia artemisiifolia (Common Ragweed) as potential neuroprotective agents. J Nat Prod. 2019;82:1128–1138. doi: 10.1021/acs.jnatprod.8b00841. [DOI] [PubMed] [Google Scholar]
- Angeloni C, Vauzour D. Natural products and neuroprotection. Int J Mol Sci. 2019;20:5570. doi: 10.3390/ijms20225570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo B, Roxo M, Borges M, Peixoto H, Crevelin E, Bertoni B, Contini S, Lopes A, Franca S, Pereira A, Wink M. Antioxidant activity of an aqueous extract from Uncaria tomentosa and its major alkaloids mitraphylline and isomitraphylline in Caenorhabditis elegans. Molecules. 2019;24:3299. doi: 10.3390/molecules24183299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher N, Tienfenthaler M, Sturm S, Stuppner H, Ausserlechner MJ, Kofler R, Konwalinka G. Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukemia cells. Br J Haematol. 2006;132:615–622. doi: 10.1111/j.1365-2141.2005.05907.x. [DOI] [PubMed] [Google Scholar]
- Bagyinszky E, Giau VV, Shim K, Suk K, An SS, Kim SY. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J Neurol Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Chen TF, Tang M-C, Chou C-H, Chiu M-J, Huang R. Dose-dependent folic acid and memantine treatments promote synergistic or additive protection against Aβ(25–35) peptide-induced apoptosis in SH-SY5Y cells mediated by mitochondria stress-associated death signals. Food Chem Toxicol. 2013;62:538–547. doi: 10.1016/j.fct.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- Frachowiak T, Baczek T, Roman K, Zbikowska B, Glerisk M, Freka I, Cisowski W. Binding of an oxindole alkaloid from Uncaria tomentosa to amyloid protein (Abeta1-40) Z Naturforsch C J Biosci. 2006;61:821–826. doi: 10.1515/znc-2006-11-1209. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sarrias A, Nunez-Sanchez MA, Tomas-Barberan FA, Espin JC. Neuroprotective effects of bioavailable polyphenol-derived metabolited against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J Agric Food Chem. 2017;65:752–758. doi: 10.1021/acs.jafc.6b04538. [DOI] [PubMed] [Google Scholar]
- Guo J, Feng X, Zhou S, Yan W, Meng D. Potential anti-Alzheimer’s disease activities of the roots of Desmodium caudatum. Ind Crops Prod. 2016;90:94–99. [Google Scholar]
- Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochem. 2005;66:5–29. doi: 10.1016/j.phytochem.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Huang J-M, Huang F-I, Yang C-R. Moscatilin ameliorates tau phosphorylation and cognitive deficits in Alzheimer’s disease models. J Nat Prod. 2019;82:1979–1988. doi: 10.1021/acs.jnatprod.9b00375. [DOI] [PubMed] [Google Scholar]
- Jakob-Roetne R, Jacobsen H. Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- Kumar D, Ganeshpurkar A, Modi G, Gupta SK, Singh SK, Kumar D. Secretase inhibitors for the treatment of Alzheimer’s disease: long road ahead. Eur J Med Chem. 2018;148:436–452. doi: 10.1016/j.ejmech.2018.02.035. [DOI] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Nudo LP, Catap ES. Immunostimulatory effects of Uncaria perrottetii (A. Rich.) Merr. (Rubiaceae) vinebark aqueous extract in Balb/C mice. J Ethnopharmacol. 2011;27:613–620. doi: 10.1016/j.jep.2010.10.044. [DOI] [PubMed] [Google Scholar]
- Okello EJ, McDougall GJ, Kumar S, Seal CJ. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ(1–42)) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691–696. doi: 10.1016/j.phymed.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Olivar JE, Sy KA, Villanueva CV, Alejandro GJD, Tan MA. Alkaloids as chemotaxonomic markers from the Philippine endemic Uncaria perrottetii and Uncaria lanosa f. philippinensis. J King Saud Univ Sci. 2018;30:283–285. [Google Scholar]
- Park SY, Kim DS. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer’s disease. J Nat Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- Peñalver P, Zodio S, Lucas R. Neuroprotective and anti-inflammatory effects of pterostilbene metabolites in human neuroblastoma SH-SY5Y and RAW 264.7 macrophage cells. J Agric Food Chem. 2020;68:1609–1620. doi: 10.1021/acs.jafc.9b07147. [DOI] [PubMed] [Google Scholar]
- Pilarski R, Filip B, Wietrzyk J, Kuraś M, Gulewicz K. Anticancer activity of the Uncaria tomentosa (Willd.) DC preparations with different oxindole alkaloid composition. Phytomedicine. 2010;17:1133–1139. doi: 10.1016/j.phymed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Poloni KM, Duarte de Oliveira IA, Tam R, Ferrari RJ. Brain MR image classification for Alzheimer’s disease diagnosis using structural hippocampal asymmetrical attributes from directional 3-D log-Gabor filter responses. Neurocomputing. 2021;419:126–135. [Google Scholar]
- Rajasekhar K, Chakrabarti M, Govindaraju T. Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chem Commun. 2015;51:13434. doi: 10.1039/c5cc05264e. [DOI] [PubMed] [Google Scholar]
- Reus LM, Stringer S, Posthuma D, Teunissen CE, Scheltens P, Pijenburg Y, Visser PJ, Tijms BM. Degree of genetic liability for Alzheimer’s disease associated with specific proteomic profiles in cerebrospinal fluid. Neurobiol Aging. 2020;93:144.e1–144.e15. doi: 10.1016/j.neurobiolaging.2020.03.012. [DOI] [PubMed] [Google Scholar]
- Ridsdale CE. A revision of Mitragyna and Uncaria (Rubiaceae) Blumea. 1978;24:43–100. [Google Scholar]
- Shim JS, Kim HG, Ju MS, Choi JG, Jeong SY, Oh MS. Effects of the hook of Uncaria rhynchophylla on neurotoxicity in the 6-hydroxydopamine model of Parkinson’s disease. J Ethnopharm. 2009;126:361–365. doi: 10.1016/j.jep.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H, Terasawa K. Evaluation of the protective effects of the alkaloids isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J Phar Pharmacol. 1999;51:715–722. doi: 10.1211/0022357991772853. [DOI] [PubMed] [Google Scholar]
- Tan MA, Lagamayo MW, Alejandro GJD, An SSA. Anti-amyloidogenic and cyclooxygenase inhibitory activity of Guettarda speciosa. Molecules. 2019;24:4112. doi: 10.3390/molecules24224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MA, Lagamayo MW, Alejandro GJD, An SSA. Neuroblastoma SH-SY5Y cytotoxicity, anti-amyloidogenic activity and cyclooxygenase inhibition of Lasianthus trichophlebus (Rubiaceae) 3 Biotech. 2020;10:152. doi: 10.1007/s13205-020-2145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MA, Gonzalez SJB, Alejandro GJD, An SSA. Neuroprotective effects of vomifoliol, isolated from Tarenna obtusifolia Merr (Rubiaceae), against amyloid-beta1-42-treated neuroblastoma SH-SY5Y cells. 3 Biotech. 2020;10:424. doi: 10.1007/s13205-020-02421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Brown RH, Cleveland DW. Decoding ALS: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D, Stankeiwicz AM, Mocan A, Sah AN, Tzvetkov NT, Huminiecki L, Horbanczuk JO, Atanasov AG. Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs. Front Aging Neurosci. 2018;10:3. doi: 10.3389/fnagi.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velander P, Wu L, Henderson F, Zhang S, Bevan DR, Xu B. Natural product-based amyloid inhibitors. Biochem Pharmacol. 2017;139:40–55. doi: 10.1016/j.bcp.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-Q, Sun X-B, Xu Y-X, Zhao H, Zhu Q-Y, Zhu C-Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010;1360:159–167. doi: 10.1016/j.brainres.2010.08.100. [DOI] [PubMed] [Google Scholar]
- Wu C, Lei H, Wang Z, Zhang W, Duan Y. Phenol red interacts with the protofibril-like oligomers of an amyloidogenic hexapeptide NFGAIL through both hydrophobic and aromatic contacts. Biophys J. 2006;91:3664–3672. doi: 10.1529/biophysj.106.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-L, Tang G-H, Guo Y-Q, Xu Y-K, Huang Z-S, Yin S. Mulberry Diels-Alder type adducts from Morus alba as multi-targeted agents for Alzheimer’s disease. Phytochem. 2019;157:82–91. doi: 10.1016/j.phytochem.2018.10.028. [DOI] [PubMed] [Google Scholar]
- Xian Y-F, Lin Z-X, Zhao M, Mao Q-Q, Ip S-P, Che C-T. Uncaria rhynchophylla ameliorates cognitive deficits induced by D-galactose in mice. Planta Med. 2011;77:1977–1983. doi: 10.1055/s-0031-1280125. [DOI] [PubMed] [Google Scholar]
- Yu H-Y, Chen Z-Y, Sun B, Liu J, Meng F-Y, Liu Y, Tian T, Jin A, Ruan H-L. Lignans from the fruit of Schisandra glaucescens with antioxidant and neuroprotective properties. J Nat Prod. 2014;77:1311–1320. doi: 10.1021/np4010536. [DOI] [PubMed] [Google Scholar]
- Yu H, Yao L, Zhou H, Qu S, Zeng X, Zhou D, Zhou Y, Li X, Liu Z. Neuroprotection against Aβ25-35-induced apoptosis by Salvia miltiorrhiza extracts in SH-SY5Y cells. Neurochem Int. 2014;75:89–95. doi: 10.1016/j.neuint.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Yu B, Yu D-Q, Liu H-M. Spirooxindoles: promising scaffolds for anticancer agents. Eur J Med Chem. 2015;97:673–698. doi: 10.1016/j.ejmech.2014.06.056. [DOI] [PubMed] [Google Scholar]
- Zeng K, Ko H, Yang H, Wang X. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology. 2010;59:542–550. doi: 10.1016/j.neuropharm.2010.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.