Highlights
-
•
Radiation is not typical in the standard of care for cardiac metastases.
-
•
MR-guided radiation uses real-time imaging and offers better soft tissue contrast.
-
•
Real-time MR-guidance allows for safe high dose radiation to cardiac metastases.
-
•
MR-guided stereotactic radiation can improve symptoms without acute toxicity.
Keywords: Cardiac metastasis, MR-guidance, SBRT, Real-time image guidance
Abstract
Aims
To assess the safety and efficacy of MR-guided stereotactic body radiation therapy (MRgSBRT) for cardiac metastases.
Materials/methods
This single institution retrospective analysis evaluated our experience with MRgSBRT for cardiac metastases. Response rate was compared between pre-RT and post-RT imaging. Symptomatic changes were also tracked and documented.
Results
Between 4/2019 and 3/2020, five patients with cardiac metastases (4 intracardiac and 1 pericardial) were treated with MRgSBRT. Median age at treatment was 73 years (range 64–80) and two patients had pre-existing cardiac disease. Histologies included melanoma and breast adenocarcinoma. Median lesion diameter was 2 cm (range 1.96–5.8 cm). Three patients were symptomatic, one of whom had pulmonary hypertension and RV enlargement. Another patient had an asymptomatic arrythmia. Median PTV prescribed dose was 40 Gy (range 40–50 Gy) and delivered in five fractions on nonconsecutive days. Median PTV volume was 53.4 cc (range 8.7–116.6 cc) and median coverage was 95% (range 84.1–100%). A uniform 3 mm margin was used for real-time gating, allowing a median 7% (range 5–10%) pixel excursion tolerance. Median follow-up was 4.7 months (range 0.9–12.3). Two patients exhibited stable disease, two had a partial response and one exhibited a complete response. All symptomatic patients experienced some relief. There were no acute adverse events, however, one patient without prior cardiac disease developed atrial fibrillation 6 months after treatment. Two patients died of causes unrelated to cardiac MRgSBRT.
Conclusion
In this largest known series of cardiac metastasis MRgSBRT, real-time image guidance enables safe treatment resulting in good response with improving presenting symptoms without acute adverse events.
1. Introduction
The heart and pericardial tissue are rare sites of malignancy. The estimated incidence of primary cardiac tumors ranges from 0.0017% to 0.33%, while the incidence of cardiac metastases may be 20–100 times more common [1], [2]. Although many histologies contribute to cardiac metastases, melanoma appears to have the greatest affinity for the heart [1]. Increasing utilization of non-invasive imaging techniques, including transthoracic echocardiography (TTE) and cardiac magnetic resonance imaging (MRI), has increased the apparent incidence of these metastases [3]. With advances in systemic therapies (particularly in melanoma), survival in the metastatic setting is improving [4], leading to increased incidence of rare metastatic sites, including the heart [5], [6].
Although radiation therapy has been used for palliation of these masses for decades [7], surgical excision has historically been one of the only definitive management options [8]. With newer advances in radiotherapy, the delivery of higher and more definitive doses of radiation to these metastases using stereotactic body radiation therapy (SBRT) has become more feasible [9]. At the same time, the emergence of MR-guided radiation therapy has allowed for real time, dynamic image guidance with the superior soft tissue delineation of MRI. This capability to clearly identify daily anatomic changes as well as to track and account for patient cardiac/respiratory motion with real-time imaging and beam gating enables extremely precise delivery of conformal high dose radiotherapy to mobile targets like cardiac metastases [10]. Here, we report a series of patients with cardiac metastases treated at our institution with MR-guided stereotactic body radiation therapy (MRgSBRT).
2. Materials/methods
This single institution retrospective study was approved by the H. Lee Moffitt Cancer Center Institutional Review Board. Sequentially treated patients with imaging-confirmed cardiac and pericardial metastases were included. Five consecutively treated patients were identified and the presence of cardiac or pericardial metastases were confirmed with cardiac MRI or TTE. Patients underwent MR simulation on the 0.35 T MR-linac (ViewRay, Inc., Mountain View, CA) and a balanced steady-state free progression (TrueFISP) imaging sequence was used to create images weighted by T2/T1 ratio. Patients were simulated with both arms up and without additional immobilization devices using both a deep inspiratory breath hold (DIBH) and free breathing techniques, evaluating breath hold reproducibility and tolerance. A representative slice of the lesion was contoured as a tracking structure on a real-time single-plane sagittal cine MRI sequence at 4 frames per second and a 3 mm gating structure was created as previously described by our group [11]. A CT simulation was then completed in the same position for electron density data. Patients were treated free breathing on non-consecutive days with real-time respiratory-gated cine MRI. A uniform 3 mm GTV to PTV expansion was used in all cases. Adaptive radiotherapy was not used in any of the cases. Pre- and post-treatment electrocardiograms (EKGs) were analyzed, where available.
3. Results
Between April 2019 and March 2020, five consecutive patients with cardiac metastases were treated with MRgSBRT in five fractions on non-consecutive days. Four patients had intracardiac metastases and one patient had a pericardial metastasis (Fig. 1). Median age at treatment was 73.8 years (range 64.3–80.1 years) and histologies included uveal melanoma, desmoplastic melanoma, breast intraductal carcinoma, and cutaneous melanoma. One patient was metastatic on diagnosis and two patients had cardiac metastases as their first site of recurrence after a median time interval of 35.0 months following initial diagnosis (range 1.8–84.4 months) Two patients had pre-existing cardiac disease (one with congestive heart failure and one with paroxysmal atrial fibrillation and sick sinus syndrome, requiring placement of a permanent pacemaker (Patient 5)). We have developed specific guidelines regarding MRgRT for patients with pacemakers based on dose to the pacemaker and whether the patient is pacemaker dependent. Three patients presented with dyspnea on exertion and two patients were asymptomatic (Table 1). One patient was on concurrent ipilimumab/nivolumab.
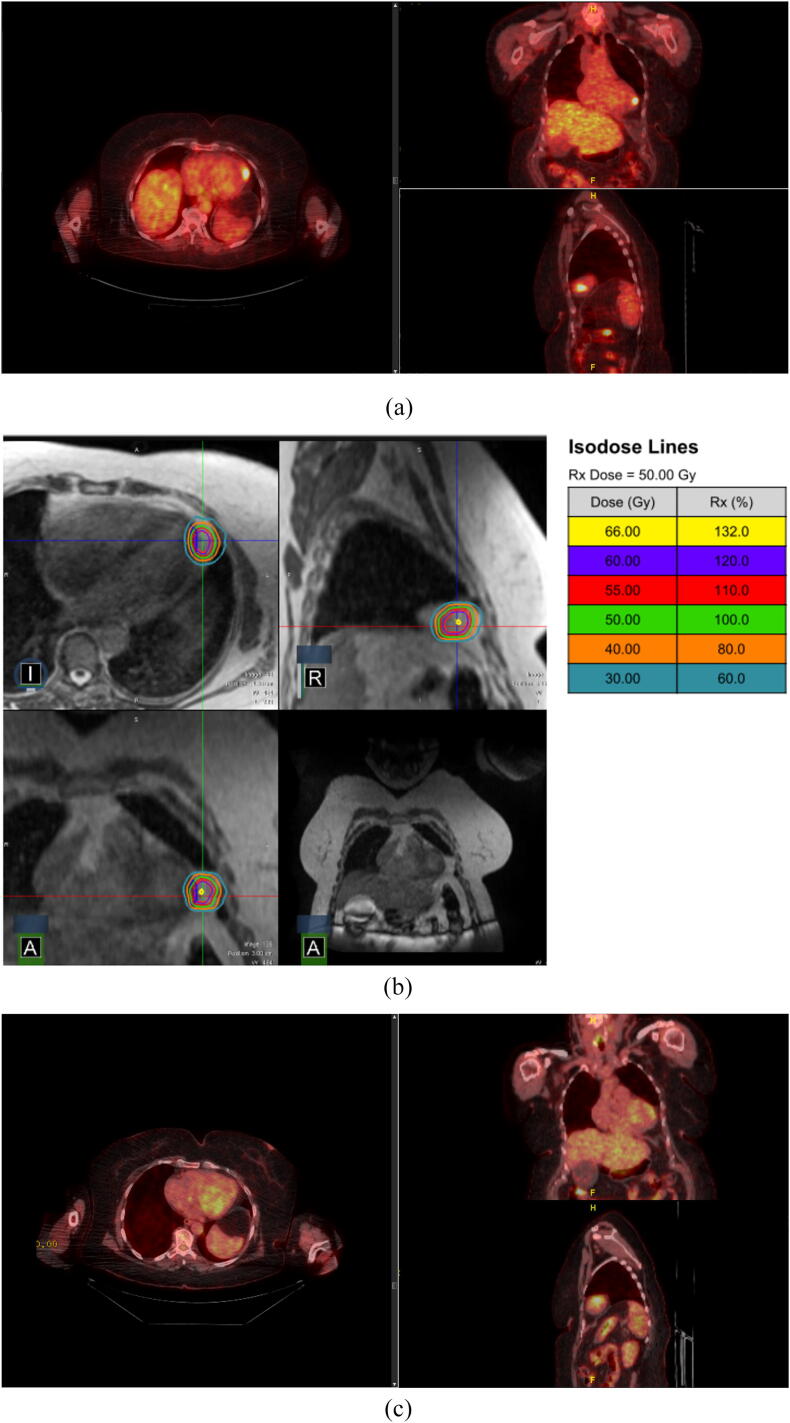
Fig. 1.
Pericardial Metastasis. Patient with a LV pericardial metastasis treated with an SIB approach (60 Gy to the GTV, 50 Gy to the PTV). (a) pre-treatment PET/CT, (b) RT plan, (c) post-treatment PET/CT.
Table 1.
Patient Characteristics.
| Age | Karnofsky Performance Status | Histology | Location | Pre-treatment signs | Pre-treatment symptoms | Concurrent systemic treatment | Post-SBRT systemic treatment | Post-treatment signs | Post-treatment symptoms | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 80.9 | 80 | Uveal melanoma | L atrium | None | DOE, palpitations | None | Pembrolizumab | None | Improved DOE, palpitations |
| Patient 2 | 64.3 | 80 | Cutaneous melanoma | L ventricle pericardium | None | None | None | Encorafenib, binimetinib | Paroxysmal atrial fibrillation | None |
| Patient 3 | 70.5 | 80 | Breast intraductal carcinoma | R ventricle | Pulmonary hypertension, RV enlargement | DOE, palpitations, chest discomfort | None | Eribulin | Reduced pulmonary hypertension, RV size | Improved palpitations, chest discomfort. Worsened DOE |
| Patient 4 | 73.8 | 90 | Desmoplastic melanoma | L atrium | Prolonged QTc | None | None | None | Reduced QTc | Fatigue |
| Patient 5 | 79.3 | 70 | Cutaneous melanoma | R atrium | Paroxysmal atrial fibrillation | DOE | Ipilimumab, nivolumab | Nivolumab | * | Fatigue, improved DOE |
RV: right ventricle, QTc: corrected QT interval, DOE: dyspnea on exertion.
Confirmatory pre-treatment imaging consisted of cardiac MRI (n = 4) and TTE (n = 2). Median size of the lesions was 2.0 cm in maximum diameter (range 1.6–5.8 cm) with a median GTV volume of 31.2 cc (2.6–41.2 cc) and median PTV volume of 53.4 cc (8.7–116.6 cc). Median dose to the PTV was 40 Gy (range 40–60 Gy) delivered in 5 fractions with a median of 95% coverage with the prescription dose (84.1–100%); three patients were treated with a simultaneous integrated boost (SIB) approach (Table 2). Patients were treated while free breathing and a uniform 3 mm margin was used for real-time gating, allowing a median 7% (range 5–10%) pixel excursion tolerance for gating.
Table 2.
Lesion Characteristics.
| Pre-Treatment Size (cm) | GTV Volume (cc) | GTV Rx Dose (Gy) | Minimum GTV dose (Gy) | Maximum GTV dose (Gy) | PTV Volume (cc) | PTV Rx Dose (Gy) | Minimum PTV dose (Gy) | Maximum PTV dose (Gy) | PTV Rx coverage (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 2.0 | 8.7 | 40 | 39.9 | 46.9 | 32.4 | 40 | 27.2 | 46.9 | 85.3 |
| Patient 2 | 2.0 | 2.6 | 60 | 57.0 | 66.7 | 8.7 | 50 | 44.1 | 66.7 | 84.1 |
| Patient 3 | 1.6 | 31.2 | 40 | 42.2 | 61.5 | 53.4 | 40 | 30.0 | 61.5 | 95.0 |
| Patient 4 | 5.8 | 87.1 | 50 | 37.8 | 60.7 | 116.6 | 40 | 31.2 | 60.7 | 96.1 |
| Patient 5 | 3.8 | 41.2 | 50 | 49.9 | 69.0 | 57.8 | 40 | 40.5 | 69.0 | 100.0 |
L: left, R: right, GTV: gross tumor volume, Rx: prescription, PTV: planning target volume.
On post-treatment imaging obtained after a median of 1.5 months (0.1–3.6), three patients experienced a response (one complete response (CR), two partial responses (PR)), while two patients had stable disease (SD). Three patients had post-treatment EKGs; one with no changes, one with new atrial fibrillation, and the last with improvement in QTc prolongation. Two previously symptomatic patients experienced improvement in their dyspnea and palpitations, one patient experienced worsening dyspnea, and two patients developed mild fatigue with no other acute adverse effects. The patient with pulmonary hypertension and RV enlargement demonstrated improvement on post-treatment TTE (Fig. 2). With a median follow-up of 4.7 months, two patients passed away, both unrelated to treatment. One experienced progression of disease with new liver metastases and another developed acute hypoxic respiratory failure secondary to Pneumocystis jiroveci pneumonia and pulmonary emboli (left atrial tumor).
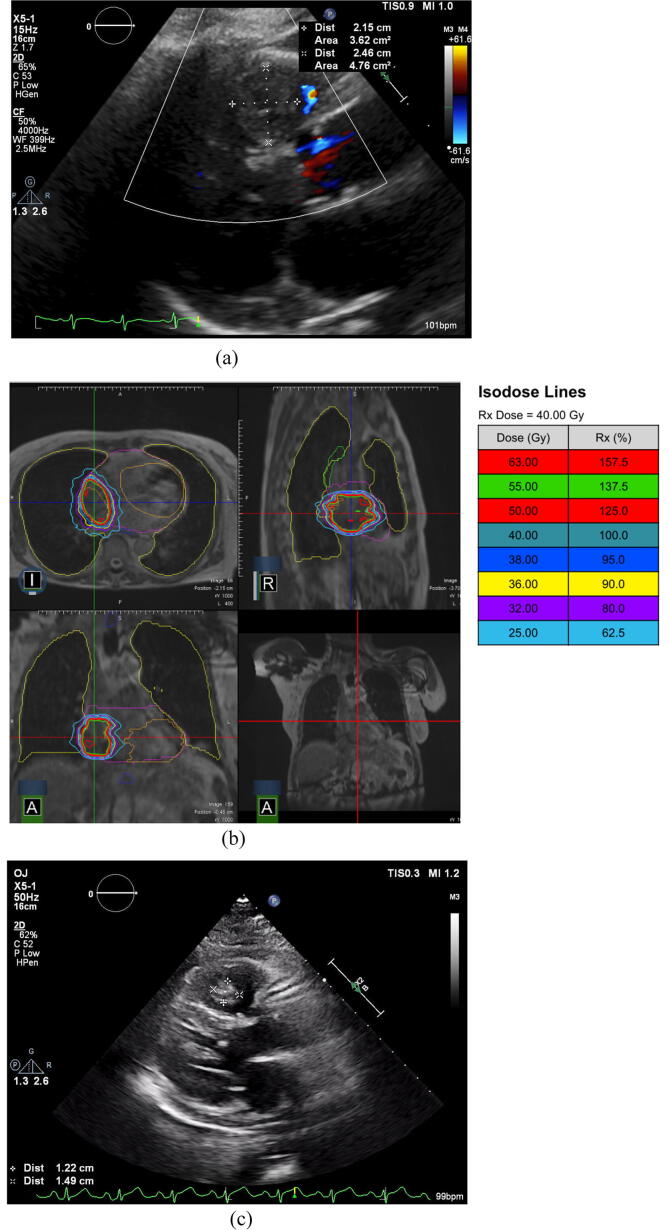
Fig. 2.
Right Ventricular Metastasis. Patient with a RV intracardiac metastasis treated to 40 Gy in 5 Fx. (a) pre-treatment TTE, (b) RT plan, (c) post-treatment TTE.
4. Discussion
In this single-institutional retrospective case series, we present, to the authors’ knowledge, the first and largest series of patients with cardiac metastases treated with MRgSBRT. Although cardiac metastases were thought to be quite rare, the combination of advances in systemic therapies and increased utilization of advanced imaging has increased the detection of these metastases [3]. Historically, surgery has been the only definitive treatment for these metastases, and even then, only in select cases [8]. Radiotherapy in this setting has historically been only for palliative intent [7]. However, recent advances in stereotactic radiotherapy and image guidance has opened the door for definitive and ablative radiotherapy [9].
Consistent with prior epidemiological data, our patients were typically in their seventh and eighth decade of life and many had melanomas [1]. Dyspnea appears to be a common presenting symptom [7], [12], but accompanying signs and symptoms appear to be highly dependent on anatomic location. Unlike previous series and cases, two patients had pre-existing cardiac disease and one patient had a permanent pacemaker. Data are lacking on cardiac SBRT in patients with pre-existing cardiac conditions. Arscott et al. [13] reported a case of a patient with metastatic urothelial carcinoma to the heart who presented in heart block because of a right ventricular metastasis and developed myocarditis and dyssynchrony requiring placement of a biventricular pacemaker, but this patient did not receive SBRT.
Treatment of these lesions with standard linear accelerators require the construction of large internal target volumes (ITVs) to account for the significant motion during the cardiac cycle, leading to a significant amount of normal tissue in the radiation field [9]. Significant doses of radiation to the heart is a well-known cause of cardiotoxicity [14] and trials of SBRT to central and ultracentral lung cancers have demonstrated a relatively high level of acute toxicities, limiting dose to central targets [15], [16]. In contrast, the largest series of cardiac and pericardial SBRT using a standard linac from Bonomo et al. did not note any development of acute toxicities or electrocardiographic changes; however only one case in this series was intracardiac [9]. In other series, the CyberKnife (Accuray, Sunnyvaile, CA) platform did allow for real-time tracking, leading to smaller target volumes, but required the invasive implantation of fiducials in many cases and did not allow real-time image guided tracking of the anatomy itself [12], [17]. Ablative radiation has also been used successfully to treat noninvasive ventricular tachycardia with no changes in left ventricular function [18].
The use of MR-guidance solves many of these problems, allowing for better soft tissue delineation and real-time gating with cine MRI without additional radiation exposure [19], [20]. As a result, we were able to harness this technology to escalate dose beyond what was previously possible [12], [17], [21], [22]. Even for lesions in excess of 100 cc in volume, patients received dose of at least 40 Gy in five fractions with minimal sequelae. One patient was also treated with concurrent dual immune checkpoint inhibitors (ICI) without evidence of increased acute toxicity. Although data are lacking with concurrent ICI use for cardiac SBRT, a report by Gabani et al. treated a primary cardiac angiosarcoma with concurrent paclitaxel and SBRT to 30 Gy in five fractions and did only note mild esophagitis acutely [21]. However, other data combining ICI with lung SBRT have noted increased rates of pneumonitis [23], requiring diligence with dose constraints.
Based on this series, MRgSBRT appears to be a good option for patients with cardiac metastases. However, limitations of this study include a small sample size, descriptive analyses, and short follow-up interval.
5. Conclusion
Overall, we demonstrate the safety and feasibility of high dose MRgSBRT for intracardiac lesions by taking advantage of real-time respiratory/cardiac motion-gated cine MRI. As many cardiac metastases either present asymptomatically or with non-specific symptoms, high clinical suspicion is required. Limitations of this study include a small sample size, descriptive analyses, and a short follow-up interval. Larger retrospective series are needed prior to prospective optimization of dose and fractionation to treat a variety of histologies.
Funding
No funding was procured or used in the preparation of this manuscript.
Clinical trail information
N/A.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
Part of this work was presented at the American Society for Radiation Oncology Annual Meeting 10/25/2020–10/28/2020
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2020.10.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Video of cine MRI during treatment.
References
- 1.Hudzik B., Miszalski-Jamka K., Glowacki J. Malignant tumors of the heart. Cancer Epidemiol. 2015;39(5):665–672. doi: 10.1016/j.canep.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Reardon M.J., Walkes J.C., Benjamin R. Therapy insight: malignant primary cardiac tumors. Nat Clin Pract Cardiovasc Med. 2006;3(10):548–553. doi: 10.1038/ncpcardio0653. [DOI] [PubMed] [Google Scholar]
- 3.Palaskas N., Thompson K., Gladish G. Evaluation and management of cardiac tumors. Curr Treat Options Cardiovasc Med. 2018;20(4):29. doi: 10.1007/s11936-018-0625-z. [DOI] [PubMed] [Google Scholar]
- 4.Wolchok J.D., Chiarion-Sileni V., Gonzalez R. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg A.D., Blankstein R., Padera R.F. Tumors metastatic to the heart. Circulation. 2013;128(16):1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.H.J., Lyle M., Menzies A.M. Metastasis-specific patterns of response and progression with anti-PD-1 treatment in metastatic melanoma. Pigment Cell Melanoma Res. 2018;31(3):404–410. doi: 10.1111/pcmr.12675. [DOI] [PubMed] [Google Scholar]
- 7.Cham W.C., Freiman A.H., Carstens P.H. Radiation therapy of cardiac and pericardial metastases. Radiology. 1975;114(3):701–704. doi: 10.1148/114.3.701. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MC, Sweeney MS, Putnam JB, Jr., et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49(4):612-7; discussion 7-8. [DOI] [PubMed]
- 9.Bonomo P., Livi L., Rampini A. Stereotactic body radiotherapy for cardiac and paracardiac metastases: University of Florence experience. Radiol Med. 2013;118(6):1055–1065. doi: 10.1007/s11547-013-0932-0. [DOI] [PubMed] [Google Scholar]
- 10.Mutic S., Dempsey J.F. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Sem Radiat Oncol. 2014;24(3):196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg S.A., Henke L.E., Shaverdian N. A multi-institutional experience of MR-Guided liver stereotactic body radaiation therapy. Adv Radiat Oncol. 2018:1–8. doi: 10.1016/j.adro.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltys S.G., Kalani M.Y., Cheshier S.H. Stereotactic radiosurgery for a cardiac sarcoma: a case report. Technol Cancer Res Treat. 2008;7(5):363–368. doi: 10.1177/153303460800700502. [DOI] [PubMed] [Google Scholar]
- 13.Arscott W.T., Lal P., Mamtani R. Long-term survival after treating cardiac metastasis with radiation and immune therapy: a case report. Cureus. 2018;10(5) doi: 10.7759/cureus.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver J.R., Shapiro C.L., Ng A. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 15.Bezjak A., Paulus R., Gaspar L.E. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non–small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 2019;37(15):1316–1325. doi: 10.1200/JCO.18.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekatli H., Haasbeek N., Dahele M. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11(7):1081–1089. doi: 10.1016/j.jtho.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Bonomo P., Cipressi S., Desideri I. Stereotactic body radiotherapy with CyberKnife for cardiac malignancies. Tumori. 2015;101(3):294–297. doi: 10.5301/tj.5000280. [DOI] [PubMed] [Google Scholar]
- 18.Cuculich P.S., Schill M.R., Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henke L.E., Contreras J.A., Green O.L. Magnetic resonance image-guided radiotherapy (MRIgRT): a 4.5-year clinical experience. Clin Oncol (Royal College of Radiologists (Great Britain)) 2018;30(11):720–727. doi: 10.1016/j.clon.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittauer K., Paliwal B., Hill P. A new era of image guidance with magnetic resonance-guided radiation therapy for abdominal and thoracic malignancies. Cureus. 2018;10(4) doi: 10.7759/cureus.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabani P., Fischer-Valuck B.W., Robinson C.G. Stereotactic body radiation therapy for the treatment of primary cardiac angiosarcoma causing hemodynamic instability. Pract Radiation Oncol. 2019;9(1):5–8. doi: 10.1016/j.prro.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Jumeau R., Vincenti M.G., Pruvot E. Curative management of a cardiac metastasis from lung cancer revealed by an electrical storm. Clin Translat Radiat Oncol. 2020;21:62–65. doi: 10.1016/j.ctro.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian S., Switchenko J.M., Buchwald Z.S. Lung stereotactic body radiation therapy and concurrent immunotherapy: a multicenter safety and toxicity analysis. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2019.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of cine MRI during treatment.