Highlights
-
•
Patients with gynecologic malignancies perceive medical cannabis relieves multiple cancer-related symptoms.
-
•
Medical cannabis is well-tolerated and perceived to have a favorable side effect profile.
-
•
Patients using medical cannabis for pain control report an associated reduction in opioid use.
Keywords: Medical cannabis, Pain, Neuropathy, Opioid reduction
Abstract
Research within a gynecologic oncology population has lagged behind the uptake in use of medical cannabis for symptom control. This study seeks to evaluate patient experience with prescribed medical cannabis obtained through licensed dispensaries in women with gynecologic malignancies.
A 43-item survey exploring patient experience with medical cannabis was administered to women with gynecologic malignancies who used medical cannabis prescribed by a gynecologic oncologist.
Thirty-six eligible patients were approached for consent, and 31 patients returned completed surveys (86%). Ninety-three percent had advanced or recurrent disease; 74% were receiving chemotherapy or immunotherapy. Eighty-three percent reported medical cannabis provided relief from cancer or treatment-related symptoms including decreased appetite (41%), insomnia (41%), neuropathy (41%), anxiety (35%), nausea (29%), joint pain (29%), bone pain (29%), abdominal pain (25%), and depression (19%). Eighty percent of patients reported medical cannabis worked the same or better than other traditional medications for management of their cancer or treatment-related symptoms, and 83% reported medical cannabis had an equivalent or better side effect profile. Of the subset of patients using medical cannabis for pain, 63% reported a reduction in opioid use.
Patients perceive that medical cannabis was useful for relief of cancer and treatment-related symptoms, suggesting medical cannabis may be a reasonable alternative or adjunct therapy. Medical cannabis was well tolerated and may have the potential to improve neuropathic pain and decrease opioid use.
1. Introduction
In 1996, California became the first state to permit the medical use of cannabis. While cannabis remains illegal and a Schedule 1 substance under federal law by the Drug Enforcement Administration classification system, 33 states have approved medical cannabis programs as of March 2020 (National Conference of State Legislatures, 2019). Several survey-based studies have been conducted regarding the clinical use of cannabis in general cancer or non-cancer populations (Pergam et al., 2017, Saadeh and Rustem, 2018, Singh et al., 2019, Zarrabi et al., 2020). With the expansion of medical cannabis across the United States, it is critical to identify whether patients with gynecologic malignancies may benefit from its use.
To our knowledge, the only existing literature specific to cannabis in gynecologic oncology patients is a survey-based study exploring the use of non-prescription cannabis products in gynecologic oncology patients (Blake et al., 2019). In the survey, approximately one third of the patients surveyed were using cannabis. None of the patients using cannabis were prescribed the therapy, and some patients reported their provider was not aware of their cannabis use. The study did not seek to assess efficacy of cannabis in symptom management or offer data specific to the limited subset of patients using cannabis obtained from medical dispensaries.
As noted by Whitcomb et al. (2020) in the Society of Gynecologic Oncology clinical practice statement, there exist barriers to conducting medical cannabis research and a paucity of literature regarding the indication, use, and effects of medical cannabis in the gynecologic oncology population (Whitcomb et al., 2020). Further research on medical cannabis is needed to guide gynecologic oncologists in their clinical practice. Our study seeks to explore the experience of women with gynecologic malignancies who were prescribed medical cannabis by a gynecologic oncologist.
2. Methods
2.1. Enrollment
We performed a single-institution survey-based study to evaluate patient experience with medical cannabis in a state with sanctioned medical cannabis dispensaries. Patients were eligible if they had a gynecologic malignancy, were at least 18 years old, were able to read and understand English, received a prescription (certification) for medical cannabis by a gynecologic oncology provider, and used medical cannabis obtained through a licensed dispensary.
To obtain a medical cannabis license in Connecticut, a physician with the privilege to certify patients must initiate the application after deeming that a patient is qualified for the state Medical Marijuana Program. To complete an application, patients must have internet access, an email address, a Connecticut mailing address, and proof of identity. Applications require a $100 registration fee. While the application does not specify a patient must be English-speaking, many of the application materials are not available in other languages.
Eligible patients were identified through a list maintained by the Connecticut State Medical Marijuana Program of patients for whom a medical cannabis license application had been submitted. Patients were approached in the inpatient and outpatient setting, and participation in the study was voluntary. All participants provided informed consent. This study was approved by the Yale University Institutional Review Board.
2.2. Study intervention
We developed a 43-question survey exploring patient experience with medical cannabis. Development of the survey was informed by gynecologic oncology providers experienced in patient use of medical cannabis and review of existing medical cannabis literature. The survey included validated items from the U.S. Department of Health and Human Services' Centers for Disease Control and Prevention (CDC) Healthy Days Measures (Moriarty et al., 2003). Novel items underwent an iterative review process by multiple providers.
The survey was administered in paper format and distributed to patients between September 2018 and December 2019. During the study period, medical cannabis was legal and recreational cannabis was partially decriminalized under Connecticut State law. Quantitative data analysis was performed using descriptive statistics.
3. Results
3.1. Enrolled patients
Between September 2018 and December 2019, 36 eligible patients were approached for consent, 34 patients provided consent, and 31 patients returned completed surveys (86%). Surveys were considered complete if over 85% of the survey items were answered. Out of 43 items total, surveys had a mean of 42 items answered (range, 38–43 items).
The demographics and characteristics of enrolled patients are summarized in Table 1. The median age of participants was 63 years (range, 24–75). Seventy-four percent (n = 23) of patients had ovarian cancer, 22% (n = 7) had uterine/endometrial cancer, and 3% (n = 1) had cervical cancer. Ninety-three percent (n = 29) had advanced stage (stage III or IV) or recurrent disease. Seventy-four percent (n = 23) were receiving active treatment with chemotherapy or immunotherapy at the time of survey, and 25% (n = 8) were not on active treatment. Of the patients on active treatment, approximately one third (35%; n = 11) were on first-line treatment, 38% (n = 12) were on second- or third-line, and 25% (n = 8) were on fourth-line or more.
Table 1.
Patient characteristics.
| Age, median (range), years | 63 (24–75) |
|---|---|
Race, n (%)
|
27 (87) 3 (9) 1 (3) |
Education, n (%)
|
1 (3) 8 (25) 14 (45) 8 (25) |
Cancer type, n (%)
|
23 (74) 7 (22) 1 (3) |
Disease stage, n (%)
|
6 (19) 2 (6) 14 (45) 9 (29) |
Regimen, n (%)
|
11 (35) 12 (38) 8 (25) |
ECOG, n (%)
|
12 (38) 12 (38) 6 (19) 1 (3) |
Treatment status, n (%)
|
23 (74) 8 (25) |
Disease status, n (%)
|
12 (38) 19 (61) |
Seventy-seven percent (n = 24) of patients had an ECOG performance status of 0 or 1, 19% (n = 6) had an ECOG of 2, and 3% (n = 1) had an ECOG of 3. None of the patients were receiving hospice care. Responses to the CDC Healthy Day Measures indicated 19% (n = 6) of patients rated their health as excellent, 41% (n = 13) as very good, 25% (n = 8) as good, and 12% (n = 4) as fair or poor.
Forty-one percent (n = 13) of patients reported having never tried cannabis recreationally, and 35% (n = 11) reported having only tried cannabis recreationally in the past. Twenty-two percent (n = 7) reported having used recreational cannabis at least yearly, with 9% (n = 3) reporting prior daily use of recreational cannabis. Forty-five percent (n = 14) reported occasional alcohol use, and all patients denied tobacco or illicit substance use.
3.2. Patterns of medical cannabis use
Forty-one percent (n = 13) of patients had used medical cannabis for greater than 6 months, 16% (n = 5) between 3 and 6 months, and 41% (n = 13) less than 3 months. Thirty-two percent (n = 10) reported using medical cannabis 6–7 days/week, 32% (n = 10) 3–5 days/week, and 29% (n = 9) 1–2 days/week or less.
Regarding change in use over time, 38% (n = 12) reported stable usage, 25% (n = 8) reported a change in amount depending on symptom burden and current condition, and 29% (n = 9) reported inability to assess due to short duration of medical cannabis use. One patient (3%) reported she required “a little more” compared to when she initiated medical cannabis therapy, and no patients reported needing “much more.”
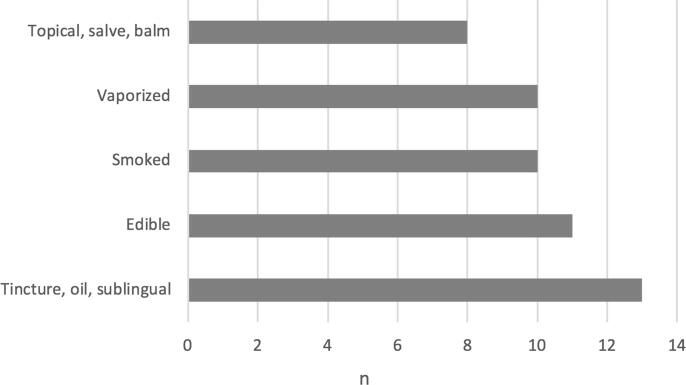
Regarding the method of therapy administration, 35% (n = 11) endorsed using more than one form of medical cannabis. Forty-one percent (n = 13) used tincture, oil, or sublingual cannabis, 35% (n = 11) used edible cannabis, 32% (n = 10) used smoked cannabis, 32% (n = 10) used vaporized cannabis, and 25% (n = 8) used topical, salve, or balm (Fig. 1). Forty-five percent (n = 14) did not use an inhaled form of medical cannabis.
Fig. 1.
Forms of medical cannabis utilized.
3.3. Perceived effect of cannabis
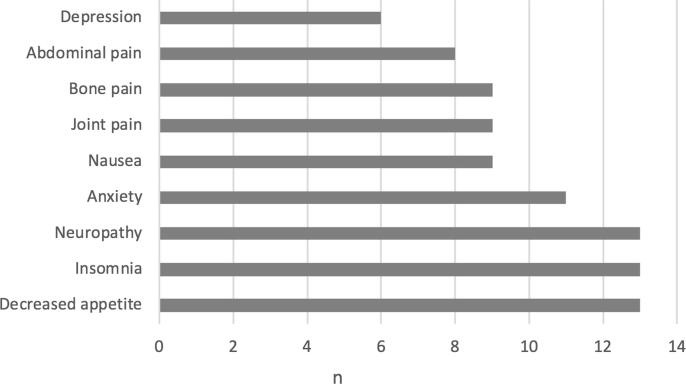
Regarding the reason for medical cannabis use, 77% (n = 24) reported using medical cannabis for management of more than one symptom. The most common reasons for use were decreased appetite (41%; n = 13), insomnia (41%; n = 13), neuropathy (41%; n = 13), anxiety (35%; n = 11), nausea (29%; n = 9), joint pain (29%; n = 9), bone pain (29%; n = 9), abdominal pain (25%; n = 8), and depression (19%; n = 6) (Fig. 2). In the subset of patients using medical cannabis while off active cancer treatment (25%; n = 8), the most common reasons for use were pain (62%; n = 5) and neuropathy (50%; n = 4).
Fig. 2.
Patient-reported symptoms addressed by medical cannabis.
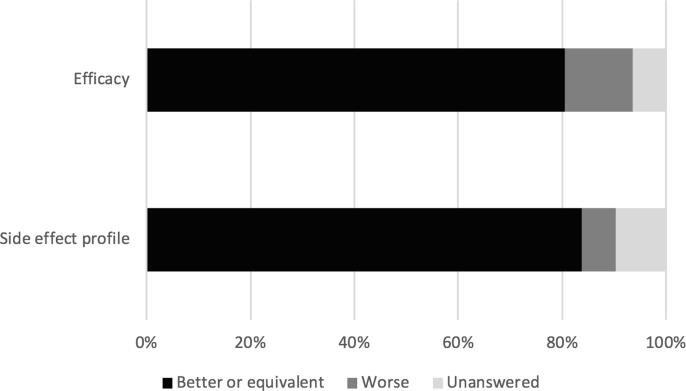
Eighty-three percent (n = 26) indicated that medical cannabis provided relief of their symptoms. Compared to other medications used for relief of cancer or treatment-related symptoms (e.g., opioids, antiemetics, anxiolytics, and sleep aids), 80% (n = 25) felt that medical cannabis worked the same or better (Fig. 3). Nine percent (n = 3) felt that medical cannabis was the only medication that could provide relief of their symptom(s). Sixty-one percent (n = 19) of patients reported using medical cannabis for pain control. Of this patient subset, the majority (63%; n = 12) reported that medical cannabis reduced their use of opioids.
Fig. 3.
Patient perception of medical cannabis compared to other medications for relief of cancer and treatment-related symptoms.
The most common side effects were dry mouth (22%; n = 7), gastrointestinal irritation (16%; n = 5), constipation (16%; n = 5), lethargy (16%; n = 5), palpitations (12%; n = 4), sweating (12%; n = 4), paranoia (12%; n = 4), and increased appetite (12%; n = 4). Eighty-three percent (n = 26) felt that the side effect profile was the same as or better than that of other medications (Fig. 3), with 64% (n = 20) reporting they experienced no adverse side effects from medical cannabis.
4. Discussion
In this survey-based study, gynecologic oncology patients prescribed medical cannabis completed a 43-item questionnaire addressing their experiences with medical cannabis. We demonstrate that patients perceived medical cannabis to be effective in relieving cancer and treatment-related symptoms. Patients reported improvement in a variety of symptoms including pain, neuropathy, nausea, insomnia, decreased appetite, and anxiety. The majority of patients in our study felt that medical cannabis was equivalent or superior in efficacy to other medications (e.g., opioids, antiemetics, anxiolytics, and sleep aids) in relieving their symptoms. Most patients felt the side effect profile was equal to or less than that of other medications used for symptom management. These data suggest medical cannabis may be a reasonable alternative or adjunct to medications frequently used for cancer or treatment-related symptoms.
The possibility that medical cannabis may be an effective therapy for neuropathy is notable given the use of taxanes as a chemotherapeutic cornerstone for ovarian and uterine malignancies. Chronic neuropathy associated with taxanes can be dose-limiting and negatively impact quality of life. An in vivo animal study demonstrated that cannabinoids reduced hyperalgesia in mice exposed to cisplatin (Harris et al., 2016), and randomized controlled trials in patients with HIV and diabetic neuropathy have also noted reduction of chronic neuropathic pain (Abrams and Guzman, 2015, Abrams et al., 2007, Wallace et al., 2015). Our study demonstrates a positive perceived effect of medical cannabis on neuropathic pain among patients both on and off active cancer treatment within a gynecologic oncology population.
The correlation between medical cannabis use and reduction in opioid use has been suggested in survey-based and epidemiologic studies. A study of individuals registered with a federally authorized Canadian cannabis producer found that over half of participants using medical cannabis as a substitute for prescribed opioids reported achieving complete cessation of opioid use (Lucas et al., 2019). Similarly, a study of California residents noted that concurrent opioid users felt medical cannabis reduced their opioid use (Reiman et al., 2017). An epidemiologic study demonstrated that states with medical cannabis programs had a decreased rate of opioid overdose mortality compared to states without such programs (Bachhuber et al., 2014). Our study demonstrates that patients with gynecologic malignancies who were prescribed medical cannabis for their malignancy symptoms reported a reduction in opioid use secondary to medical cannabis use.
Regarding the potential for misuse with medical cannabis, patients did not report needing increasing amounts of medical cannabis to achieve the same symptomatic relief, and none of the patients reported concurrent illicit drug use. Many patients denied having used cannabis recreationally in the past. These data suggest that medical cannabis was not being used for recreational purposes, nor were patients developing tolerance or abusive habits.
This single-institution survey-based study is limited by small sample size as well as lack of a formally validated survey. However, the use of a survey partially or entirely composed of author-designed items is similar to other literature exploring patient experience with medical cannabis (Saadeh and Rustem, 2018, Zarrabi et al., 2020, Blake et al., 2019), as no formally-validated comprehensive measure is available. Further, given the use of medical cannabis obtained through dispensaries, no standardized form of medical cannabis was evaluated, which may hinder the ability of a provider to make specific recommendations. Additionally, the small sample size precludes meaningful comparison between different forms of medical cannabis. This study may be biased towards resource-rich patients who can navigate the complex medical cannabis certification process and who present to care more frequently.
This study takes an important step toward addressing the paucity of research on medical cannabis in the field of gynecology oncology. Given that a limited number of clinicians qualify to prescribe medical cannabis and only 33 states in the United States allow the use of medical cannabis, experience with medical cannabis is sparse and will continue in this way for the foreseeable future. Our study helps to advance the field as it is the first to highlight the experiences of patients prescribed medical cannabis by physicians in gynecologic oncology.
Our survey reflects patient-perceived effects of medical cannabis; a randomized controlled trial with a placebo arm is necessary to evaluate efficacy and validate patient perceptions. Additional areas of research may explore the ability of medical cannabis to mitigate chemotherapy-induced neuropathic pain and reduce opioid use, as well as better characterize adverse effects and identify potential for abuse.
Author Contributions
GA, GSY, GS, BM, JB, PES, and ESR were responsible for the conception and design of the study. GA, EMW, GSY, JT, BZ, CH, CKA, GM, GH, MA, DS, VA, ADS, PES, and ESR contributed significantly to acquisition of data. GA and EMW conducted data analysis and interpretation. GA and EMW drafted the manuscript. All authors contributed to manuscript revisions and provided final approval.
Declaration of Competing Interest
Gloria Huang serves on the advisory board for Tesaro/GSK and BMS/Pfizer Alliance and has received speaker’s honoraria from AstraZeneca. Alessandro D. Santin serves on the advisory board for Merck and Tesaro and reports grants from R-Pharma, Gilead, Genentech, Boheringer, Puma, and Immunomedics. All other authors declare no conflicts of interest.
References
- Abrams D.I., Guzman M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015;97:575–586. doi: 10.1002/cpt.108. [DOI] [PubMed] [Google Scholar]
- Abrams D.I., Jay C.A., Shade S.B., Vizoso H., Reda H., Press S. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Bachhuber M.A., Saloner B., Cunningham C.O., Barry C.L. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern. Med. 2014;174:1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake E.A., Ross M., Ihenacho U., Figueroa L., Silverstein E., Flink D. Non-prescription cannabis use for symptom management amongst women with gynecologic malignancies. Gynecol. Oncol. Rep. 2019;30 doi: 10.1016/j.gore.2019.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H.M., Sufka K.J., Gul W., ElSohly M.A. Effects of Delta-9-Tetrahydrocannabinol and Cannabidiol on Cisplatin-Induced Neuropathy in Mice. Planta Med. 2016;82:1169–1172. doi: 10.1055/s-0042-106303. [DOI] [PubMed] [Google Scholar]
- Lucas P., Baron E.P., Jikomes N. Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm. Reduct. J. 2019;16:9. doi: 10.1186/s12954-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty D.G., Zack M.M., Kobau R. The Centers for Disease Control and Prevention's Healthy Days Measures - population tracking of perceived physical and mental health over time. Health Qual. Life Outcomes. 2003;1:37. doi: 10.1186/1477-7525-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Conference of State Legislatures. State Medical Marijuana Laws. 2019. (Accessed 11 January, 2020, at https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.).
- Pergam, S.A., Woodfield, M.C., Lee, C.M., Cheng, G.S., Baker, K.K., Marquis, S.R., et al., 2017. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 123, 4488-4497. [DOI] [PMC free article] [PubMed]
- Reiman A., Welty M., Solomon P. Cannabis as a Substitute for Opioid-Based Pain Medication: Patient Self-Report. Cannabis Cannabinoid. Res. 2017;2:160–166. doi: 10.1089/can.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeh C.E., Rustem D.R. Medical Marijuana Use in a Community Cancer Center. J. Oncol. Pract. 2018;14:e566–e578. doi: 10.1200/JOP.18.00057. [DOI] [PubMed] [Google Scholar]
- Singh V., Zarrabi A.J., Curseen K.A., Sniecinski R., Welsh J.W., McKenzie-Brown A.M. Concerns of Patients With Cancer on Accessing Cannabis Products in a State With Restrictive Medical Marijuana Laws: A Survey Study. J. Oncol. Pract. 2019;15:531–538. doi: 10.1200/JOP.19.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M.S., Marcotte T.D., Umlauf A., Gouaux B., Atkinson J.H. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J Pain. 2015;16:616–627. doi: 10.1016/j.jpain.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb B., Lutman C., Pearl M., Medlin E., Prendergast E., Robison K. Use of cannabinoids in cancer patients: A Society of Gynecologic Oncology (SGO) clinical practice statement. Gynecol. Oncol. 2020;157:307–311. doi: 10.1016/j.ygyno.2019.12.013. [DOI] [PubMed] [Google Scholar]
- Zarrabi A.J., Welsh J.W., Sniecinski R., Curseen K., Gillespie T., Baer W. Perception of Benefits and Harms of Medical Cannabis among Seriously Ill Patients in an Outpatient Palliative Care Practice. J. Palliat. Med. 2020;23:558–562. doi: 10.1089/jpm.2019.0211. [DOI] [PubMed] [Google Scholar]