Abstract
Background
The subclavian artery is an alternative access route for transcatheter aortic valve implantation (TAVI), with a potential advantage in patients unsuitable for traditional access routes such as the femoral artery. This study aimed to determine the safety and efficacy of the trans-subclavian (TSc) compared to the trans-femoral (TF) approach.
Methods
A systematic review was conducted on two online databases: Embase and Medline. The initial search returned 508 titles. Nine observational studies were included: n = 2938 patients (2382 TF and 556 TSc).
Results
Both TSc and TF groups were comparable for: 30-day mortality (Odds ratio, OR 0.75, 95% CI 0.49 – 1.16, p = 0.195); in-hospital stroke (OR 1.05, 95% CI 0.60–1.85, p = 0.859); myocardial infarction (OR 1.97, 95% CI 0.74–5.23, p = 0.176); paravalvular leaks (OR 1.20, 95% CI 0.76–1.90, p = 0.439); rates of postoperative permanent pacemaker implantation (OR 1.49, 95% CI 0.92–2.41, p = 0.105); in-hospital bleeding and meta-analysis demonstrated no significant difference between access points (OR 3.44, 95% CI 0.35–34.22, p = 0.292). Procedural time was found to be longer in the TSc group (SMD 1.02; 95% CI 0.815–1.219, p < 0.001). Major vascular complications were significantly higher in the TF group (OR 0.55, 95% CI 0.32–0.94, p = 0.029). Meta regression found no influence of the covariates on the outcomes.
Conclusion
Subclavian access is both a safe and feasible alternative access route for TAVI with lower risks of major vascular complications. This study supports the use of subclavian access as a viable alternative in patient groups where transfemoral TAVI is contraindicated.
Keywords: Transcatheter aortic valve, Femoral artery, Subclavian artery, Vascular complications
1. Introduction
Since the first transcatheter aortic valve implantation (TAVI) in 2002 [1], the most common vascular access route has been via the lower limbs. TAVI via the femoral artery is considered the least invasive option and the preferred route for most patients, mainly due to the size and calibre of the vessel, favourable surface anatomy and complimenting patient positioning [2].
However, given the multiple comorbidities often associated with patients requiring TAVI, peripheral vascular disease is not infrequent, which has a preponderance for distressing the lower limb vessels [3].
For these patient groups there may be appropriate arterial access in the upper limbs allowing for alternative access routes Multiple access routes for TAVI are possible [4]. In addition, patients with less than optimal pulmonary function or frailty may suffer from poor recovery after TAVI via the transapical or trans-aortic route, which are more invasive methods.
Most of the major trials have exclusively used trans-femoral access routes and excluded patients with unfavourable femoral access vessels, which makes robust comparative data quite limited [5], [6]. This systematic review and meta-analysis aims to use data from numerous comparative studies in the literature to compares clinical outcomes and safety of trans-subclavian TAVI versus trans-femoral TAVI.
2. Materials and methods
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [7]
2.1. Literature search and selection criteria
The following online databases were searched to identify all randomized controlled trials (RCTs), prospective and retrospective studies from 2002 onwards: Medline (via Ovid) and Embase. Since the first TAVI procedure was reported by Cribier and colleagues in 2002 [1], studies published prior to 2002 were excluded. The databases were searched with a combination of the keywords and their variations: (transcatheter aortic valve replacement OR TAVI) AND (trans-subclavian or axillary artery) (more detailed outline of the search strategy can be found in the Appendix 1). The last date of the search was 4 August 2019. The references of included studies and relevant review articles were also analysed for relevant titles.
2.2. Inclusion and exclusion criteria
All studies that demonstrated clear descriptors of the access routes for TAVI and provided comparative data between both access via the subclavian artery and access via the femoral artery, were eligible. Studies that provided data on short with or without long-term outcomes were included (study eligibility criteria outlined in Appendix 2). Outcomes assessed included short-term complications (30-day mortality, MI, stroke), access complications, paravalvular leak, permanent pacemaker implantation, and procedural time.
Studies that compared TAVI to other treatment methods for AS, such as SAVR or best medical therapy were excluded, unless this was part of a multivariate analysis where the two access routes for TAVI were also compared. Case reports/series were excluded, as were purely descriptive and “how to” papers merely detailing the methods of performing the procedure.
2.2.1. Study selection
Two reviewers (AA and NH) performed an independent initial screening of citations by title and abstract. If predefined study eligibility criteria were met or the abstract was inconclusive, full-texts of the relevant manuscript titles were obtained and assessed for relevance. Disagreements between reviewers were solved by consensus.
2.2.2. Data extraction
An electronic data spreadsheet was used to obtain study and procedural characteristics, baseline characteristics of the patient population, and outcomes parameters of interest. A second reviewer checked extracted data for accuracy and completeness (AA).
2.2.3. Data synthesis and analysis
The odds ratio was used as the main summary statistic, which was calculated with 95% confidence intervals (CI). Since we anticipated clinical heterogeneity across studies, we employed and reported results from random-effects models [8] in the text; forest plots also depict results from random-effects models. Heterogeneity across studies was assessed by visual inspection of the forest plots and calculation of I2 statistics [9], [10]. In addition, fixed-effects meta-analysis was conducted for comparison (Appendix). Funnel plot analysis was used to assess for publication bias. Data was analysed using Stata 13.0 software (Stata Corp., College station, TX).
3. Results
The search initially generated 508 titles. From these, 484 studies were excluded due to the criteria employed (Fig. 1A). Eventually, 9 eligible studies [11], [12], [13], [14], [15], [16], [17], [18], [19] comparing the safety and efficacy of TAVI between TF and TSc access routes were included. These studies included two propensity-matched studies [15], [16], three retrospective cohort studies [12], [13], [17], two prospective cohort studies [11], [14] and two registry studies [18], [19] (Table 1). The total number of participants in the studies were 7,237 which comprised of 5,624 and 791 in TSc and TF groups, respectively.
Fig. 1A.
Flow diagram demonstrating study selection process.
Table 1.
Patient characteristics in included studies.
| Author, year | N Use of VARC-2 criteria |
Age years Mean ± SD/ Median (IQR) |
Female N (%) |
Log Euro SCORE % Mean ± SD/Median (IQR) |
STS risk score % Mean ± SD |
NYHA III or IV N (%) |
LVEF % Mean ± SD/Median (IQR) |
AF N (%) |
DM N (%) |
CAD N (%) |
Prior MI N (%) |
PVD N (%) |
Prior PPM N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gleason, 2018 Propensity matched |
TSc/TAx: 202 TF: 202 VARC |
TSc/TAx: 80.8 ± 8.1 TF: 80.2 ± 9.7 |
TSc/Tax: 73 (36.1) TF: 83 (41.1) |
TSc/TAx 20.7 ± 14.3 TF: 19.4 ± 15.0 |
TSc/TAx 9.7 ± 5.9 TF: 9.8 ± 5.5 |
179 (88.6) 181 (89.6) |
NR | TSc/TAx 98 (48.5) TF: 106 (52.5) |
TSc/TAx 87 (43.1) TF: 87 (43.1) |
TSc/TAx 165 (81.7) TF: 169 (83.7) |
TSc/TAx: 64 (31.7) TF: 63 (31.2) |
TSc/TAx: 122 (60.4) TF: 117 (57.9) |
NR |
| Petronio, 2012 Propensity matched |
TSc: 141 TF: 141 VARC |
TSc: 83.0 (78.9–87.0) TF: 83.0 (78.6–86.1) |
TSc: 55 (39.0) TF: 60 (42.3) |
TSc: 23.7 (15.8–33.6) TF: 23.3 (13.5–32.7) |
NR | TSc: 102 (72.3) TF: 96 (68.0) |
TSc: 54 (41–60) TF: 52 (40–60) |
NR | NR | TSc: 83 (58.9) TF: 69 (48.9) |
NR | TSc: 120 (85.1) TF: 29 (20.6) |
NR |
| Muensterer, 2013 |
TSc: 40 TF: 301 VARC |
TSc: 79.5 ± 8.5 TF: 80.2 ± 7.0 |
TSc: 17 (42.5) TF: 166 (55.1) |
TSc: 21.5 ± 12.2 TF: 19.2 ± 12.8 |
TSc: 6.6 ± 5.6 TF: 5.9 ± 4.1 |
TSc: 40 (100%) TF: 287 (95.3) |
NR | NR | NR | TSc: 24 (60.0%) TF: 157 (52.2) |
NR | NR | NR |
| Doshi, 2017 |
TAx: 16 TF: 347 VARC |
TSc: 78 (72–84) TF: 83 (78–86) |
TSc: 4 (25) TF: 157 (45) |
TSc: 19 (15–24) TF: 14 (10–24) |
NR | NR | NR | TSc: 6 (38) TF: 87 (25) |
TSc: 6 (38) TF: 107 (31) |
NR | TSc: 7 (44) TF: 77 (22) |
TSc: 13 (81) TF: 73 (21) |
TSc: 5 (31) TF: 70 (20) |
| Blackman 2013 | All patients: 1620. Sapien TF: 387. Sapien TA: 408. CoreValve TF: 704. CoreValve TS: 94 | Sapien TF: 82.2 ± 7.4. Sapien TA: 81.8 ± 6.9. CoreValve TF: 81.1 ± 7.6. CoreValve TS: 82.0 ± 6.5 | Sapien TF: 197 (50.9). Sapien TA: 216 (52.9). CoreValve TF: 376 (53.4). CoreValve TS: 64(68.1) | Sapien TF: 17.7 ± 11.1 vs Sapien TA: 22.5 ± 12.9 (p < 0.001). CoreValve TF: 19.5 ± 14.2 vs CoreValve TS: 25.9 ± 16.9 (p < 0.01) | NR | NR | NR | NR | Sapien TF: 83 (21.4), Sapien TA: 79 (19.4). CoreValve TF: 158 (22.4) vs CoreValve TS: 23 (24.5) | Sapien TF: 184 (47.5), Sapien TA: 225(55.1), CoreValve TF: 299 (42.5), CoreValve TS: 48(51.1) | Sapien TF: 81(20.9), Sapien TA: 81(19.9). CoreValve TF: 158(22.4), CoreValve TS: 24 (25.3) | Sapien TF: 56(14.5), Sapien TA: 179(43.9) [p < 0.0001]. CoreValve TF: 136(19.3) vs CoreValve TS: 52(55.3) (p < 0.01) | NR |
| Anselmi 2018 |
843 patients VARC |
TAVI: 81 years ± 7.4 years | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Frohlich 2015 | TSc: 188 TF: 2828 |
TSc: 83 (78–86). TF: 83 (77–87). |
TSc: 65 (35) TF: 1377 (49) |
TSc: 22 (14–34) TF: 17 (11–26) |
NR | NR | TSc: >50% = 99 (53%), 20–39%= 68 (36%), <30%= 19 (10%) TF: >50%= 1714 (61%), 30–49%= 818 (29%), <30%= 272 (10%) |
TSc: 32 (17) TF: 591 (21) |
TSc: 45 (23) TF: 629 (23) |
TSc: 94 (51) TF: 1155 (42) |
TSc: 52 (28) TF: 622 (22) |
NR | NR |
| Eltchaninoff 2011 | TSc: 12 TF: 161 |
TSc: 75.5 ± 11.0. TF: 82.9 ± 6.7. |
TSc: 6 (50) TF: 75 (46.6) |
TSc: 24.6 ± 14.5 TF: 25.5 ± 11.3 |
TSc: 21 ± 17.2 TF: 19.0 ± 12.7 |
TSC: 6 (50) TF: 123 (76.3) |
TSc: 51 ± 12 TF: 49 ± 14 |
NR | TSc: 1 (8.3) TF: 46 (28.6) |
TSc: 6 (50) TF: 62 (38.5) |
TSc: 3 (25) TF: 42 (26.1) |
NR | TSc: 3 (25) TF: 28 (17.4) |
| Moynagh 2011 | TSc: 35 TF: 253 |
TSc: 80.6 ± 4.9. TF: 81.7 ± 6.4. |
NR | TSc: 25.0 ± 14.7 TF: 19.1 ± 12.3 |
NR | NR | TSc: <50%= 23 (65.7%) TF: <50%= 94 (37.2%) |
NR | NR | TSc: 26 (74.2) TF: 148 (58.5) |
TSc: 12 (34.3) TF: 41 (16.2) |
TSc: 26 (74.2) TF: 54 (21.3) |
NR |
Abbreviations: AF = Atrial fibrillation; CAD = Coronary artery disease; DM = Diabetes mellitus; Log euroSCORE = Logistic European System for Cardiac Operative Risk Evaluation; LVEF = Left ventricular ejection fraction; MI = Myocardial infarction; NYHA = New York Heart Association; N = Number of patients; NR = Not reported; PPM = Permanent Pacemaker; PVD = Peripheral vascular disease, STS = Score Society of Thoracic Surgeons; TSc: Trans-subclavian; TF: transfemoral; VARC: Valve Academic Research Consortium Criteria
A funnel plot analysis was conducted to assess publication bias within the studies included for meta-analysis. There was no evidence of publication bias (Fig. 1B).
Fig. 1B.
Funnel plot analysis demonstrating low evidence of publication bias.
The majority of studies reported patient characteristics including: age (9/9), the proportion of female patients (7/9), diabetes mellitus (6/9), atrial fibrillation (4/9), coronary artery disease (8/9), prior myocardial infarction (6/9), peripheral vascular disease (5/9), Log Euro SCORE (8/9), STS Risk Score (3/9) (Table 1).
3.1. Short-term complications
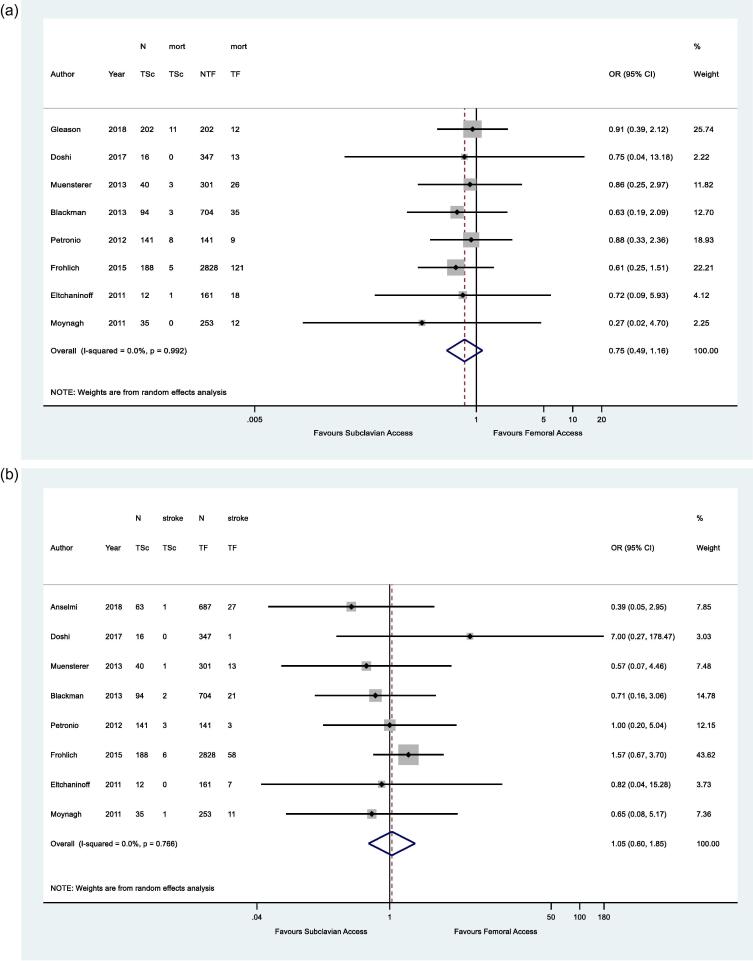
Meta-analysis of the main outcomes was conducted. The 30-day mortality of patients undergoing TAVI through TSc and TF access were comparable (Odds ratio, OR 0.75, 95% CI 0.49 – 1.16, p = 0.195) with little evidence of heterogeneity in the data (I2 = 0.0%, p = 0.992) (Fig. 2A). Similarly, the incidence of post-procedural stroke was found to be non-significantly different between TSc and TF access (OR 1.05, 95% CI 0.60–1.85, p = 0.859) (Fig. 2B). Post-procedural myocardial infarction was reported in 5 studies. Meta-analysis did not yield a difference between TSc and TF access (OR 1.97, 95% CI 0.74–5.23, p = 0.176) (Fig. 2C).
Fig. 2.
Forest plots: meta-analysis of the incidence of short-term complications following following transcatheter aortic valve implantation in two vascular access groups: transfemoral (TF) and trans-subclavian (TSc). A 30-day mortality. B post-procedural stroke. C post-procedural myocardial infarction. D major in-hospital bleeding. E major vascular complications. D major in-hospital bleeding. E major vascular complications. F early paravalvular leak. G permanent pacemaker (PPM) insertion.
3.2. Complications relating to access
Major bleeding in relation to the access sites were only reported in 3 out of 9 studies with a total of 641 and 169 participants in TF and TSc cohorts, respectively. The meta-analysis showed there were no difference between the access points (OR 3.44, 95% CI 0.35–34.22, p = 0.292) (Fig. 2D). The incidence of major vascular complications were significantly higher in TF access compared to TSc (OR 0.55, 95% CI 0.32–0.94, p = 0.029) (Fig. 2E).
3.3. Paravalvular leak and pacemaker insertion
Paravalvular Leak (PVL) as assessed by short or early follow-up echocardiography did not differ between the two access groups (OR 1.20, 95% CI 0.76–1.90, p = 0.439) (Fig. 2F). Similarly, the rates of permanent pacemaker implantation (PPI) showed similar rates between TSc and TF groups (OR 1.49, 95% CI 0.92–2.41, p = 0.105) (Fig. 2G).
3.4. Procedural time
The procedural time was only reported in two studies. Meta-analysis found that procedures via TSc access took significantly longer than TF (Standardised mean difference 1.02, 95% CI 0.815–1.219, p < 0.001) (Appendix 3).
3.5. Meta regression
Owing to heterogeneity in a number of areas in the meta-analyses, the influence of relevant covariates on the outcomes of interest were used using meta-regression. The effects of between-group variations in gender, peripheral vascular disease, previous MI, Euroscore and NYHA class were assessed sequentially on the procedural mortality, stroke and major vascular complications. None of the covariates were found to influence any of the short-term outcomes assessed (p > 0.05) (Table 2).
Table 2.
Meta-regression analysis demonstrating the influence of covariates on the main outcome measures.
| Covariate | Coefficient of variance | 95% CI | Standard error | P value | |
|---|---|---|---|---|---|
| 30-day mortality | Gender | −0.011 | −0.090–0.066 | 0.030 | 0.721 |
| Euroscore | 0.005 | −0.28–0.29 | 0.12 | 0.966 | |
| PVD | 0.019 | -0.088–0.13 | 0.033 | 0.605 | |
| Previous MI | 0.056 | −0.16–0.27 | 0.077 | 0.510 | |
| DM | 0.018 | −0.068–0.10 | 0.027 | 0.560 | |
| NYHA class | 0.00060 | −0.13–0.13 | 0.0092 | 0.963 | |
| Stroke | Gender | −0.049 | −0.17 – 0.068 | 0.042 | 0.311 |
| Euroscore | −0.19 | −0.59 – 0.20 | 0.15 | 0.264 | |
| PVD | 0.037 | −0.26–0.33 | 0.068 | 0.635 | |
| DM | 0.13 | −0.44 – 0.70 | 0.13 | 0.430 | |
| Major vascular complication | Gender | 0.0022 | −0.23 – 0.27 | 0.078 | 0.789 |
| Euroscore | 0.014 | −0.35 – 0.38 | 0.13 | 0.916 | |
| PVD | 0.22 | −2.61 – 3.05 | 0.22 | 0.504 | |
| DM | −0.23 | −1.45 – 1.41 | 0.11 | 0.873 |
For all outcomes, the results of fixed-effects meta-analysis (Appendix) were found to be comparable with the aforementioned results arising from random-effects models.
4. Discussion
This systematic review and meta-analysis has demonstrated early evidence for a good safety profile for TAVI via subclavian artery access (TSc), despite its less frequent use compared to the trans-femoral (TF) approach. Consideration of access routes for TAVI ought to be patient-specific and consider potential risks related to device delivery and comorbid complications.
The recent PARTNER 3 trial [20] exclusively used the trans-femoral approach for device delivery. Despite the landmark results which highlight the advantages of TAVI over surgical valve replacement with respect to one-year outcomes (mortality, stroke or rehospitalisation), the trial was very selective of its patients.
TAVI via the femoral artery is contraindicated in patients with excessive atherosclerosis, calcifications, or tortuosity of common femoral arteries or iliac arteries and should be considered cautiously in patients with an aneurysm of the thoracic or abdominal aorta [4]. Prior surgery or percutaneous access to the femoral arteries also causes excessive scarring and impedes access of the prosthesis [21]. Of note, the recent international guidelines emphasise that open surgery is still a satisfactory option whenever no favourable access is available for TAVI, primarily transfemoral [22]. This gives impetus for the interventional team to consider all potential access routes prior to deeming patients unsuitable for TAVI.
TAVI via subclavian access was first reported in the literature in 2008 [4]. Most procedures via subclavian access are performed using the left subclavian artery (LSA): the right subclavian is usually more difficult to access. After branching off the aortic arch, the LSA usually continues its course through the superior thoracic aperture passing through the scalene muscles [7]. The artery then passes between the first rib and clavicle before passing across the lateral border of the first rib where it becomes anatomically defined as the axillary artery [4].
In all of the studies discussed, gaining femoral access was considered to be the first-choice route that was used for patients requiring TAVI. Subclavian access was only considered for patients who had contraindications for TAVI via the femoral artery [6]. Separate criteria were also used for patients selected to undergo the TSc approach [20].
Four of the reports discussed how subclavian access was obtained for patients undergoing the TSc approach. Although obtaining femoral access varies between surgical cutdown and percutaneous approaches, trans-subclavian access is primarily obtained via surgical cutdown; Gleason et al [20] stated 96% of TSc cases used surgical cutdown, whilst all TSc patients in reports from Petronio et al, Muensterer et al (with a 5–6 cm incision above or below the clavicle) and Doshi et al were accessed via surgical cutdown [15], [16], [17]. Surgically accessing the artery takes longer than using closure devices for percutaneous access, resulting in longer overall procedural time for TSc compared to TF. However, can be crucial to allow visualisation of surrounding structures, such as the brachial plexus and pleura, thus avoiding iatrogenic complications [16].
The present study has important implications, as it highlights a significantly lower rate of major vascular complications with the trans-subclavian approach. Similarly, one of the largest propensity-matched reports from Petronio et al [11], demonstrated a significantly lower incidence of minor vascular complications. The trans-subclavian approach has been reported to provide a more direct access to the aortic valve compared with femoral access [15]. With adoption of direct introduction of 14F CoreValve Evolute R delivery system without a sheath, trans-subclavian access allows a better manoeuvrability and improved accuracy in the positioning and deployment of the prosthesis. This was demonstrated in a propensity-matched study which showed a significant lower rate of bleeding events in the subclavian group compared to trans-femoral access [11]. The reduced bleeding rate may in turn confer a reduced rate of vascular complications with the TSc approach. Evidence also suggests a difference in vascular microarchitecture between subclavian and femoral arteries: subclavian vessels are more elastic in nature compared to the higher constituency of smooth muscle within the tunica media of the femoral artery [23]. Other possible explanations could be the altered calibre of the conduit vessel or altered post-procedural nursing of the puncture site, although this has not been formerly reported.
In a recent large meta-analysis [24], Wagner and colleagues found the rate of major vascular complications to be higher following TAVI compared to SAVR. The majority of studies included TF access routes predominantly, although other access routes (e.g. TSc, trans-apical) were not excluded. This was in addition to higher incidences of paravalvular leak and new pacemaker insertion [24]. In a large propensity-matched study, Giannini et al [25] performed a propensity-matched analysis between Evolut R and the older Corevalve device. Amongst its findings, the investigators demonstrated a decrease in major vascular complications over time due to the increasing rate of TSc access routes as well as the use of the newer generation device. Our findings also concur with a recently published, albeit smaller, meta-analysis on the subject [26], which found similar rates of vascular complications following both TSc and TF approaches. This adds to the novelty of the present study.
5. Limitations
The results of this study ought to be taken with caution. First, our meta-analysis data are limited by the small number of studies included in this systematic review, with the studies being observational studies as opposed to Randomised Controlled Trials. Despite the multiple reports of the trans-subclavian approach, very few studies provide adequate comparative data against established practice for analysis. Furthermore, the level of heterogeneity detected in our analysis is at least moderate, which may reflect varying levels of pre-existing vascular disease (although meta-regression found no influence on the main outcomes). In addition to this, there is limited data available as to what defines a major vascular complication. The Valve Academic Research Consortium (VARC-2) criteria to assess outcomes was used in five out the nine studies in this meta-analysis [11], [12], [13], [15], [16]; the fact that not all nine studies used this standard criteria could be a further source of potential bias during comparison. Specific complications, such as Brachial plexus injury and haemo/pneumothorax, are also not comparable between the two access routes due to their anatomical location. This systematic review, like others of its kind, may suffer from inherent publication biases in the literature.
6. Conclusion
Our results encourage the consideration of subclavian access for transcatheter aortic valves as a potential first line option, particularly in patient groups with radiological signs of femoral disease precluding access via the lower limbs. Future randomised studies assessing comparing the safety and efficacy of both routes are warranted.
Funding
None
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100668.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cribier A., Eltchaninoff H., Tron C., Bauer F., Derumeaux G., Bash A., Borenstein N. Percutaneous implantation of prosthetic heart valves: From animal model to first human implantation in calcific aortic stenosis. J. Am. Coll. Cardiol. 2003;41(6):508. doi: 10.1016/S0735-1097(03)82753-5. [DOI] [Google Scholar]
- 2.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., III, Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., Rigolin V.H., Sundt T.M., III, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol. 2017;70(2):252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Hayashida K., Lefvre T., Chevalier B., Hovasse T., Romano M., Garot P. Transfemoral aortic valve implantation: New criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4(8):851–858. doi: 10.1016/j.jcin.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Overtchouk P., Modine T. A comparison of alternative access routes for transcatheter aortic valve implantation. Expert Rev Cardiovasc Ther. 2018;16(10):749–756. doi: 10.1080/14779072.2018.1524295. [DOI] [PubMed] [Google Scholar]
- 5.Mack M.J., Leon M.B., Smith C.R., Miller D.C., Moses J.W., Tuzcu E.M. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 6.Webb J.G., Mack M.J., White J.M., Dvir D., Blanke P., Herrmann H.C. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol. 2017;69(18):2253–2262. doi: 10.1016/j.jacc.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;7(3):177–188. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Statist. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Petronio A.S., De Carlo M., Bedogni F., Maisano F., Ettori F., Klugmann S. 2-year results of CoreValve implantation through the subclavian access: A propensity-matched comparison with the femoral access. J Am Coll Cardiol. 2012;60(6):502–507. doi: 10.1016/j.jacc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Muensterer A., Mazzitelli D., Ruge H., Wagner A., Hettich I., Piazza N. Safety and efficacy of the subclavian access route for TAVI in cases of missing transfemoral access. Clin Res Cardiol. 2013;102(9):627–636. doi: 10.1007/s00392-013-0575-0. [DOI] [PubMed] [Google Scholar]
- 13.Doshi S.N., George S., Kwok C.S., Mechery A., Mamas M., Ludman P.F. A feasibility study of transaxillary TAVI with the lotus valve. Catheter Cardiovasc Interv. 2018;92(3):542–549. doi: 10.1002/ccd.27409. [DOI] [PubMed] [Google Scholar]
- 14.Blackman D.J., Baxter P.D., Gale C.P., Moat N.E., Maccarthy P.A., Hildick-Smith D. Do Outcomes from transcatheter aortic valve implantation vary according to access route and valve type? the UK TAVI registry. J Interv Cardiol. 2014;27(1):86–95. doi: 10.1111/joic.12084. [DOI] [PubMed] [Google Scholar]
- 15.Anselmi A., Tomasi J., Giardinelli F., Bedossa M., Rosier S., Verhoye J.P. Safety and effectiveness of the transsubclavian approach for transcatheter aortic valve implantation with the 14-F CoreValve Evolut R device. J Cardiovasc Med. 2018;19(11):664–668. doi: 10.2459/JCM.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 16.Gleason T.G., Schindler J.T., Hagberg R.C., Deeb G.M., Adams D.H., Conte J.V. Subclavian/Axillary Access for Self-Expanding Transcatheter Aortic Valve Replacement Renders Equivalent Outcomes as Transfemoral. Ann Thorac Surg. 2018;105(2):477–483. doi: 10.1016/j.athoracsur.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Moynagh A.M., Scott D.J.A., Baumbach A., Khavandi A., Brecker S.J., Laborde J.C. CoreValve transcatheter aortic valve implantation via the subclavian artery: Comparison with the transfemoral approach. J Am Coll Cardiol. 2011;57(5):634–635. doi: 10.1016/j.jacc.2010.08.642. [DOI] [PubMed] [Google Scholar]
- 18.Fröhlich G.M., Baxter P.D., Malkin C.J., Scott D.J.A., Moat N.E., Hildick-Smith D. Comparative Survival after Transapical, Direct Aortic, and Subclavian Transcatheter Aortic Valve Implantation (Data from the UK TAVI Registry) Am J Cardiol. 2015;116(10):1555–1559. doi: 10.1016/j.amjcard.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Eltchaninoff H., Prat A., Gilard M., Leguerrier A., Blanchard D., Fournial G. Transcatheter aortic valve implantation: Early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32(2):191–197. doi: 10.1093/eurheartj/ehq261. [DOI] [PubMed] [Google Scholar]
- 20.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 21.Marcus A.J., Lotzof K., Howard A. Access to the Superficial Femoral Artery in the Presence of a “Hostile Groin”: A Prospective Study. Cardiovasc Intervent Radiol. 2007;30(3):351–354. doi: 10.1007/s00270-005-0347-y. [DOI] [PubMed] [Google Scholar]
- 22.Falk V., Baumgartner H., Bax J.J., De Bonis M., Hamm C., Holm P.J. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017;52(4):616–664. doi: 10.1093/ejcts/ezx324. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer U., Ho Y., Frerker C., Schewel D., Sanchez-Quintana D., Schofer J. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: “the Hamburg Sankt Georg approach”. JACC Cardiovasc Interv. 2012;5(5):477–486. doi: 10.1016/j.jcin.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Wagner G., Steiner S., Gartlehner G., Arfsten H., Wildner B., Mayr H. Comparison of transcatheter aortic valve implantation with other approaches to treat aortic valve stenosis: A systematic review and meta-analysis. Syst Rev. 2019;8(1):44. doi: 10.1186/s13643-019-0954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini C., De Carlo M., Tamburino C., Ettori F., Latib A.M., Bedogni F. Transcathether aortic valve implantation with the new repositionable self-expandable Evolut R versus CoreValve system: A case-matched comparison. Int J Cardiol. 2017;243:126–131. doi: 10.1016/j.ijcard.2017.05.095. [DOI] [PubMed] [Google Scholar]
- 26.Amat-Santos I.J., Rojas P., Gutiérrez H., Vera S., Castrodeza J., Tobar J. Transubclavian approach: A competitive access for transcatheter aortic valve implantation as compared to transfemoral. Catheter Cardiovasc Interv. 2018;92(5):935–944. doi: 10.1002/ccd.27485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.