Abstract
Several studies have documented the hypolipidemic effect of anthocyanin-rich plants in vitro and in vivo. The objective of this study was to elucidate the inhibitory activity of anthocyanin-rich fraction from Thai berries against fat digestive enzymes. The ability of Thai berries to bind bile acid, disrupt cholesterol micellization and the cholesterol uptake into Caco-2 cells was also determined. The content of total phenolics, flavonoid and anthocyanin in Prunus domestica L. (TPE), Antidesma bunius (L.) Spreng, Syzygium cumini (L.) Skeels, and Syzygium nervosum A. Cunn. Ex DC was 222.7–283.5 mg gallic acid equivalents, 91.2–184.3 mg catechin equivalents, and 37.9–49.5 mg cyanidin-3-glucoside equivalents/g extract, respectively. The anthocyanin-rich fraction of all extracts inhibited pancreatic lipase and cholesterol esterase with the IC50 values of 90.6–181.7 μg/mL and 288.7–455.0 μg/mL, respectively. Additionally, all extracts could bind primary and secondary bile acids (16.4–36.6%) and reduce the solubility of cholesterol in artificial micelles (53.0–67.6%). Interestingly, TPE was the most potent extract on interfering the key steps of lipid digestion among the tested extracts. In addition, TPE (0.10–0.50 mg/mL) significantly reduced the cholesterol uptake into Caco-2 cells in a concentration-dependent manner. These results demonstrate a new insight into the role of anthocyanin-rich Thai berry extract on interfering the key steps of lipid digestion and absorption.
Keywords: Thai berries, Dyslipidemia, Anthocyanins, Lipid digestion and absorption, Natural product chemistry, Plant products, Diet, Pharmaceutical science, Natural product, Biochemistry, Alternative medicine, Evidence-based medicine
Thai berries; Dyslipidemia; Anthocyanins; Lipid digestion and absorption; Natural product chemistry; Plant products; Diet; Pharmaceutical science; Natural product; Biochemistry; Alternative medicine; Evidence-based medicine
1. Introduction
Over the past several decades, the prevalence of dyslipidemia is dramatically increasing as a result of excessive consumption of high fat diets together with low physical activity [1]. Dyslipidemia is one of the modifiable risk factors for development of insulin resistance, stroke and cardiovascular diseases [2]. Current data suggest that treating abnormal blood lipids by pharmacological agents are important therapeutic priorities for reducing risk of vascular events [3]. Nowadays, using lipid-lowering drugs in conjunction with dietary and behavior exercise has been shown to be an effective strategy for the management of dyslipidemia [4]. However, these drugs may cause adverse effects such as myopathy, hepatotoxicity, gastrointestinal disorders [5]. Therefore, there is now growing evidence that polyphenol-rich natural plants are an alternative source for treatment of dyslipidemia due to their health benefits with less side effects [6].
Anthocyanins, a subclass of polyphenol family, are responsible for red and purple pigment in fruits and vegetables. Previous studies demonstrated that anthocyanin-rich fruits improve lipid metabolism disorders in rats [7, 8, 9]. Especially, berries, the excellent source of anthocyanins, were shown to have favorable effects on reduction of blood glucose and lipid profiles in the subjects [10, 11]. Today, berries including Prunus domestica L. (Thai plum or Luknhai), Antidesma bunius (L.) Spreng (Mamao), Syzygium cumini (L.) Skeels (Lukwha), and Syzygium nervosum A. Cunn. Ex DC (Makiang) are widely grown and distributed in many provinces of Thailand. The phytochemical compounds identified in the ripe pulp of Thai berries are mainly anthocyanins including cyanidin and its derivatives [12, 13, 14, 15]. In food manufacturing, their pulps are usually used as a raw material for dried-fruit snack, jam, soft-drink, and wine. In addition, Thai berries have been found to possess pharmacological activities such as antioxidant [12, 16, 17, 18] and anti-diabetic activity [19, 20]. However, scientific evidence regarding the antihyperlipidemic activity of anthocyanin-rich Thai berries on the key steps of lipid digestion and absorption remains unknown. Hence, the aim of this study was to determine the effect of anthocyanin-rich fraction from Thai berry extracts including Prunus domestica L. (TPE), Antidesma bunius (L.) Spreng (MME), Syzygium cumini (L.) Skeels (LWE), and Syzygium nervosum A. Cunn. Ex DC (MKE) on the inhibition of pancreatic lipase, cholesterol esterase, binding bile acids and the interfere of cholesterol micellization and cholesterol uptake into Caco-2 cells.
2. Material and methods
2.1. Chemicals
p-Nitrophenylbutylrate (p-NPB), oleic acid, lipase from porcine pancreas type II, 4-methylumbelliferyl (4-MUO), phosphatidylcholine, taurocholic acid, glycodeoxycholic acid, taurodeoxycholic acid, and porcine cholesterol esterase were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cholesterol test kit was purchased from HUMAN GmbH Co. (Wiesbaden, Germany). A total bile acid test kit was purchased from GenWay Biotech Inc. (San Diego, CA, USA). High and low-glucose Dulbecco's modified Eagle medium (DMEM), non-essential amino acids, and fetal bovine serum (FBS) were purchased from Hyclone Laboratories (South Logan, Utah, USA). 22-(N–N7-nitrobenz-2-oxa-1, 3-diazol-4-yl) amino)-23, 24-bisnor-5-cholen-3-ol (NBD-cholesterol) was obtained from Invitrogen (Eugene, OR, USA).
2.2. The preparation and extraction of Thai berries
The ripe fruit of Thai berries (Prunus domestica L., Antidesma bunius (L.) Spreng, Syzygium cumini (L.) Skeels, and Syzygium nervosum A. Cunn. Ex DC) were collected from Maehongsorn, Sakonnakhon, Lumpang, and Phrae provinces, respectively. They were authenticated by a taxonomist at Department of Botany, Faculty of Science, Chulalongkorn University, Thailand (Voucher specimen: BCU015912 (Prunus domestica L.), BCU015866 (Antidesma bunius (L.) Spreng), BCU015910 (Syzygium cumini (L.) Skeels), and BCU015918 (Syzygium nervosum A. Cunn)). The pulp of samples (100 g) was separately blended with 200 mL of distilled water twice and filtered through cheesecloth. Thereafter, the aqueous solution was centrifuged at 4000 rpm for 5 min at 4 °C to remove residues before re-filtered under vacuum through Whatman No.1 filter paper. The extract solution was frozen at -20 °C, and further lyophilized at -40 °C with 0.5 psi for 48 h. The isolation of anthocyanin fraction was prepared by using C18 solid-phase extraction (Stratar C18-E, 6 g capacity: Phenomenex Ltd., Macclesfield, UK) according to previous studies [21, 22]. Briefly, the extract solution was dissolved in distilled water. Then, C18 cartridges were preconditioned with 0.2% (v/v) formic acid in acetonitrile and 0.2% (v/v) formic acid in distilled water sequentially. The extract solution was eluted through the preconditioned C18 column and washed with 0.2% (v/v) formic acid in distilled water. Finally, the absorbed compounds were eluted by 80% acetonitrile in distilled water. The eluents were dried under the rotary evaporator at 50 °C, pressure gradient with 600 for 15 min. The dried extract was kept at -20 °C until use.
2.3. Determination of total phenolics content
The content of total phenolic was determined using the Folin-Ciocalteu method as described in the previous report [23]. The anthocyanin-rich extracts (0.125–0.250 mg) were dissolved in distilled water (1 mL). The solution (50 μL) was mixed with 50 μL Folin-Ciocalteu reagent (1:10). After 5 min of incubation at room temperature, 10% (w/v) Na2CO3 (50 μL) was added and then incubated for 30 min at room temperature. The absorbance was measured at 760 nm. Gallic acid (0–80 μg/mL) was used as a standard.
2.4. Determination of total flavonoids content
The content of total flavonoid was performed by a colorimetric assay as a previously described method with minor modifications [24]. The anthocyanin-rich extracts (0.25–0.50 mg/mL) were dissolved in distilled water. Then, the extracts (50 μL) were mixed with 50 μL of 5% (w/v) NaNO2 and incubated for 5 min at room temperature before adding 30 μL of 2% (w/v) AlCl3. The reaction was incubated for 5 min at room temperature. After addition with 20 μL of 1 M NaOH for 5 min, the absorbance was determined at 510 nm. Catechin (0–200 μg/mL) was used as a standard.
2.5. Determination of total anthocyanin content
The content of total anthocyanins was determined by pH differential method following a previous study [24]. The aqueous extract (500 μL) was mixed with 500 μL of different buffers including 0.025 M potassium chloride (KCl, pH 1.0) buffer and 0.4 M sodium acetate (CH3COONa, pH 4.5) buffer. The mixture was kept in dark area for 20 min at room temperature. The absorbance was measured at 510 and 700 nm and calculated by following equation: A = (Abs510 – Abs700)pH1.0 – (Abs510 – Abs700)pH4.5. The content of TA was expressed as mg of cyanidin-3-glucoside per g of extract.
2.6. Determination of pancreatic lipase activity
The assay of pancreatic lipase was performed following the method of Mäkynen et al. [23]. The porcine pancreatic lipase (7.5 mg/mL) and 0.2 mM 4-MUO were prepared in 0.1 M PBS, pH 7.0, whereas the extracts were dissolved in distilled water with various concentration. To determine the lipase activity, the solution of anthocyanin-rich extract (5 μL) was mixed with 50 μL of 4-MUO solution. Then, the enzyme solution (45 μL) was added to the mixture in order to initiate the reaction. The mixture was immediately incubated at 37 °C for 20 min before adding 100 μL of 0.1 M sodium citrate (pH 4.2) to stop the reaction. The absorbance of fluorescence was read at the excitation wavelength of 355 nm and the emission wavelength of 460 nm. Orlistat in 1% DMSO was used as a positive control.
2.7. Determination of cholesterol esterase activity
Th assay of cholesterol esterase was performed according to the method of Mäkynen et al. [23]. The anthocyanin-rich extract dissolved in distilled water (5 μL) was mixed with 150 μL of 100 mM PBS (100 mM NaCl, pH 7.0) containing 0.2 mM p-NPB and 5.16 mM taurocholic acid. After incubation with 45 μL of cholesterol esterase solution (0.125 μg/mL) for 20 min at room temperature, the absorbance of the mixture (200 μL) was immediately measured at 405 nm.
2.8. Determination of bile acid binding
The assay of bile acid binding was done according to a previous report [23]. Taurocholic acid (TCA), glycodeoxycholic acid (GDA), and taurodeoxycholic acid (TDA) were used in this experiment. In brief, 20 μL of the anthocyanin-rich extract dissolved in distilled water (1 mg/mL) was incubated with 180 μL of 2 mM bile acid in 0.1 M PBS, pH 7.4 for 90 min at 37 °C. The mixture (200 μL) was centrifuged at 5000 rpm for 5 min. The supernatant was analyzed using a bile acid analysis kit. Cholestyramine (1 mg/mL) was used as a positive control. The absorbance was read at 540 nm using a microplate reader at 37 °C. The results were expressed as the percentage of bile acid binding.
2.9. Determination of cholesterol micellization
The preparation of artificial micelle solution was performed according to a previously described method [23]. The solution containing 2 mM cholesterol, 1 mM oleic acid, and 2.4 mM phosphatidylcholine was completely dissolved in methanol and then dried under nitrogen gas. After adding 15 mM PBS (containing 6.6 mM taurocholate acid, pH 7.4), the suspension was sonicated twice using a sonicator for 20 min. The micelle solution was dropped in the incubator at 37 °C overnight. Thereafter, 20 μL of anthocyanin-rich extract was mixed with the micelle solution and incubated for 2 h at 37 °C. Finally, 200 μL of the mixture was then centrifuged at 10,000 rpm for 20 min. The supernatant was collected for determining free cholesterol using total cholesterol test kits. After incubation for 10 min at room temperature, the absorbance was measured at 500 nm. Gallic acid was used as a positive control.
2.10. Caco-2 cell culture
Caco-2 cells were purchased from American Tissue Culture Collection (ATCC). Cells used in this study were at the passage number from 30 to 50. They were maintained in high glucose Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% non-essential amino acids at 37 °C in a 90% humidified incubator with 5% CO2. Cells at 80% confluence were seeded for the experiments.
2.11. Cell viability assay
The viability of Caco-2 cells was assessed using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) or MTT assay [25]. Cells were seeded in 96-well plates at a density of 1 × 104 cells/mL. They were grown for 48 h before cells were treated with various concentration of TPE (0.10, 0.25, 0.50, and 1.00 mg/mL) for 2 h at 37 °C in an incubator with 90% humidified atmosphere containing 5% CO2. The culture medium was used as the control. After the incubation, the supernatant was replaced with 100 μL of fresh serum free medium containing 0.5 mg/mL of MTT and incubated for 2.5 h at 37 °C. Then, the solution was substituted with DMSO in order to dissolve the purple formazan crystals. The absorbance was read at 570 nm.
2.12. Cholesterol uptake
The cholesterol uptake assay was performed in Caco-2 cells according to a previous study [26] with slight modification. Caco-2 cells (2.5 × 104 cells/well) were seeded in a 24-well plate and cultured for 6–7 days for the differentiated period. Then, cells were starved in low-glucose DMEM for 24 h. After further starvation with Hanks' balanced salt solution (HBSS; containing 140 mM NaCl, 5 mM KCl, 1.2 mM Na2HPO4, 2 mM CaCl2, 1.2 mM MgSO4, 20 mM HEPES, and 0.2% BSA, pH 7.4) for 1 h, cells were treated with TPE (0.1–0.5 mg/mL) or ezetimibe (0.05–0.10 mg/mL), or combination of TPE and ezetimibe (0.05 mg/mL). The cholesterol uptake was started by adding 0.025 mM NBD-cholesterol in HBSS containing 0.5 mM taurocholic acid. After incubation at 37 °C for 1 h, cells were washed twice with cold HBSS. The fluorescent intensity was determined at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. Cells were lysed by cold lysis buffer (10 mM Tris-HCl, 1% Triton-X-100, 1 mM EDTA, 0.1% SDS, pH 7.4). The determination of protein was done by BCA colorimetric assay using bovine serum albumin (BSA) as a standard.
2.13. Statistical analysis
The results are expressed as mean ± SEM. Data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple-range test. Correlation analysis was obtained by Pearson correlation test. The statistical significance was considered at p-value < 0.05 using SPSS statistics 16.0 (SPSS Inc., Chicago, IL., USA).
3. Results
3.1. Phytochemical contents of aqueous Thai berry extracts
The results in Table 1 demonstrate that TPE and MKE had the highest and lowest content of total phenolics and total flavonoids, respectively. In addition, the highest content of total anthocyanins was observed in TPE and MME.
Table 1.
Phytochemical contents of anthocyanin-rich fraction from Thai berry extracts.
| Samples | Total phenolics |
Total flavonoids |
Total anthocyanins |
|---|---|---|---|
| (mg GAE/g extract) | (mg CE/g extract) | (mg C3G/g extract) | |
| TPE | 283.5 ± 0.4a | 184.3 ± 0.7a | 48.8 ± 0.6a |
| MKE | 222.7 ± 1.4b | 91.2 ± 2.4b | 37.9 ± 0.2b |
| MME | 276.6 ± 0.6c | 135.6 ± 1.4c | 49.5 ± 0.2a |
| LWE | 268.2 ± 0.6d | 106.8 ± 2.5d | 44.0 ± 0.4c |
Values are expressed as mean ± SEM (n = 3). The means with different superscripted letters in the same column are significantly different (p < 0.05). Abbreviations: TPE, Prunus domestica L.; MME, Antidesma bunius (L.) Spreng; LWE, Syzygium cumini (L.) Skeels; MKE, Syzygium nervosum A. Cunn. Ex DC; GAE, Gallic acid equivalents; CE, Catechin equivalents; C3G, cyanidin-3-glucoside.
3.2. Inhibitory effects of Thai berry extracts against pancreatic lipase and cholesterol esterase
The IC50 values of Thai berry extracts against pancreatic lipase and cholesterol esterase are shown in Table 2. It was found that TPE and MME exhibited the highest inhibitory activity against pancreatic lipase and cholesterol esterase, respectively. The order of inhibitory activity was TPE > MME > MKE > LWE against pancreatic lipase and MME > MKE > TPE > LWE against pancreatic cholesterol esterase. However, all anthocyanin-rich fractions of Thai berries were less potent than orlistat (IC50 value = 1.8 μg/mL).
Table 2.
The IC50 values of anthocyanin-rich fraction from Thai berry extracts against pancreatic lipase and cholesterol esterase.
| Sample | IC50 values (μg/mL) |
|
|---|---|---|
| Pancreatic lipase activity (Lipase) | Cholesterol esterase activity (CEase) | |
| TPE | 90.6 ± 0.4b | 417.7 ± 2.4a |
| MKE | 146.6 ± 2.1c | 333.8 ± 4.5b |
| MME | 118.7 ± 1.1d | 288.7 ± 1.7c |
| LWE | 181.7 ± 0.8e | 455.0 ± 2.2d |
| Orlistat | 1.8 ± 0.0a | NA |
Values are expressed as mean ± SEM (n = 3). NA = not determined. Means within the same column were statistical analyzed using one-way analysis of variance (ANOVA), followed by Duncan's multiple-range test. The significant differences of each assay are represented with different superscripted letters (p < 0.05). Abbreviations: TPE, Prunus domestica L.; MME, Antidesma bunius (L.) Spreng; LWE, Syzygium cumini (L.) Skeels; MKE, Syzygium nervosum A. Cunn. Ex DC.
3.3. Bile acids binding of Thai berry extracts
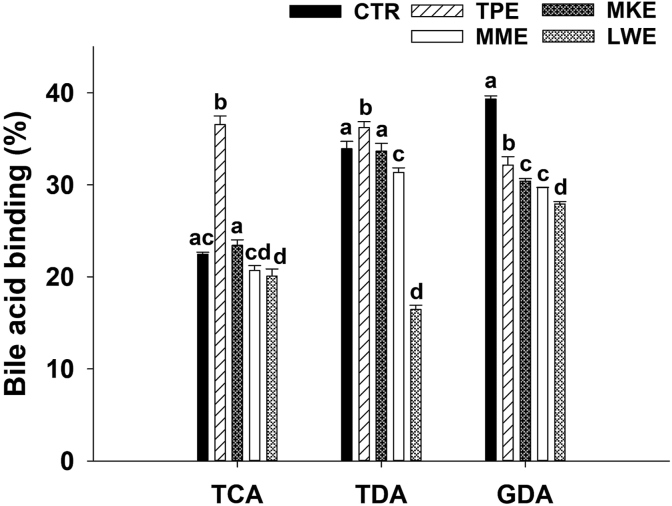
The percentage of bile acid binding of Thai berry extracts is presented in Figure 1. It was found that all extracts (1 mg/mL) had the ability to bind taurocholic acid (TCA, 20.1–36.6%), taurodeoxycholic acid (TDA, 16.4–36.2%) and glycodeoxycholic acid (GDA, 27.9–32.2%). Interestingly, TPE had the highest ability to bind bile acids among all Thai berry extracts. Additionally, TPE exhibited greater binding efficacy to TCA and TDA than cholestyramine at the same concentration (1 mg/mL).
Figure 1.
Effect of cholestyramine (CTR) and anthocyanin-rich fraction from Thai berry extracts (1 mg/mL) on bile acid binding. Data are expressed as mean ± SEM (n = 3). The significant differences of each bile acid are presented with different superscripted letters (p < 0.05). Abbreviations: TPE, Prunus domestica L.; MME, Antidesma bunius (L.) Spreng; LWE, Syzygium cumini (L.) Skeels; MKE, Syzygium nervosum A. Cunn. Ex DC; TCA, taurocholic acid; TDA, taurodeoxycholic acid; GDA, glycodeoxycholic acid.
3.4. Effects of Thai berry extracts on cholesterol micellization
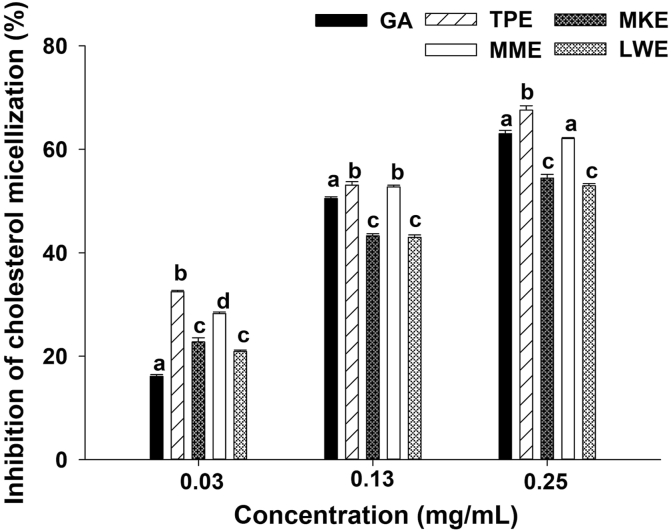
As shown in Figure 2, Thai berry extracts reduced the solubility of cholesterol in mixed micelles. Interestingly, TPE (0.03–0.25 mg/mL) showed the most effective extract to reduce cholesterol solubility in mixed micelles among all Thai berry extracts. The order of cholesterol micelle inhibition was TPE > MME > MKE = LWE at the highest concentration (0.25 mg/mL).
Figure 2.
The inhibitory effects of gallic acid (GA) and anthocyanin-rich fraction from Thai berry extracts (0.03–0.25 mg/mL) on cholesterol micellization. Data are expressed as mean ± SEM (n = 3). The significant differences are presented with different superscripted letters (p < 0.05). Abbreviations: TPE, Prunus domestica L.; MME, Antidesma bunius (L.) Spreng; LWE, Syzygium cumini (L.) Skeels; MKE, Syzygium nervosum A. Cunn. Ex DC.
3.5. Correlation between activities of lipid digestion and absorption and phytochemicals in Thai berry extracts
The results from Pearson's correlation coefficients between the variables are presented in Table 3. It was found that the percentage of binding of taurocholic acid (TCA) had the strongest positive correlation with total phenolic content (TP, r = 0.777) and total anthocyanin content (TA, r = 0.683). In addition, TP, TA and total flavonoid content (TF) also had the strong negative associations with the IC50 values of pancreatic lipase (r = -0.899, -0.959 and -0.667, respectively). Moreover, a strong positive correlation was also found between TF (r = 0.948) and the percentage inhibition of cholesterol micellization. The moderate negative correlation was found between TP (r = -0.574) and TA (r = -0.530) and the IC50 values of cholesterol esterase.
Table 3.
Pearson's correlation analyses of % taurocholic acid binding (TCA), IC50 values of pancreatic lipase (Lipase) and pancreatic cholesterol esterase (CEase), % cholesterol micelle formation, and phytochemical compounds including total phenolic (TP), total flavonoid (TF), and total anthocyanin (TA) content of Thai berry extracts.
| Parameters | TCA | Lipase | CEase | Micelle |
|---|---|---|---|---|
| TP | .777∗∗ | -.899∗∗ | -.574∗ | .075 |
| TF | .159 | -.667∗∗ | -.115 | .868∗∗ |
| TA | .683∗∗ | -.959∗∗ | -.530∗ | .204 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
3.6. Effect of TPE on cell viability in Caco-2 cells
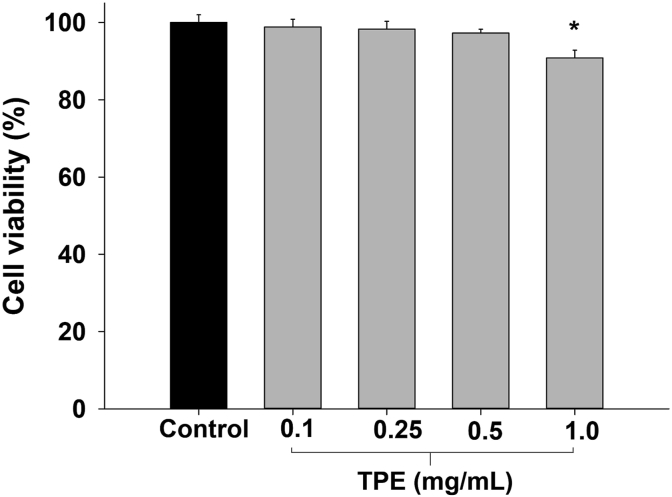
In this study, TPE was chosen to study the inhibitory effect on the cholesterol uptake in Caco-2 cells because it had the highest content of phytochemical compounds and demonstrated the most effective lipid-lowering activity. In this study, TPE (0.1–1 mg/mL) were tested for cell cytotoxicity. As shown in Figure 3, TPE at the maximum concentration of 1 mg/mL caused a significant reduction of cell viability (90.8 ± 2.1%) when compared to the control (0.1% DMSO). Therefore, the concentration of TPE at 0.10–0.50 mg/mL were selected for the cholesterol uptake assay into Caco-2 cells.
Figure 3.
Effect of anthocyanin-rich fraction of Prunus domestica L. (TPE) on viability of Caco-2 cells after 2 h of incubation. Data are expressed as mean ± SEM (n = 3). ∗p < 0.05 compared to the control group.
3.7. Effect of TPE on the uptake of cholesterol into Caco-2 cells
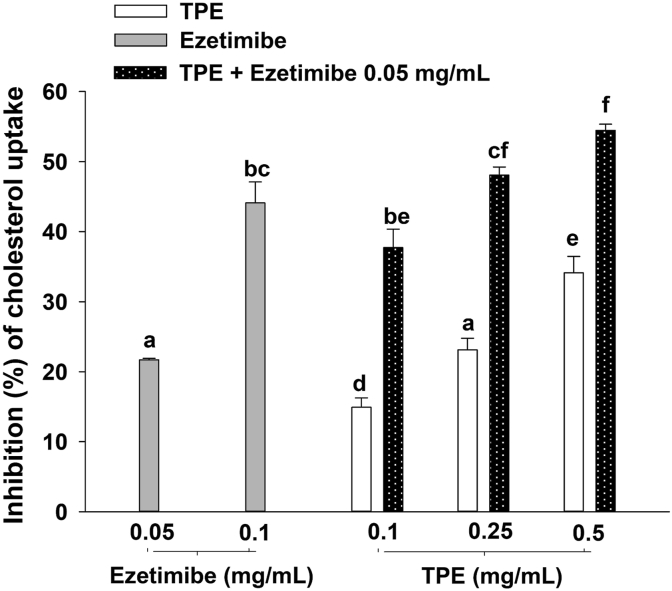
As shown in Figure 4, TPE (0.10, 0.25, and 0.5 mg/mL) significantly reduced the uptake of cholesterol into Caco-2 cells in a concentration-dependent manner (14.9–34.1%). At the concentration of 0.1 mg/mL, TPE was less potent than ezetimibe, a positive control in this assay. Furthermore, the combination of TPE (0.1 mg/mL, 14.9 ± 1.3%) and ezetimibe (0.05 mg/mL, 21.7 ± 0.2%) demonstrated additive inhibition on uptake of cholesterol into Caco-2 cells (37.7 ± 2.7%). This additive effect was the similar percentage inhibition as ezetimibe at concentration of 0.1 mg/mL (44.1 ± 3.0%).
Figure 4.
Effect of anthocyanin-rich fraction of Prunus domestica L. (TPE) on cholesterol uptake into Caco-2 cells. Data are expressed as mean ± SEM (n = 3). Significant differences are presented with different superscripted letters (p < 0.05).
4. Discussion
Strong evidence revealed the lipid-lowering activity of anthocyanins in animal and human studies [10, 27, 28]. Especially, the interfering key step of lipid digestion and absorption is one of the therapeutic options to improve hyperlipidemia [29]. For example, inhibition of pancreatic lipase and cholesterol esterase causes a delay in triglyceride and dietary cholesterol digestion into free fatty acid and free cholesterol, respectively [29, 30]. Moreover, the decreased binding ability of bile acid could disrupt the formation of cholesterol micelles, leading to reduction of the cholesterol absorption into the small intestine [31]. This is the first study to show the effect of anthocyanin-rich fraction from Thai berry extract including Prunus domestica (TPE), Antidesma bunius (MME), Syzygium cumini (LWE), and Syzygium nervosum (MKE) on the key steps of lipid digestion and absorption. Especially, the fraction of Prunus domestica was the highest effective extract on inhibition of pancreatic lipase, reduction of bile acid binding, and disruption of cholesterol micelle formation. We also found that the fraction of Antidesma bunius exhibited the highest inhibition against pancreatic cholesterol esterase.
Several studies support that lipid-lowering activity may be attributed, in part, to the presence of phytochemical compounds [37, 38]. Our findings showed that phytochemical compounds were detected from the remaining fraction after removing organic acids and sugars. Interestingly, the fraction of Thai berry extracts had higher content of anthocyanins than previous studies of Prunus domestica [32, 33], Antidesma bunius [12, 34], Syzygium cumini [35], and Syzygium nervosum [15, 36]. In the current study, the correlations between phytochemical compounds in the extract and the lipid-lowering activity were also found. This finding is consistent with previous studies indicating a significant relationship between total flavonoid content in traditional Thai medical herbs and pancreatic lipase inhibition [39, 40]. A study of green, black, and dark tea polyphenols demonstrated a significant positive correlation between phenolic compounds and the bile acid-binding ability [41]. Therefore, the present findings suggest that the interference with lipid digestion and absorption may be attributed to the content of phytochemical compounds in Thai berries. To evaluate the clinical benefits, human studies are needed to investigate the efficacy of Thai-berries consumption on postprandial lipid profiles.
The cholesterol uptake is mediated by NPC1L1 protein transporter at brush border of the small intestine [42]. This study presented that Prunus domestica extract had the ability to reduce the cholesterol uptake into Caco-2 cells. Many studies reported that natural pigments from plant extracts interrupted the cholesterol uptake into Caco-2 cells such as curcumin [43], black rice anthocyanins [26], and riceberry rice [21]. Yao et al. reported that cyanidin-3-glucoside (C3G) and peonidin-3-glucoside (P3G) reduced cholesterol uptake into Caco-2 cells [26]. In addition, C3G and P3G are identified in the pulp of Prunus domestica [32]. It is suggested that these compounds may contribute to inhibit cholesterol uptake into Caco-2 cells. Interestingly, the combination of Prunus domestica and ezetimibe produced the additive inhibition of cholesterol uptake into Caco-2 cells. This effect may help to increase the efficacy of ezetimibe on the management of postprandial cholesterol. Previous studies revealed that the inhibitory effect of plant polyphenols on the cholesterol uptake might be due to the downregulation of NPC1L1 in Caco-2 cells [21, 43, 44, 45]. The further study is needed to investigate the effect of Prunus domestica on the mRNA and protein expression of NPC1L1 in long-term period.
5. Conclusions
The current study presents the effect of anthocyanin-rich fraction from Thai berry extracts including Prunus domestica, Antidesma bunius, Syzygium cumini, and Syzygium nervosum on the interference of key step of lipid digestion and absorption. The anthocyanin-rich fraction of Prunus domestica demonstrates the most effective extract on inhibition of pancreatic lipase, binding of bile acids, reduction of the cholesterol micelle formation among all Thai berry extracts. In addition, the anthocyanin-rich fraction of Prunus domestica reduces the cholesterol uptake into Caco-2 cells. It also produces additive effect on inhibition of cholesterol uptake when combination with ezetimibe. These findings suggest that the anthocyanin-rich fraction from Thai berries could be promising natural sources for interfering the key steps of lipid digestion and cholesterol absorption.
Declarations
Author contribution statement
S. Ngamukote and S. Adisakwattana: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
N. Chamnansilpa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
P. Aksornchu: Performed the experiments.
T. Thilavech: Analyzed and interpreted the data; Wrote the paper.
K. Mäkynen and W. Dahlan: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Grant for International Research Integration: Chula Research Scholar, and Grant for Join Funding (CU-GRS-62-04-37-01) and 90th Anniversary Chulalongkorn University, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to thank the Department of Nutrition and Dietetics, Faculty of Allied Health Sciences and the Halal Science Center, Chulalongkorn University for providing the facilities and instruments.
References
- 1.Tietge U.J. Hyperlipidemia and cardiovascular disease: inflammation, dyslipidemia, and atherosclerosis. Curr. Opin. Lipidol. 2014;25:94–95. doi: 10.1097/MOL.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 2.Mahdavi H., Kim J.B., Safarpour S. Dyslipidemia and cardiovascular diseases. Curr. Opin. Lipidol. 2009;20:157–158. doi: 10.1097/MOL.0b013e32832956ed. [DOI] [PubMed] [Google Scholar]
- 3.Doroodchi H., Abdolrasulnia M., Foster J.A. Knowledge and attitudes of primary care physicians in the management of patients at risk for cardiovascular events. BMC Fam. Pract. 2008;9:42. doi: 10.1186/1471-2296-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinsier R.L., Hunter G.R., Heini A.F. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am. J. Med. 1998;105:145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 5.Graham D.J., Staffa J.A., Shatin D. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. J. Am. Med. Assoc. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 6.Basu A., Rhone M., Lyons T.J. Berries: emerging impact on cardiovascular health. Nutr. Rev. 2010;68:168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011;2:1–7. doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes-Hernández T.Y., Giampieri F., Gasparrini M. Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients. 2017;9:621. doi: 10.3390/nu9060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S., Lee M.S., Chang E. Mulberry fruit extract promotes serum HDL-cholesterol levels and suppresses hepatic microRNA-33 expression in rats fed high cholesterol/cholic acid diet. Nutrients. 2020;12:1499. doi: 10.3390/nu12051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin Y., Xia M., Ma J. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009;90:485–492. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 11.Basu A., Wilkinson M., Penugonda K. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: baseline and post intervention effects. Nutr. J. 2009;8:43. doi: 10.1186/1475-2891-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorjong S., Butkhup L., Samappito S. Phytochemicals and antioxidant capacities of Mao-Luang (Antidesma bunius L.) cultivars from Northeastern Thailand. Food Chem. 2015;181:248–255. doi: 10.1016/j.foodchem.2015.02.093. [DOI] [PubMed] [Google Scholar]
- 13.Usenik V., Stampar F., Kastelec D. Phytochemicals in fruits of two Prunus domestica L. plum cultivars during ripening. J. Sci. Food Agric. 2013;93:681–692. doi: 10.1002/jsfa.5783. [DOI] [PubMed] [Google Scholar]
- 14.Chhikara N., Kaur R., Jaglan S. Bioactive compounds and pharmacological and food applications of Syzygium cumini - a review. Food Funct. 2018;9:6096–6115. doi: 10.1039/c8fo00654g. [DOI] [PubMed] [Google Scholar]
- 15.Poontawee W., Natakankitkul S., Wongmekiat O. Protective effect of Cleistocalyx nervosum var. paniala fruit extract against oxidative renal damage caused by cadmium. Molecules. 2016;21:133. doi: 10.3390/molecules21020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charoensin S., Taya S., Wongpornchai S. Assessment of genotoxicity and antigenotoxicity of an aqueous extract of Cleistocalyx nervosum var. paniala in in vitro and in vivo models. Interdiscipl. Toxicol. 2012;5:201–206. doi: 10.2478/v10102-012-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igwe E.O., Charlton K.E. A systematic review on the health effects of Plums (Prunus domestica and Prunus salicina) Phytother Res. 2016;30:701–731. doi: 10.1002/ptr.5581. [DOI] [PubMed] [Google Scholar]
- 18.Ayyanar M., Subash-Babu P. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012;2:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utsunomiya H., Yamakawa T., Kamei J. Anti-hyperglycemic effects of plum in a rat model of obesity and type 2 diabetes, Wistar fatty rat. Biomed. Res. 2005;26:193–200. doi: 10.2220/biomedres.26.193. [DOI] [PubMed] [Google Scholar]
- 20.Elya B., Basah K., Mun'im A. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. BioMed Res. Int. 2011;2012 doi: 10.1155/2012/281078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poosri S., Thilavech T., Pasukamonset P. Studies on Riceberry rice (Oryza sativa L.) extract on the key steps related to carbohydrate and lipid digestion and absorption: a new source of natural bioactive substances. NFS J. 2019;17:17–23. [Google Scholar]
- 22.Grussu D., Stewart D., McDougall G.J. Berry polyphenols inhibit alpha-amylase in vitro: identifying active components in rowanberry and raspberry. J. Agric. Food Chem. 2011;59:2324–2331. doi: 10.1021/jf1045359. [DOI] [PubMed] [Google Scholar]
- 23.Mäkynen K., Jitsaardkul S., Tachasamran P. Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chem. 2013;139:735–743. doi: 10.1016/j.foodchem.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Sariburun E., Sahin S., Demir C. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010;75:C328–335. doi: 10.1111/j.1750-3841.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 25.Peng L., He Z., Chen W. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 26.Yao S.-L., Xu Y., Zhang Y.-Y. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. 2013;4:1602–1608. doi: 10.1039/c3fo60196j. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Suarez J.M., Giampieri F., Tulipani S. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014;25:289–294. doi: 10.1016/j.jnutbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi A., Okazaki Y., Nakamoto A. Dietary anthocyanin-rich Haskap phytochemicals inhibit postprandial hyperlipidemia and hyperglycemia in rats. J. Oleo Sci. 2014;63:201–209. doi: 10.5650/jos.ess13196. [DOI] [PubMed] [Google Scholar]
- 29.Tucci S.A., Boyland E.J., Halford J.C. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab. Syndr. Obes. 2010;3:125–143. doi: 10.2147/dmsott.s7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers-Payne S.C., Hui D.Y., Brockman H.L. Cholesterol esterase: a cholesterol transfer protein. Biochemistry. 1995;34:3942–3947. doi: 10.1021/bi00012a011. [DOI] [PubMed] [Google Scholar]
- 31.Chiang J.Y.L. Bile acid metabolism and signaling. Comp. Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usenik V., Štampar F., Veberič R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009;114:529–534. [Google Scholar]
- 33.Miletic N., Popovic B., Mitrovic O. Phenolic content and antioxidant capacity of fruits of plum cv.'Stanley'('Prunus domestica'L.) as influenced by maturity stage and on-tree ripening. Aust. J. Crop. Sci. 2012;6:681. [Google Scholar]
- 34.Islary A., Sarmah J., Basumatary S. Nutritional value, phytochemicals and antioxidant properties of two wild edible fruits (Eugenia operculata Roxb. and Antidesma bunius L.) from Assam, North-East India. Med. J. Nutr. Metab. 2017;10:29–40. [Google Scholar]
- 35.Chaudhary B., Mukhopadhyay K. Solvent optimization for anthocyanin extraction from Syzygium cumini L. Skeels using response surface methodology. Int. J. Food Sci. Nutr. 2013;64:363–371. doi: 10.3109/09637486.2012.738647. [DOI] [PubMed] [Google Scholar]
- 36.Jansom C., Bhamarapravati S., Itharat A. Major anthocyanin from ripe berries of Cleistocalyx nervosum var. paniala. Thammasat Med. J. 2008;8:365. [Google Scholar]
- 37.Ngamukote S., Makynen K., Thilawech T. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules. 2011;16:5054–5061. doi: 10.3390/molecules16065054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo S.I., Noh S.K. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sompong W., Muangngam N., Kongpatpharnich A. The inhibitory activity of herbal medicines on the keys enzymes and steps related to carbohydrate and lipid digestion. BMC Compl. Alternative Med. 2016;16:439. doi: 10.1186/s12906-016-1424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dechakhamphu A., Wongchum N. Screening for anti-pancreatic lipase properties of 28 traditional Thai medicinal herbs. Asian Pac. J. Trop. Biomed. 2015;5:1042–1045. [Google Scholar]
- 41.Wu Z., Teng J., Huang L. Stability, antioxidant activity and in vitro bile acid-binding of green, black and dark tea polyphenols during simulated in vitro gastrointestinal digestion. RSC Adv. 2015;5:92089–92095. [Google Scholar]
- 42.Phan C.T., Tso P. Intestinal lipid absorption and transport. Front. Biosci. 2001;6:D299–319. doi: 10.2741/phan. [DOI] [PubMed] [Google Scholar]
- 43.Feng D., Ohlsson L., Duan R.D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010;9:40. doi: 10.1186/1476-511X-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thilavech T., Adisakwattana S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Compl. Alternative Med. 2019;19:242. doi: 10.1186/s12906-019-2664-8. 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanotti I., Dall'Asta M., Mena P. Atheroprotective effects of (poly)phenols: a focus on cell cholesterol metabolism. Food Funct. 2015;6:13–31. doi: 10.1039/c4fo00670d. [DOI] [PubMed] [Google Scholar]