Summary
Hepatocholangiocarcinoma, fibrolamellar carcinoma, hepatic haemangioendothelioma and hepatic angiosarcoma represent less than 5% of primary liver cancers. Fibrolamellar carcinoma and hepatic haemangioendothelioma are driven by unique somatic genetic alterations (DNAJB1-PRKCA and CAMTA1-WWTR1 fusions, respectively), while the pathogenesis of hepatocholangiocarcinoma remains more complex, as suggested by its histological diversity. Histology is the gold standard for diagnosis, which remains challenging even in an expert centre because of the low incidences of these liver cancers. Resection, when feasible, is the cornerstone of treatment, together with liver transplantation for hepatic haemangioendothelioma. The role of locoregional therapies and systemic treatments remains poorly studied. In this review, we aim to describe the recent advances in terms of diagnosis and clinical management of these rare primary liver cancers.
Keywords: Mixed tumor, Hepatocholangiocarcinoma, Fibrolamellar carcinoma, Hepatic hemangioendothelioma, Hepatocellular carcinoma, Hepatic angiosarcoma
Abbreviations: 5-FU, 5-Fluorouracil; AFP, alpha-fetoprotein; APHE, arterial phase hyperenhancement; CA19-9, carbohydrate antigen 19-9; CCA, cholangiocarcinoma; CEUS, contrast-enhanced ultrasound; cHCC-CCA, combined hepatocholangiocarcinoma; CK, cytokeratin; CLC, cholangiolocellular carcinoma; EpCAM, epithelial cell adhesion molecule; FISH, fluorescence in situ hybridisation; FLC, fibrolamellar carcinoma; HAS, hepatic angiosarcoma; HCC, hepatocellular carcinoma; HEH, hepatic epithelioid haemangioendothelioma; HepPar1, hepatocyte specific antigen antibody; iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemistry; LI-RADS, liver imaging reporting and data system; LT, liver transplantation; RT-PCR, reverse transcription PCR; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolisation; WHO, World Health Organization
Key points.
-
•
Recent consensus has reclassified pure cholangiolocarcinoma in CCA whereas cHCC-CCA are characterised histologically by the presence of 2 distinct morphological patterns in the same lesion.
-
•
A unique genetic alteration drives the pathogenesis of fibrolamellar carcinoma (DNAJB1-PRKCA fusion) and hepatic haemangioendothelioma (CAMTA1-WWTR1 fusion).
-
•
The combination of imaging and histology, mainly using tumour and non-tumour biopsy, are required for the diagnosis of rare PLCs.
-
•
When feasible, liver resection is the main treatment for rare PLCs.
-
•
No systemic or locoregional therapies are currently validated for the treatment of any unresectable rare PLC.
-
•
Liver transplantation is validated for hepatic epithelioid haemangioendothelioma even in a metastatic setting, whereas this is still an area of research for small cHCC-CCA.
Background
Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) account, respectively, for 85% and 10% of all primary liver cancers (PLCs). Large cohort studies and randomised controlled trials are available and have enabled the development of international guidelines for the management of HCC and CCA. In contrast, prospective studies and clinical trials are lacking for rare PLCs, such as combined hepatocholangiocarcinoma (cHCC-CCA), fibrolamellar carcinoma (FLC), hepatic epithelioid haemangioendothelioma (HEH) and hepatic angiosarcoma (HAS) due to their scarcity. Herein, we summarise recent advances in our understanding of the pathophysiology of rare PLCs, as well as discussing the latest developments in clinical management.
Combined hepatocholangiocarcinoma
A matter of definition
cHCC-CCA is characterised at histology by the presence of 2 distinct morphological patterns in the same lesion: HCC and intrahepatic CCA (iCCA).1,2 Several classifications have been proposed (Table S1)3 and were used sequentially in the literature, leading to confusion. The discussion about terminology, based on recent morphological and molecular advances, has led to the exclusion of several types of PLC from the definition of cHCC-CCA: collision tumours, hepatoblastoma, typical HCCs with immunohistochemical expression of progenitor markers, typical iCCAs with immunohistochemical expression of hepatocytic markers. Whether intermediate cell carcinoma and cholangiolocarcinoma (CLC) should be classified as cHCC-CCAs is also debated.
A recent proposal aimed at achieving a consensus terminology4 divided PLCs that cannot be defined as either HCC or iCCA into 3 classes:
-
1.
Tumours with hepatocytic and cholangiocytic histology, mixed with a transition or separated areas within the same tumour, which can be considered cHCC-CCA.
-
2.
PLCs completely composed of “intermediate cells” (intermediate cell carcinoma) – small cells of intermediate size (between that of stem cells and hepatocytes) with transitional morphology between hepatocytes and cholangiocytes. Whether these cells can be considered a subtype of cHCC-CCA is still a matter of discussion.5,6
-
3.
PLCs composed of pure CLC; if the main component of PLC is >80% CLC, they can be reclassified as small duct iCCAs.6
According to this classification, the concept of “stem cell” phenotypes based on immunohistochemistry (epithelial cell adhesion molecule [EpCAM], cytokeratin (CK)19 and CD56) is not considered as a sub-category per se, but rather as a feature that can be present in different types of PLC.5 Moreover, all subtypes of PLC could be associated, in the same lesion, with minor histological components observed in more than half of cHCC-CCAs.7,8 If different subtypes are present, a precise description is recommended and the percentage of each tumour type present should be assessed in surgical specimens.5
Epidemiology and risk factors
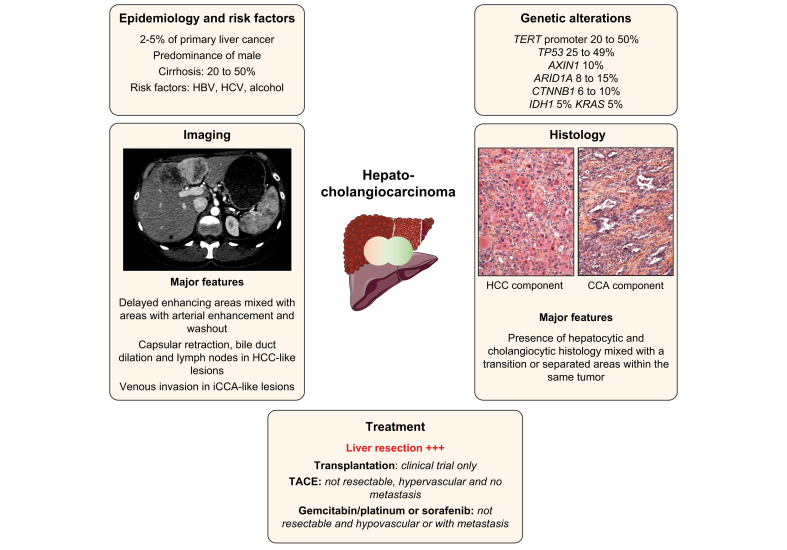
Data from large databases reported that nearly 0.75% of all PLCs are cHCC-CCAs, with an incidence of 0.05/100,000 in the general population.[9], [10], [11] The incidence in monocentric studies based on resection or necropsies varies from 2.4% to 5.3% of PLCs and the 2018 World Health Organization (WHO) classification estimates the frequency at 2%–5% (Fig. 1).1,5,6,[12], [13], [14], [15], [16]
Fig. 1.
Main characteristics of combined hepatocholangiocarcinoma.
Representation of the main genetic alterations, as well as clinical, histopathological, and radiological features of combined hepatocholangiocarcinoma; treatment strategies are also shown. CCA, cholangiocarcinoma; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolisation.
Risk factors associated with cHCC-CCA are shared with other PLCs and include HBV, HCV, alcohol consumption, cirrhosis and male sex (predominance of up to 79%) (Table 1). The association with cirrhosis has been reported in several surgical series in eastern countries (26% to 81%), where it is mostly associated with HBV infection.5,13,15,[17], [18], [19], [20] In Western countries, cHCC-CCA has been associated with cirrhosis in 52% of cases, with HCV the leading cause in Spain (43%) and the USA (23%) and alcohol the leading cause in France (40%).14,[21], [22], [23] These data are consistent with a systematic review identifying cirrhosis in 51.7% of liver explants or surgical specimens.19 It seems that cHCC-CCA stands at the crossroads between iCCA (low rate of cirrhosis and HBV/HCV infection) and HCC (high rate of cirrhosis and HBV/HCV infection) in terms of underlying liver disease.
Table 1.
Recent data on risk factors of hepatocholangiocarcinoma.
| Author | Country | Numbers of patients | Advanced fibrosis | HCV | HBV | Alcohol | Metabolic syndrome |
|---|---|---|---|---|---|---|---|
| Sasaki et al. 201727 | Japan | 53 | 14/24 (58%) | 9/19 (47%) | 9/44 (21%) | 2/19 (11%) | 3/19 (16%) |
| Zhou et al. 2017153 | China | 144 | 91/144 (63.2%) | – | 101/144 (70%) | 29/144 (20%) | – |
| Xue et al. 201929 | China | 121 | 54/115 (47%) | 2/115 (2%) | 89/115 (77%) | – | – |
| Okumura et al. 2020154 | Japan | 89 | 30/89 (34%) | 29/89 (33%) | 37/89 (43%) | – | – |
| Gentile et al. 201919 | Systematic Review | 437 | 226/437 (52%) | 39/437 (9%) | 264/437 (60%) | – | – |
| Wells et al. 201522 | USA | 39 | 12/39 (31%) | 9/39 (23%) | 0/39 | 3/39(8%) | 2/39 (5%) |
| Gigante et al. 201923 | France | 20 | 10/20 (50%) | 1/20 (4%) | 3/20 (15%) | 8/20 (40%) | 6/20 (30%) |
| De Martin 2020∗55 | France | 31 | 31/31 (100%)∗ | – | – | 40/75 (53%) | – |
| Holzner 202054 | USA | 47 | 20/47 (43%) | 15/47 (32%) | 22/47 (47%) | – | – |
We included recent studies with histologically confirmed (Goodman transitional type (type II)/Allen and Lisa type B or C/WHO classical type tumours and stem cell type with exception of CLC, studies already included in the systematic review (Gentile et al. 2019) are not shown.
Study including only lesions on cirrhosis. Data about risk factor prevalence are relatives to the entire cohort of cHCC-CCA and iCCA.
Genetic landscape of cHCC-CCA
A whole-genome sequencing analysis of liver cancers displaying biliary phenotype, including cHCC-CCA, reported a median number of 60 to 70 non-synonymous coding mutations per tumour (Table 2).24 TERT promoter, TP53, ARID1A and ARID2 mutations are more frequent in cHCC-CCA; PBRM1, BAP1, KRAS, IDH1 and FGFR2 mutations in iCCA; and CTNNB1 mutations in HCC (Fig. 1). The high heterogeneity in terms of techniques and HCC-CCA classification in these studies needs to be underlined.[25], [26], [27], [28] Genomic analysis also suggests an impact of viral hepatitis (HCV and HBV) on the genetic landscape of cHCC-CCA that seems closer to HCC than iCCA in terms of genomic profiles and prevalence of TERT promoter mutations.24
Table 2.
Genomic alterations in rare primary liver cancers.
| Study | Classification | Type of analysis | N patients Subtypes |
Fibrosis (F3-F4) | Somatic genetic alterations |
|---|---|---|---|---|---|
| Hepatocholangiocarcinoma | |||||
| Cazals-Hatem et al. 2004155 | Lisa et Allen 1949 | Target sanger sequencing | 14 mixed, 1 fibrolamellar HCC 3 collision tumours | 3/15 | TP53 |
| Fujimoto et al. 201524 | WHO 2010 |
WGS and RNA-seq | 30 Liver cancer with biliary phenotype 7cHCC-CCA +2CLC |
4/9 | TERT promoter 53%, PBMR1 20%, ARID2 27% |
| Sasaki et al. 201727 | WHO 2010 |
Target sanger sequencing + IHC | 53 mixed tumours 4 CT, 4 TS, 20 INT, 25 CLC |
38/53 | cHCC-CCA: TERT 50%, TP53 25%, KRAS 50% ARID1A 0% Intermediate: TERT 42%, TP53 58%, , KRAS 5%, ARID1A 11% |
| Moeini et al. 201728 | WHO 2010 |
Microarray, DNA copy number, WES |
18 mixed tumours 6 CLC/8SC/4CT |
10/18 | CLC: TP53 and IDH1 cHCC-CCA: TP53, TERT promoter, BRAF, FGFR2-BICC1 fusion |
| Liu et al. 201825 |
WHO 2010 |
WGS, WES and RNA-seq | 4 cHCC-CCA not specified | n.a. | TP53, CTNNB1 and ARID1A |
| Wang et al. 201831 |
WHO 2010 |
WES | 7 cHCC-CCA | n.a. | TP53 and ARID2 |
| Xue et al. 201929 |
Lisa et Allen 1949 | WES, WGS, RNA-seq, | 121 tumours: 6 separate type, 56 combined type, 59 mixed type. | 54/115 | TP53 49% , TERT promoter 23%, AXIN 10%, KMT2D 9%, KEAP1 8%, ARID1A 8%, RB1 8%, CTNNB1 6%, IDH1 5% |
| Joseph et al. 201926 | Consensus 2019 |
Target next-generation sequencing | 20CT | 15/18 | TP53 (80%), TERT (70%), ARID1A (15%), CTNNB1 (10%), AXIN1 (10%), KRAS (5%) |
| Sasaki et al. 201930 | Consensus 2019 |
Target sequencing + IHC | 9 CT | 6/9 | TP53 (66%), TERT promoter (33%), KRAS (22%) |
| Fibrolamellar carcinoma | |||||
| Honeyman et al. 201479 | n.a. | RNA-seq | 15 FLC | 0 | DNAJB1-PRKACA fusion (100%) |
| Cornella et al. 201580 | n.a. | FISH WES |
78 FLC | 0 |
DNAJB1-PRKACA fusion (79%) BRCA2 (4.2%) |
| Graham et al. 201581 | n.a. | RT-PCR FISH |
26 FLC | 0 | DNAJB1-PRKACA fusion (100%) |
| Graham et al. 201886 | n.a. | FISH NGS |
3 FLC without DNAJB1-PRKACA fusion | 0 | PRKAR1A (100%) in patients with Carney syndrome and FLC |
| Graham et al. 2018156 | n.a. | FISH | 104 typical FLC, 12 probable FLC and 9 unlikely FLC | 0 | 99% DNAJB1-PRKACA fusion in typical, 75% in probable and 0% in unlikely FLC |
| Hepatic haemangioendothelioma | |||||
| Tanas et al. 2011113 |
n.a. | RNA-seq FISH |
47 haemangioendothelioma (hepatic and non-hepatic) | 0 | 89% WWTR1-CAMTA1 fusion |
| Errani et al. 2011115 | n.a. | FISH | 17 haemangioendothelioma (hepatic and non-hepatic) | 0 | 100% WWTR1-CAMTA1 fusion |
| Antonescu et al. 2013116 | n.a. | FISH | 10 haemangioendothelioma without WWTR1-CAMTA1 | 0 | 100% YAP1-TFE3 fusion (in tumours without WWTR1-CAMTA1 fusion) |
| Flucke et al. 2014157 | n.a. | FISH RT-PCR |
35 haemangioendothelioma (hepatic and non-hepatic) | 0 | 94% WWTR1-CAMTA1 and 6% YAP1-TFE3 fusion |
| Patel et al. 2015117 | n.a. | RT-PCR | 18 haemangioendothelioma (hepatic and non-hepatic) | 0 | 78% WWTR1-CAMTA1 and 6% YAP1-TFE3 fusion |
Molecular alterations of HAS were not represented as very few data are currently available in the literature. CLC, cholangiolocarcinoma; CT, classical type; IHC, immunohistochemistry; INT, intermediate subtype; SC, stem cell subtype; TS, typical subtype; WES, whole-exome sequencing; WGS, whole-genome sequencing.
The largest genetic study on cHCC-CCA was performed on 133 patients in Asia. Similar to the genomic alterations observed in HCC, TP53, TERT promoter, AXIN1, KMT2D, ARID1A were the most common mutations in cHCC-CCAs; the frequency of TERT promoter mutations (23%) was lower than in HCCs (40–60%) but higher than in iCCAs (0–8%). The analysis of mutational signatures identified an exposure to aristolochic acid, aflatoxin B1 and hepatitis B. Epithelial-mesenchymal transition, EpCAM, and KRT19 genes were mostly expressed in “combined” type cHCC-CCA with an enrichment of KRAS mutations. Xenobiotic and bile acid metabolism and overexpression of alpha-fetoprotein (AFP), glypican 3, and spalt-like transcription factor 4 were more frequently observed in “mixed” type cHCC-CC according to the Allen Classification. The authors suggest that “mixed” type cHCC-ICC could be more similar to HCC and “combined” type cHCC-CCA more similar to iCCA.29
Analysis of genetic landscapes shows that CLC has a different genetic profile to pure cHCC-CCA with more ARID1A and less TERT promoter mutations.30 Another genomic analysis confirms that CLC looks like a biliary-derived molecular entity harbouring chromosomal stability and activation of TGFβ pathway with biliary features.28
In terms of tumour heterogeneity, comparing the iCCA and HCC components confirms the monoclonal origin of cHCC-CCA but also shows a significant intratumor genetic heterogeneity that overlaps with morphological heterogeneity.29,31 One study identified TERT promoter mutations in both HCC and iCCA components suggestive of an early event in carcinogenesis, whereas mutations in other driver genes such as TP53 harboured intratumoural heterogeneity.26
In terms of the cell of origin, the disruption of p53 in mice promotes dedifferentiation of mature hepatocytes into nestin-positive progenitor cells that could give rise to HCC or iCCA under the influence of Wnt and Notch.32,33 Overexpression of nestin was identified in 81.3% of human cHCC-CCAs and was associated with a poor clinical outcome.29 Moreover, a cell line derived from cHCC-CCA can differentiate into either HCC or iCCA under different growth conditions.34,35 These results are consistent with the hypothesis that cHCC-CCA can derive from hepatic progenitor cells that express markers of both lineages (hepatocytes and biliary cells).36,37
These data suggest that i) cHCC-CCA is monoclonal, deriving from a common cell of origin; ii) cHCC-CCA genomic features may be more similar to HCC than iCCA, even if some cHCC-CCA harboured genomic features closer to iCCA; iii) risk factors can be associated with specific genetic features in cHCC-CCA; and iv) CLC has a different molecular profile that is similar to iCCA.
Diagnosis
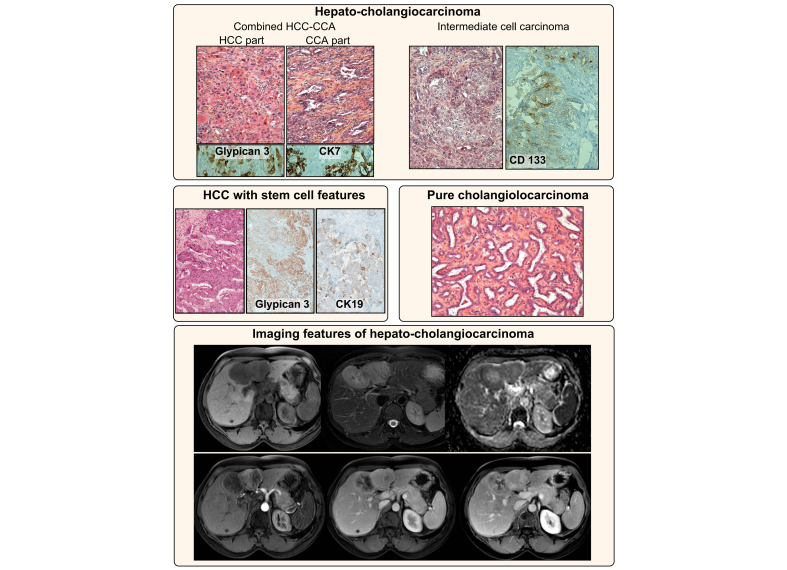
The diagnosis of cHCC-CCA is based on histology from biopsies or surgical specimens (Fig. 2).5,6 Immunohistochemical markers are not mandatory but can be helpful to better characterise PLCs: hepatocyte markers (HepPar1, AFP and glypican 3); cholangiocyte markers (CK19, CK7) and “stem cell” markers (EpCAM, CK19, CD133).5,23 These markers should be considered in the context of morphological analysis, especially “stem cells markers” that could be expressed by all PLCs. In the pre-surgical setting, liver biopsy had an estimated 48% sensitivity and 100% specificity for the diagnosis of cHCC-CCA.23
Fig. 2.
Histological and radiological features of hepatocholangiocarcinoma.
Classical cHCC-CCA (upper panel, left) with the HCC (positive for glypican 3) and CCA (positive for CK7) components, as well as intermediate cell carcinoma (positive for CD133) (upper panel, right). HCC with stem cell features (middle panel, left) at immunohistochemistry (positive for glypican 3 and for CK19) and cholangiolocarcinoma recently reclassified as iCCA (middle panel right). An example of an MRI of cHCC-CCA with a well-delineated heterogeneous lesion with capsular retraction. The lesion harboured progressive delayed enhancing areas mixed with areas with arterial enhancement and washout. cHCC-CCA, combined hepatocholangiocarcinoma; CCA, cholangiocarcinoma; HCC, hepatocellular carcinoma.
Sometimes the discordance between imaging and serum tumour markers (imaging suggestive of HCC with increased serum carbohydrate antigen 19-9 [CA19-9] or hypovascular nodule suggestive of iCCA with increased AFP) could raise the suspicion of a cHCC-CCA.13,18 However, serum biomarkers alone are not reliable for the diagnosis of cHCC-CCA, with elevation of serum CA19-9 and AFP only observed in 45% of cases and with limited specificity.22,38
Even though histology remains the gold standard for the diagnosis of cHCC-CCA, radiology (abdominal CT or MRI with contrast agent injection) may help guide the diagnosis (Fig. 2). Hallmarks of HCC (arterial phase hyperenhancement [APHE] and washout) are observed in a minority of cHCC-CCAs.22,23,39 Nevertheless, recent studies using the American College of Radiology's liver imaging reporting and data system (LI-RADS) have reported misclassification of cHCC-CCA as HCC in 26% to 54% of cases when using major radiological features.40,41 Notably, 88% of these patients could be reclassified as having malignant tumours that are not HCC (LI-RADS M category) after addition of ancillary features such as rim/peripheral APHE, progressive central enhancement on portal venous and delayed phase images, predominantly peripheral washout appearance, liver surface retraction, biliary obstruction and marked diffusion restriction.40 The depiction of these features explains why the main differential diagnosis is often iCCA, and why performance of imaging is often insufficient.39,[42], [43], [44], [45], [46] The association of HCC features with CCA features (appearance of iCCA with portal venous invasion, or appearance of HCC with biliary dilation or enlarged lymph nodes) may guide the diagnosis. Finally, contrast-enhanced ultrasound (CEUS) also harbours an insufficient specificity for the diagnosis of cHCC-CCA, since tumours exhibit various degrees of heterogeneous APHE with washout.47,48
Imaging has a limited diagnostic performance alone, with a sensitivity of only 48% and a specificity of 81%, though the combination of imaging and biopsy can improve the sensitivity (60%) and specificity (82%).23 Overall, radiology is fundamental to guide liver biopsy (especially possible multiple biopsies in heterogeneous tumours) and to perform tumour staging.
Treatments
Liver resection
Liver resection is currently the most effective curative-intent therapy for cHCC-CCA. According to state-of-the-art principles for oncologic liver surgery, liver resection aims to completely remove the lesion with adequate margins and with a sufficient liver remnant volume. This requires a multi-parametric evaluation of the patient, tumour and underlying liver disease.49 A resection margin >10 mm has been associated with prolonged disease-free survival.50 Major hepatectomy can be proposed if a sufficient liver remnant volume has been secured in order to limit the risk of postoperative liver failure.49 In patients with cirrhosis, evaluation of the degree of portal hypertension should also be performed as clinically significant portal hypertension represents an absolute contraindication to major hepatectomy.51 Furthermore, a lymphatic pattern of tumour spread in cHCC-CCA requires a routine hilar lymphadenectomy.52 The need for routine lymphadenectomy should currently restrict the use of the laparoscopic approach only to centres with extensive expertise both in liver surgery and laparoscopy.53
In a systematic review that included 437 patients with cHCC-CCA, liver resection led to an average disease-free survival of 14.2 months in patients with cHCC-CCA, 43.1 months in those with HCC, and 17.8 months in those with iCCA, corresponding to an average overall survival of 37, 67 and 32 months, respectively.19 Outcomes after liver resection for cHCC-CCA are similar to those for iCCA and worse than in patients with HCC, mainly due to early tumour recurrence,14,18 although a recent study identified no difference in outcomes after adjustment for cirrhosis and tumour size.54
Liver transplantation
The role of liver transplantation (LT) in the treatment of small iCCA or cHCC-CCA remains controversial. A systematic review of retrospective studies on LT for cHCC-CCA reported a median disease-free survival of 14.2 months and a median overall survival of 37.1 months.19 These results were discouraging and, in many countries, cHCC-CCA is still a contraindication for LT.
In contrast, recent studies with similar inclusion criteria reported more positive outcomes. The first study was conducted in Spain on 42 patients undergoing LT for HCC, with an incidental diagnosis of HCC-CCA or iCCA, who were stratified according to tumour size and number. The 5-year survival rates were similar between cHCC-CCA and HCC controls (78% vs. 86%). Patients with multinodular or uninodular tumours larger than 2 cm had the worst outcomes.21 The second retrospective study analysed patients treated by resection (n = 26) or LT (n = 95) for iCCA and cHCC-CCA <5 cm developed on cirrhosis. Overall survival (67% at 5 years) and recurrence-free survival (75% at 5 years) were better in patients treated by LT than in patients treated by resection. Survival was similar in patients with iCCA or cHCC-CCA.55
Recent retrospective data suggest that transplantation improves survival compared to resection in cirrhotic patients with cHCC-CCA, if tumour size is <5 cm.21,55,56 One of the main drawbacks of these studies is that cHCC-CCA was identified incidentally on the explant and intention-to-treat analyses of LT for cHCC-CCA diagnosed before inclusion on the waiting list are lacking. A recent consensus concluded that there is not enough evidence to propose LT for cHCC-CCA but that this approach should be explored in clinical trials.57 Moreover, LT should also be discussed according to national guidelines.
Locoregional treatments
The effectiveness of transarterial chemoembolisation (TACE) on cHCC-CCA has been analysed in retrospective studies in a limited number of patients. In a study of 50 patients, TACE induced a partial response or stable disease in 70% of cases, mainly in tumours with APHE, leading to a median overall survival of 12.3 months.58
Better outcomes were reported in a cohort of patients treated by TACE for recurrence after liver resection. As expected, cHCC-CCAs with a non-rim APHE pattern at imaging are associated with a better radiological response rate (36% vs. 0%) and survival (52.8 vs. 12.4 months) compared to tumours with a rim APHE pattern.59,60 Data on radio-embolisation (selective internal radiation therapy [SIRT]) and chemotherapy for unresectable iCCA show that 22% of patients can be downstaged for surgical intervention.61 In 1 study, SIRT was associated with a 55% radiological response rate, 65% disease control rate, and a median overall survival of 9.3 months in 21 patients, suggesting a possible role for SIRT in cHCC-CCA.62
Altogether, few data are currently available to support the value of intra-arterial treatments in patients with cHCC-CCA, even if some retrospective data suggest a possible role in selected patients with tumours showing APHE.
Systemic treatments
Data on systemic treatments for unresectable cHCC-CCA are limited to retrospective series testing the first-line treatments approved for advanced HCC (sorafenib) and CCA (gemcitabine/platinum regimens).63,64
A multicentre Japanese study in 36 patients with unresectable cHCC-CCA analysed different first-line systemic treatments. The median overall survival with gemcitabine/cisplatin, fluorouracil/cisplatin and sorafenib was 11.9, 10.2 and 3.5 months, respectively, suggesting that sorafenib was associated with reduced survival.65 A French multicentric study included 30 patients treated with gemcitabine and oxaliplatin or cisplatin ± bevacizumab. Eight patients (28.6%) had a partial response with a median progression-free survival of 9.0 months and an overall survival of 16.2 months.66 The largest series available was a monocentric cohort of 68 patients with unresectable cHCC-CCA who received mainly gemcitabine-based regimens (57/68), of whom 23.5% received gemcitabine ± fluoropyrimidine and 60.3% gemcitabine with platinum. Overall survival was 11.5 months in patients receiving gemcitabine/platinum therapy and 9.6 months in the 7 patients treated with sorafenib alone.67 Currently, no data are available regarding the use of atezolizumab/bevacizumab, lenvatinib, cabozantinib and ramucirumab in cHCC-CCA.
To summarise, systemic treatments based on gemcitabine/platinum regimens are the most widely used, but their use is not supported by a high level of evidence. The role of sorafenib remains unknown.
Fibrolamellar carcinoma
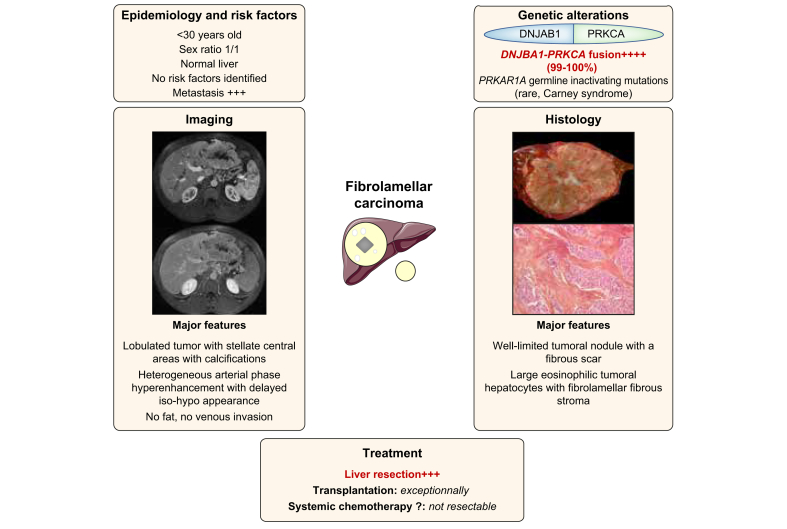
FLC is a rare PLC derived from hepatocytes that occurs in young adults (sex ratio 1:1) on normal liver (Fig. 3).[68], [69], [70], [71], [72], [73] It is characterised by eosinophilic polygonal cells and prominent nucleoli, with fibrotic tissue surrounding tumour cells on histology.68,73,74 No risk factors for FLC development have been identified so far. Most FLCs are diagnosed before 40 years, with the median age of diagnosis ranging from 20–29 years.[77], [78], [79] FLCs are larger (9-13 cm) with a higher rate of lymph node invasion (43–46%) compared to HCC.71,[75], [76], [77] The most frequent sites of metastases are the lung (50%), bone (19.2%), and brain (1.9%).78
Fig. 3.
Main characteristics of fibrolamellar carcinoma.
Representation of the main genetic alterations, as well as clinical, histopathological, and radiological features of fibrolamellar carcinoma; treatment strategies are also shown.
Pathophysiology
At the molecular level, DNAJB1-PRKCA fusion – due to a focal deletion in the chromosome 19 – is identified in almost all FLCs and is considered highly specific but not pathognomonic (Table 2).[79], [80], [81] The same fusion was identified in intraductal oncocytic papillary neoplasms of the pancreas and bile duct.82,83 A subset of HCC with fibrolamellar-like features has been shown to occur in non-cirrhotic livers, but in older patients, and was characterised by both BAP1 alterations and an aberrant activation of the protein kinase A pathway due to a chromosome gain of PRKACA combined with a loss of PRKAR2A (the inhibitory regulatory subunit of protein kinase A).84 These tumours also expressed neuroendocrine and pancreatic markers pointing to a potential hepato-pancreatic progenitor. Finally, GNAS mutations leading to protein kinase A activation were observed in a subset of hepatocellular adenomas with a fibrous stroma.85 All these data suggested that protein kinase A activation in the liver was associated with “fibrolamellar-like” features and underlined a link between the activation of protein kinase A and a hepato-pancreatic progenitor lineage. Finally, rare cases of FLC arising in patients with Carney complex were related to germline inactivating mutations in PRKAR1A (Table 2).86 PRKCA from the DNAJB1-PRKCA fusion has a conserved tyrosine kinase domain and an enhanced cAMP-stimulated protein kinase A activity. It leads to a constitutive activation of the protein kinase A pathway and promoted the malignant transformation of hepatocytes in a mouse model.87,88 As DNAJB1-PRKACA fusion is a genetic footprint of FLC, it could be used to confirm the diagnosis of FLC using fluorescence in situ hybridisation (FISH) or reverse transcription PCR (RT-PCR) in clinical practice.81,89
Diagnosis
Most of the time diagnosis is made in a symptomatic patient with abdominal pain and weight loss.74 Rarely, obstructive jaundice, gynecomastia in males, encephalopathy, ascites, acute liver failure, recurrent thrombophlebitis, anaemia, hypoglycaemia or Budd-Chiari syndrome can reveal FLC.[90], [91], [92] Differential diagnosis consists of primary liver tumours with fibrosis, such as some subtypes of HCC (especially BAP1 mutated HCC), CCA or focal nodular hyperplasia.
The diagnosis of FLC could be suspected on CT and MRI based on the clinical context (young patient without chronic liver disease). FLCs are usually large and lobulated heterogeneous lesions with a central stellate scar seen in 65–70% of cases and tumour calcifications and abdominal lymphadenopathy observed in half of cases.93,94 On MRI, FLC show T1-weighted hypointensity and T2-weighted hyperintensity with a central area showing hypointensity on both T1- and T2-weighted images.93 FLCs exhibit heterogeneous APHE, with a variable enhancement pattern on portal venous and delayed phases.91 Noticeably, FLCs never contain fat, and do not invade hepatic or portal veins in contrast to classical HCC. FLCs are also hypointense on the hepatobiliary phase (using hepatobiliary contrast agents).95 FLCs do not usually produce detectable AFP and less than 10% of patients have AFP levels above 200 ng/ml.90
Tumour and non-tumour liver biopsy is usually advised in clinical practice, with the exception of patients eligible for front-line surgery irrespective of the results of biopsy.92,96 High rates of CK7- and CD68-positive staining on liver samples and low rates of glypican 3-positive staining could differentiate FLC from regular HCC.97,98
Treatment
In a systematic review including 575 patients, those treated with partial hepatectomy (55%) had 5-year overall survival rates of 70%.77 Liver resection was associated with a better overall survival in patients with FLC compared to patients with classical HCC (median overall survival of 84.9 vs. 42.9 months, respectively). However, no significant difference in 5-year survival could be observed when focusing on patients without cirrhosis, suggesting that the difference observed in the overall population was likely related to the severity of the underlying liver disease.70,72,78 Currently, liver resection remains the most effective curative-intent treatment option for FLC; aggressive initial surgical resection along with regional lymphadenectomy is advised.76,77
In contrast, results of LT are impaired by a high rate of tumour recurrence leading to a 5-year overall survival of 35%.77 However, the absence of selection criteria for patients treated by LT limits the interpretation of these studies.72,77,99 Slightly better results were recently reported in 63 patients undergoing LT, with an overall survival rate of 48% at 5 years.100 As for other indications, LT should be discussed according to national guidelines.
Patients with unresectable disease (20 to 25% at diagnosis) are treated with various combinations of systemic therapy, with or without locoregional therapies. The role of TACE or SIRT alone is also poorly studied. Chemotherapy regimens included 5-fluorouracil (5-FU) + cisplatin or irinotecan, doxorubicin and gemcitabine + oxaliplatin, but few patients exhibited a radiological response.101 Sorafenib was associated with stable disease in 4 out of 9 patients and 1 patient achieved a complete response with an anti-PD1 antibody.102 Moreover, aurora kinase A inhibitors had a limited antitumour effect in a phase II clinical trial.103 Shutdown of the PRKCA pathway and targeting the DNABJ1-PRKACA fusion is an appealing therapeutic avenue. While several therapeutic options have been proposed in FLC, such as inhibitors of the kinase pocket of the fusion protein or the combination of Hsp70 and MEK inhibitor,104,105 currently no efficient targeted therapy has been validated.
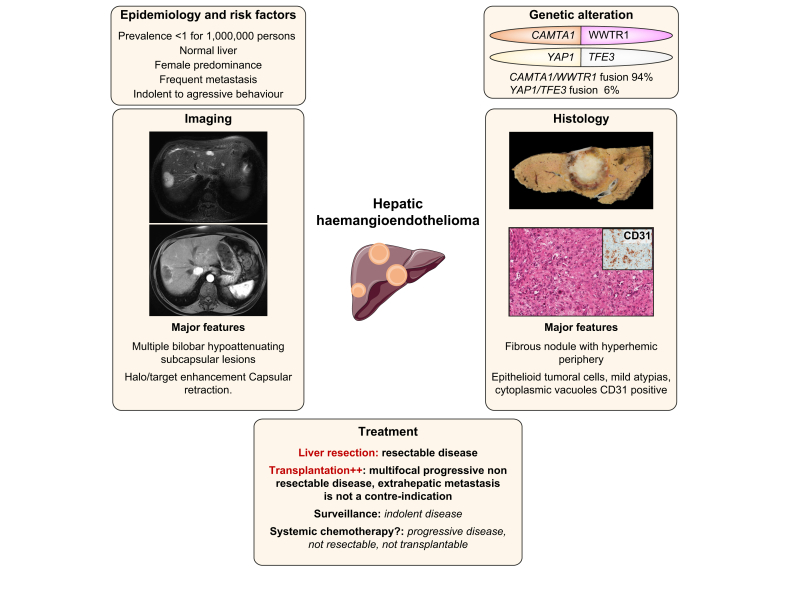
Hepatic epithelioid haemangioendothelioma
HEH is a rare vascular tumour that develops on normal liver, characterised by epithelioid and histiocytoid vascular endothelial cells in a fibrotic stroma (Fig. 4).106 Tumour cells are positive for endothelial markers (factor VIII-related antigen, CD34 and CD31) on immunohistochemistry.106 Tumour cells likely invade pre-existing vascular channels including centrilobular veins at the periphery. Some risk factors have been suggested in the literature such as oral contraception, vinyl chloride, thorotrast, asbestos, or viral hepatitis even if the level of evidence is low.107,108
Fig. 4.
Main characteristics of hepatic haemangioendothelioma.
Representation of the main genetic alterations, as well as clinical, histopathological, and radiological features of hepatic haemangioendothelioma; treatment strategies are also shown.
Haemangioendothelioma was described in 1982 as a vascular neoplasia affecting different organs, with a prevalence of less than 1 per million.[109], [110], [111] The most common organs involved are the liver alone (21%), the liver and lung (18%), the lung alone (12%) and bone alone (14%), but any site in the body can be affected.107,112 HEH is more frequent in females (61% to 80%).107,110 The clinical behaviour is heterogeneous, ranging from indolent to aggressive behaviour.112
Pathophysiology
The CAMTA1-WWTR1 gene fusion, resulting from a translocation t(1;3)(p36.3;q25) involving CAMTA1 (a calmodulin-binding transcription activator) and WWTR1 (coding for TAZ – a transcriptional coactivator) is pathognomonic of HEH (Table 2).113 In cellulo, CAMTA1-WWTR1 fusion results in nuclear localisation of the fusion protein and leads to constitutive activation of the hippo pathway through a TAZ-dependent transcriptomic programme.114 Around 90% of HEHs harbour the CAMTA1-WWTR1 gene fusion which has been consistently identified in haemangioendothelioma, irrespective of the primary site.115 Moreover, a rare YAP1-TFE3 fusion has been identified in HEHs without CAMTA1-WWTR1 fusion (Table 2).116,117 Detection of CAMTA1-WWTR1 fusion by FISH or RT-PCR, or nuclear CAMTA1 expression at immunohistochemistry, is useful to confirm the diagnosis of HEH, as this fusion has not been identified in other human tumours.117,118
Diagnosis
A systemic review including 402 patients with HEH reported that 25% were asymptomatic, while right upper quadrant pain (48.6%), hepatomegaly (20.4%) and weight loss (15.6%) were the most frequent symptoms at diagnosis. Extrahepatic metastases were observed in 36.6% of patients.108 HEH could be nodular or diffuse and nodular lesions are usually multiple and affect both lobes of the liver.
A HEH should be suspected in cases of multifocal nodules (88%), which are sometimes coalescent, or the presence of nodules in subcapsular regions (up to 96%) with a capsular retraction (50 to 80%) on imaging.[119], [120], [121] Presence of ring enhancement at the tumour periphery on arterial phase is observed in 33% of patients, with a target appearance on the portal venous phase in 69% of cases – explained by central fibrosis with a concentric layer of tumour cells and a peripheral avascular rim on histology. On MRI, HEH harboured a target appearance on the T2-weighted sequences in 67% and on the diffusion-weighted sequences in 61% of patients.121
Histology is the gold standard for the diagnosis of HEH, with the help of immunohistochemistry (endothelial markers: factor VIII-related antigen, CD34 and CD31). The differential diagnosis with hepatic angiosarcoma is sometimes difficult to perform at histology and identification of CAMTA1-WWTR1 fusion could be useful to confirm the diagnosis of HEH.122
Treatment
Therapeutic options in HEH depend on tumour burden, extrahepatic metastasis, resectability, age and comorbidities. The pattern of progression (stability vs. slow or rapid progression) should also be used to guide therapeutic decisions.
A comprehensive review of the literature reported the use of LT in 44.8% of patients, followed by no treatment in 24.8%, chemotherapy or radiotherapy in 21% and liver resection in 9.4%.108 Results from a multicentre database showed that LT led to a 5-year overall survival rate of 77.2% in 131 patients with HEH.123 Moreover, patients with extrahepatic metastasis could achieve prolonged survival after LT (up to 78% at 10 years).124
Risk factors for recurrence after LT were macrovascular invasion, waiting time of less than 3 months and lymph node metastases.125 A retrospective study suggested that similar outcomes could be achieved with resection or LT in HEH, although more patients at advanced stages were treated by LT126 As HEH is often a bilobar disease rarely amenable to liver resection, LT might be the best option even for patients with extrahepatic metastasis.127
In non-resectable or non-transplantable patients, different systemic treatments such as interferon alpha, thalidomide, doxorubicin, intra-arterial 5-fluorouracil and bevacizumab have been used in a very limited number of cases.122 In a pilot study of 15 patients affected by HEH of different localisation, sorafenib was associated with a median progression-free survival of 6 months.128 In a subset of patients with indolent disease, careful follow-up can be an option, with recent data reporting 10-year overall survival of 41% in selected patients.129,130 As no systemic treatment is currently approved for the treatment of HEH, a better understanding of the biological consequences of CAMT1-WWTR1 fusion is needed to identify new therapeutic targets.
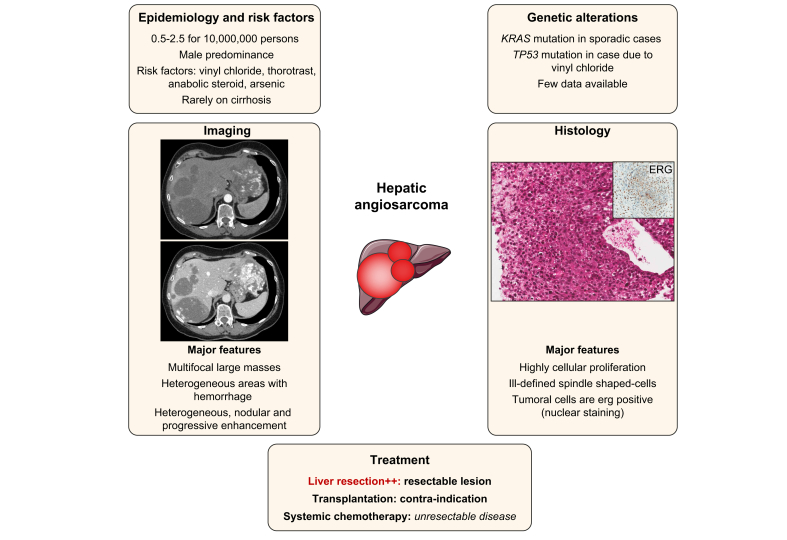
Hepatic angiosarcoma
HAS is a high-grade aggressive mesenchymal malignancy, defining a subtype of soft-tissue sarcoma, composed of malignant endothelial cells of vascular or lymphatic origin that develop mostly on normal liver (Fig. 5). HAS is extremely rare, with an incidence estimated at 0.5–2.5 cases per 10,000,000 people, and more commonly develops in males (ratio 3:1).[131], [132], [133] In the 60s, 25% of HASs were associated with environmental risk factors such as vinyl chloride monomer, thorotrast, anabolic steroids and arsenic.134 When associated with vinyl chloride monomer, HAS can develop on cirrhosis (up to 20% to 43%).[135], [136], [137] Notably, the incidence of HAS declined following controls on vinyl chloride exposure in workers in the 70s.138,139
Fig. 5.
Main characteristics of hepatic angiosarcoma.
Representation of the main genetic alterations, as well as clinical, histopathological, and radiological features of hepatic angiosarcoma; treatment strategies are also shown.
Pathophysiology
Overall, few data on molecular analysis are currently available: KRAS mutations have been described in sporadic cases, TP53 mutations in vinyl chloride-related HAS, and recently a ROS1-GOPC/FIG fusion has been identified in 1 HAS.[140], [141], [142]
Diagnosis
Most of the time, patients with HAS have symptoms at presentation such as abdominal pain, fatigue, weight loss, hepatosplenomegaly, ascites, jaundice, and anaemia. The intraperitoneal rupture of HASs has been reported in 15–27% of patients.143 HASs have a very aggressive behaviour; poor prognostic factors are older age, large tumour size and high Ki-67 index.131,133 In a recent systematic review of 219 patients, the average age at onset was 56.7 years and distant metastases were frequent. The median overall survival was 6 months, with a 2-year survival rate of 17.3%.144
At contrast-enhanced imaging, HAS is usually multifocal with heterogeneous patterns, such as a progressive enhancement without washout at the portal and delayed phase. Progressive centripetal or diffuse “flash-fill” enhancement pattern (“reverse haemangioma”) with centrifugal enhancement have also been reported.136,145 HASs often contain haemorrhagic areas resulting in heterogeneous lesions on MRI, with hyperintense zones on T1WI and hypointense zones on T2WI.145
Some controversy exists about the performance of liver biopsy and about a potential high risk of bleeding .137,143,146 However, histology remains the gold standard for the diagnosis of HAS and liver biopsy is required to confirm the diagnosis.133 HAS is heterogeneous at histology, ranging from well-defined anastomotic vessels (vasoformative) to solid sheets of epithelioid or spindled cells without vasoformation, with different patterns sometimes mixed in the same tumours.131 HASs express ERG (erythroblast transformation specific-related gene) and endothelial markers such as CD31 and CD34.147
Treatment
Surgical resection seems the best therapeutic option, leading to a median overall survival of between 17 and 19 months.143,148 Survival was limited in studies assessing LT (around 6 months), with most of patients dying from tumour recurrence, explaining why HAS is a contraindication to LT.149,150 It is important to note that only 30% of patients had a known pre-LT diagnosis of HAS.126
Transarterial embolisation is frequently used to treat tumour bleeding with a limited impact on survival.144 There is no approved chemotherapy regimen for non-resectable liver HAS. ESMO guidelines on sarcomas report that angiosarcomas in general are sensitive to taxanes, reporting gemcitabine as an option alone or in combination with docetaxel.151
In a phase II trial including 3 primary liver angiosarcomas in patients with metastatic or unresectable disease, weekly paclitaxel led to progression-free survival rates of 74% and 45% at 2 and 4 months, respectively, with a median overall survival of 8 months.152 Palliative chemotherapy, such as 5-FU with doxorubicin or ifosfamide, carboplatin, bevacizumab or sorafenib, has been used in case reports or small series with limited radiological response and poor survival.133 Due to the rarity of this cancer, the management of HAS should be made in centres with multidisciplinary expertise on sarcomas.
Conclusion
Despite several advances in recent decades, mainly in the field of pathophysiology, the diagnosis of rare PLCs remains challenging, and the prospective collection of dedicated clinical data, as well as trial recruitment, remain limited. Moreover, grants dedicated to these PLCs are lacking and pharmaceutical companies are rarely interested in the development of new drugs for these patients. To bypass these limitations, large international consortiums are needed to raise grants to run large prospective cohorts and to better define rare PLCs in terms of pathophysiology and clinical behaviour. This cooperative network will also be the basis of future clinical trials.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
Elia Gigante: writing, revision and approval of the manuscript. Valérie Paradis: writing, revision and approval of the manuscript. Maxime Ronot: writing, revision and approval of the manuscript. François Cauchy: writing, revision and approval of the manuscript. Olivier Soubrane: revision and approval of the manuscript. Nathalie Ganne-Carrié : writing, revision and approval of the manuscript. Jean-Charles Nault: writing, revision and approval of the manuscript.
Conflicts of interests
Jean-Charles Nault received research grant from Bayer for Inserm UMR1148, Nathalie Ganne-Carrié received personal fees from Bayer, Gilead, Ipsen and Shionogi. Elia Gigante, François Cauchy, Maxime Ronot, Valérie Paradis, Olivier soubrane: none to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100174.
Supplementary data
References
- 1.Allen R.A., Lisa J.R. Combined liver cell ahd bile duct carcinoma. Am J Pathol. 1949;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 2.Koster H., Kasman L.P. Primary duplex liver carcinoma. Am J Surg. 1932;17:237–241. [Google Scholar]
- 3.Theise N Nakashima O., Park Y.N., Nakanuma Y. Combined hepatocellular-cholangiocarcinoma. In: Bosman F.T., Carnoiro F., Hruba R.H., Theise N.D., editors. WHO classification of tumors of the digestive system. IARC Press; Lyon: 2010. pp. 225–227. [Google Scholar]
- 4.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2019 doi: 10.1111/his.13975. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt E., Aishima S., Clavien P.-A., Fowler K., Goodman Z., Gores G. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113–126. doi: 10.1002/hep.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sempoux C., Kakar S., Kondo F., Schirmacher P. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. In: Arends M.J., Fukuyama M., Klimstra D.S., editors. WHO Classification of Tumours: Digestive System Tumours. 5th. IARC; Lyon: 2019. p. 260. [Google Scholar]
- 7.Sasaki M., Sato H., Kakuda Y., Sato Y., Choi J.H., Nakanuma Y. Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular-cholangiocarcinoma. Liver Int. 2015;35:1024–1035. doi: 10.1111/liv.12563. [DOI] [PubMed] [Google Scholar]
- 8.Akiba J., Nakashima O., Hattori S., Tanikawa K., Takenaka M., Nakayama M. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496–505. doi: 10.1097/PAS.0b013e31827332b0. [DOI] [PubMed] [Google Scholar]
- 9.Garancini M., Goffredo P., Pagni F., Romano F., Roman S., Sosa J.A. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952–959. doi: 10.1002/lt.23897. [DOI] [PubMed] [Google Scholar]
- 10.Ramai D., Ofosu A., Lai J.K., Reddy M., Adler D.G. Combined hepatocellular cholangiocarcinoma: a population-based retrospective study. Am J Gastroenterol. 2019;114:1496–1501. doi: 10.14309/ajg.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M., Izumi N., Kubo S., Kokudo N., Sakamoto M., Shiina S. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2020;50:15–46. doi: 10.1111/hepr.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman Z.D., Ishak K.G., Langloss J.M., Sesterhenn I.A., Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Ng I.O., Shek T.W., Nicholls J., Ma L.T. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34–40. doi: 10.1111/j.1440-1746.1998.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 14.Jarnagin W.R., Weber S., Tickoo S.K., Koea J.B., Obiekwe S., Fong Y. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040–2046. doi: 10.1002/cncr.10392. [DOI] [PubMed] [Google Scholar]
- 15.Lee W.-S., Lee K.-W., Heo J.-S., Kim S.-J., Choi S.-H., Kim Y.-I. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892–897. doi: 10.1007/s00595-006-3276-8. [DOI] [PubMed] [Google Scholar]
- 16.Edmondson H.A., Steiner P.E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi J., Nakashima O., Tanaka M., Hisaka T., Takazawa T., Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996;11:758–764. doi: 10.1111/j.1440-1746.1996.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu C.-L., Fan S.-T., Lo C.-M., Ng I.O.-L., Lam C.-M., Poon R.T.-P. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg. 2003;138:86–90. [PubMed] [Google Scholar]
- 19.Gentile D., Donadon M., Lleo A., Aghemo A., Roncalli M., di Tommaso L. Surgical treatment of hepatocholangiocarcinoma: a systematic review. Liver Cancer. 2020;9:15–27. doi: 10.1159/000503719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y.-M., Zhang X.-F., Wu L.-P., Sui C.-J., Yang J.-M. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-control study. World J Gastroenterol. 2014;20:12615–12620. doi: 10.3748/wjg.v20.i35.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapisochin G., de Lope C.R., Gastaca M., de Urbina J.O., López-Andujar R., Palacios F. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish Matched Cohort Multicenter Study. Ann Surg. 2014:259. doi: 10.1097/SLA.0000000000000494. https://journals.lww.com/annalsofsurgery/Fulltext/2014/05000/Intrahepatic_Cholangiocarcinoma_or_Mixed.17.aspx Available from: [DOI] [PubMed] [Google Scholar]
- 22.Wells M.L., Venkatesh S.K., Chandan V.S., Fidler J.L., Fletcher J.G., Johnson G.B. Biphenotypic hepatic tumors: imaging findings and review of literature. Abdom Imaging. 2015;40:2293–2305. doi: 10.1007/s00261-015-0433-9. [DOI] [PubMed] [Google Scholar]
- 23.Gigante E., Ronot M., Bertin C., Ciolina M., Bouattour M., Dondero F. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019;39:2386–2396. doi: 10.1111/liv.14261. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto A., Furuta M., Shiraishi Y., Gotoh K., Kawakami Y., Arihiro K. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120. doi: 10.1038/ncomms7120. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z.-H., Lian B.-F., Dong Q.-Z., Sun H., Wei J.-W., Sheng Y.-Y. Whole-exome mutational and transcriptional landscapes of combined hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma reveal molecular diversity. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2360–2368. doi: 10.1016/j.bbadis.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Joseph N.M., Tsokos C.G., Umetsu S.E., Shain A.H., Kelley R.K., Onodera C. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol. 2019;248:164–178. doi: 10.1002/path.5243. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki M., Sato Y., Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology. 2017;70:423–434. doi: 10.1111/his.13084. [DOI] [PubMed] [Google Scholar]
- 28.Moeini A., Sia D., Zhang Z., Camprecios G., Stueck A., Dong H. Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66:952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Xue R., Chen L., Zhang C., Fujita M., Li R., Yan S.-M. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell. 2019;35:932–947.e8. doi: 10.1016/j.ccell.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki M., Sato Y., Nakanuma Y. Cholangiolocellular carcinoma with “Ductal Plate Malformation” pattern may Be characterized by ARID1A genetic alterations. Am J Surg Pathol. 2019;43:352–360. doi: 10.1097/PAS.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 31.Wang A., Wu L., Lin J., Han L., Bian J., Wu Y. Whole-exome sequencing reveals the origin and evolution of hepato-cholangiocarcinoma. Nat Commun. 2018;9:894. doi: 10.1038/s41467-018-03276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz S.-F., Lechel A., Obenauf A.C., Begus-Nahrmann Y., Kraus J.M., Hoffmann E.M. Disruption of Trp53 in livers of mice induces formation of carcinomas with bilineal differentiation. Gastroenterology. 2012;142:1229–1239.e3. doi: 10.1053/j.gastro.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Tschaharganeh D.F., Xue W., Calvisi D.F., Evert M., Michurina T.V., Dow L.E. p53-dependent Nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell. 2014;158:579–592. doi: 10.1016/j.cell.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano H., Iemura A., Haramaki M., Momosaki S., Ogasawara S., Higaki K. A human combined hepatocellular and cholangiocarcinoma cell line (KMCH-2) that shows the features of hepatocellular carcinoma or cholangiocarcinoma under different growth conditions. J Hepatol. 1996;24:413–422. doi: 10.1016/s0168-8278(96)80161-9. [DOI] [PubMed] [Google Scholar]
- 35.Fujii H., Zhu X.G., Matsumoto T., Inagaki M., Tokusashi Y., Miyokawa N. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol. 2000;31:1011–1017. doi: 10.1053/hupa.2000.9782. [DOI] [PubMed] [Google Scholar]
- 36.Roskams T.A., Libbrecht L., Desmet V.J. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 37.Yeh M.M. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485–1492. doi: 10.1111/j.1440-1746.2010.06430.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Li Y., Yu G. Diagnostic value of serum biomarkers in combined hepatocelluar-cholangiocarcinoma. J Coll Physicians Surg Pak. 2020;30:263–267. doi: 10.29271/jcpsp.2020.03.263. [DOI] [PubMed] [Google Scholar]
- 39.Potretzke T.A., Tan B.R., Doyle M.B., Brunt E.M., Heiken J.P., Fowler K.J. Imaging features of biphenotypic primary liver carcinoma (hepatocholangiocarcinoma) and the potential to mimic hepatocellular carcinoma: LI-RADS analysis of CT and MRI features in 61 cases. AJR Am J Roentgenol. 2016;207:25–31. doi: 10.2214/AJR.15.14997. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell D.G., Bruix J., Sherman M., Sirlin C.B. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056–1065. doi: 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- 41.Jeon S.K., Joo I., Lee D.H., Lee S.M., Kang H.-J., Lee K.-B. Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol. 2019;29:373–382. doi: 10.1007/s00330-018-5605-x. [DOI] [PubMed] [Google Scholar]
- 42.Hwang J., Kim Y.K., Park M.J., Lee M.H., Kim S.H., Lee W.J. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012;36:881–889. doi: 10.1002/jmri.23728. [DOI] [PubMed] [Google Scholar]
- 43.Nishie A., Yoshimitsu K., Asayama Y., Irie H., Aibe H., Tajima T. Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. Am J Roentgenol. 2005;184:1157–1162. doi: 10.2214/ajr.184.4.01841157. [DOI] [PubMed] [Google Scholar]
- 44.Willekens I., Hoorens A., Geers C., Op de Beeck B., Vandenbroucke F., de Mey J. Combined hepatocellular and cholangiocellular carcinoma presenting with radiological characteristics of focal nodular hyperplasia. World J Gastroenterol. 2009;15:3940–3943. doi: 10.3748/wjg.15.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Campos R.O.P., Semelka R.C., Azevedo R.M., Ramalho M., Heredia V., Armao D.M. Combined hepatocellular carcinoma-cholangiocarcinoma: report of MR appearance in eleven patients. J Magn Reson Imaging. 2012;36:1139–1147. doi: 10.1002/jmri.23754. [DOI] [PubMed] [Google Scholar]
- 46.Fowler K.J., Sheybani A., Parker R.A., Doherty S., Brunt E M., Chapman W.C. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. Am J Roentgenol. 2013;201:332–339. doi: 10.2214/AJR.12.9488. [DOI] [PubMed] [Google Scholar]
- 47.Li R., Yang D., Tang C.-L., Cai P., Ma K.-S., Ding S.-Y. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158. doi: 10.1186/s12885-016-2156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagrini E., Iavarone M., Stefanini F., Tovoli F., Vavassori S., Maggioni M. Imaging of combined hepatocellular-cholangiocarcinoma in cirrhosis and risk of false diagnosis of hepatocellular carcinoma. United European Gastroenterol J. 2019;7:69–77. doi: 10.1177/2050640618815378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal S., Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- 50.Ma K.W., Chok K.S.H. Importance of surgical margin in the outcomes of hepatocholangiocarcinoma. World J Hepatol. 2017;9:635–641. doi: 10.4254/wjh.v9.i13.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cucchetti A., Piscaglia F., Grigioni A.D., Ravaioli M., Cescon M., Zanello M. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;6(52):880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 52.Bagante F., Spolverato G., Weiss M., Alexandrescu S., Marques H.P., Aldrighetti L. Surgical management of intrahepatic cholangiocarcinoma in patients with cirrhosis: impact of lymphadenectomy on peri-operative outcomes. World J Surg. 2018;42:2551–2560. doi: 10.1007/s00268-017-4453-1. [DOI] [PubMed] [Google Scholar]
- 53.Yoh T., Cauchy F., Soubrane O. Oncological resection for liver malignancies: can the laparoscopic approach provide benefits? Ann Surg. 2020 doi: 10.1097/SLA.0000000000003851. [DOI] [PubMed] [Google Scholar]
- 54.Holzner M.L., Tabrizian P., Parvin-Nejad F.P., Fei K., Gunasekaran G., Rocha C. Resection of mixed hepatocellular-cholangiocarcinoma, hepatocellular carcinoma, and intrahepatic cholangiocarcinoma: a Western Center Experience. Liver Transpl. 2020;26:888–898. doi: 10.1002/lt.25786. [DOI] [PubMed] [Google Scholar]
- 55.De Martin E., Rayar M., Golse N., Dupeux M., Gelli M., Gnemmi V. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl. 2020;26:785–798. doi: 10.1002/lt.25737. [DOI] [PubMed] [Google Scholar]
- 56.Lunsford K.E., Court C., Seok Lee Y., Lu D.S., Naini B.V., Harlander-Locke M.P. Propensity-matched analysis of patients with mixed hepatocellular-cholangiocarcinoma and hepatocellular carcinoma undergoing liver transplantation. Liver Transpl. 2018;24:1384–1397. doi: 10.1002/lt.25058. [DOI] [PubMed] [Google Scholar]
- 57.Sapisochin G., Javle M., Lerut J., Ohtsuka M., Ghobrial M., Hibi T. Liver transplantation for cholangiocarcinoma and mixed hepatocellular-cholangiocarcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1125–1130. doi: 10.1097/TP.0000000000003212. [DOI] [PubMed] [Google Scholar]
- 58.Kim J.H., Yoon H.-K., Ko G.-Y., Gwon D.I., Jang C.S., Song H.-Y. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255:270–277. doi: 10.1148/radiol.09091076. [DOI] [PubMed] [Google Scholar]
- 59.Yoon Y.-I., Hwang S., Lee Y.-J., Kim K.-H., Ahn C.-S., Moon D.-B. Postresection outcomes of combined hepatocellular carcinoma-cholangiocarcinoma, hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2016;20:411–420. doi: 10.1007/s11605-015-3045-3. [DOI] [PubMed] [Google Scholar]
- 60.Na S.K., Choi G.H., Lee H.C., Shin Y.M., An J., Lee D. The effectiveness of transarterial chemoembolization in recurrent hepatocellular-cholangiocarcinoma after resection. PLoS One. 2018;13:e0198138. doi: 10.1371/journal.pone.0198138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edeline J., Touchefeu Y., Guiu B., Farge O., Tougeron D., Baumgaertner I. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2019;6:51–59. doi: 10.1001/jamaoncol.2019.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malone C.D., Gibby W., Tsai R., Kim S.K., Lancia S., Akinwande O. Outcomes of Yttrium-90 radioembolization for unresectable combined biphenotypic hepatocellular-cholangiocarcinoma. J Vasc Interv Radiol. 2020;31:701–709. doi: 10.1016/j.jvir.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 63.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.-F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 64.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2017;15:95. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi S., Terashima T., Shiba S., Yoshida Y., Yamada I., Iwadou S. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109:2549–2557. doi: 10.1111/cas.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salimon M., Prieux-Klotz C., Tougeron D., Hautefeuille V., Caulet M., Gournay J. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018;118:325–330. doi: 10.1038/bjc.2017.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trikalinos N.A., Zhou A., Doyle M.B.M., Fowler K.J., Morton A., Vachharajani N. Systemic therapy for combined hepatocellular-cholangiocarcinoma: a single-institution experience. J Natl Compr Canc Netw. 2018;16:1193–1199. doi: 10.6004/jnccn.2018.7053. [DOI] [PubMed] [Google Scholar]
- 68.Lin C.-C., Yang H.-M. Fibrolamellar carcinoma: a concise review. Arch Pathol Lab Med. 2018;142:1141–1145. doi: 10.5858/arpa.2017-0083-RS. [DOI] [PubMed] [Google Scholar]
- 69.Lalazar G., Simon S.M. Fibrolamellar carcinoma: recent advances and unresolved questions on the molecular mechanisms. Semin Liver Dis. 2018;38:51–59. doi: 10.1055/s-0037-1621710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramai D., Ofosu A., Lai J.K., Gao Z.-H., Adler D.G. Fibrolamellar hepatocellular carcinoma: a population-based observational study. Dig Dis Sci. 2020 doi: 10.1007/s10620-020-06135-3. [DOI] [PubMed] [Google Scholar]
- 71.Mayo S.C., Mavros M.N., Nathan H., Cosgrove D., Herman J.M., Kamel I. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg. 2014;218:196–205. doi: 10.1016/j.jamcollsurg.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Njei B., Konjeti V.R., Ditah I. Prognosis of patients with fibrolamellar hepatocellular carcinoma versus conventional hepatocellular carcinoma: a systematic review and meta-analysis. Gastrointest Cancer Res. 2014;7:49–54. [PMC free article] [PubMed] [Google Scholar]
- 73.Edmondson H.A. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–186. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 74.Craig J.R., Peters R.L., Edmondson H.A., Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46:372–379. doi: 10.1002/1097-0142(19800715)46:2<372::aid-cncr2820460227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 75.Yamashita S., Vauthey J.-N., Kaseb A.O., Aloia T.A., Conrad C., Hassan M.M. Prognosis of fibrolamellar carcinoma compared to non-cirrhotic conventional hepatocellular carcinoma. J Gastrointest Surg. 2016;20:1725–1731. doi: 10.1007/s11605-016-3216-x. [DOI] [PubMed] [Google Scholar]
- 76.Stipa F., Yoon S.S., Liau K.H., Fong Y., Jarnagin W.R., D'Angelica M. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331–1338. doi: 10.1002/cncr.21703. [DOI] [PubMed] [Google Scholar]
- 77.Mavros M.N., Mayo S.C., Hyder O., Pawlik T.M. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820–830. doi: 10.1016/j.jamcollsurg.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Assi H.A., Mukherjee S., Machiorlatti M., Vesely S., Pareek V., Hatoum H. Predictors of outcome in patients with fibrolamellar carcinoma: analysis of the National Cancer Database. Anticancer Res. 2020;40:847–855. doi: 10.21873/anticanres.14017. [DOI] [PubMed] [Google Scholar]
- 79.Honeyman J.N., Simon E.P., Robine N., Chiaroni-Clarke R., Darcy D.G., Lim I.I.P. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–1014. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cornella H., Alsinet C., Sayols S., Zhang Z., Hao K., Cabellos L. Unique genomic profile of fibrolamellar hepatocellular carcinoma. Gastroenterology. 2015;148:806–818.e10. doi: 10.1053/j.gastro.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graham R.P., Jin L., Knutson D.L., Kloft-Nelson S.M., Greipp P.T., Waldburger N. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol. 2015;28:822–829. doi: 10.1038/modpathol.2015.4. [DOI] [PubMed] [Google Scholar]
- 82.Singhi A.D., Wood L.D., Parks E., Torbenson M.S., Felsenstein M., Hruban R.H. Recurrent rearrangements in PRKACA and PRKACB in intraductal oncocytic papillary neoplasms of the pancreas and bile duct. Gastroenterology. 2020;158:573–582.e2. doi: 10.1053/j.gastro.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vyas M., Hechtman J.F., Zhang Y., Benayed R., Yavas A., Askan G. DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Mod Pathol. 2020;33:648–656. doi: 10.1038/s41379-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirsch T.Z., Negulescu A., Gupta B., Caruso S., Noblet B., Couchy G. BAP1 mutations define a homogeneous subgroup of hepatocellular carcinoma with fibrolamellar-like features and activated PKA. J Hepatol. 2020;72:924–936. doi: 10.1016/j.jhep.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 85.Nault J.C., Fabre M., Couchy G., Pilati C., Jeannot E., Tran Van Nhieu J. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol. 2012;56:184–191. doi: 10.1016/j.jhep.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 86.Graham R.P., Lackner C., Terracciano L., González-Cantú Y., Maleszewski J.J., Greipp P.T. Fibrolamellar carcinoma in the Carney complex: PRKAR1A loss instead of the classic DNAJB1-PRKACA fusion. Hepatology. 2018;68:1441–1447. doi: 10.1002/hep.29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riggle K.M., Riehle K.J., Kenerson H.L., Turnham R., Homma M.K., Kazami M. Enhanced cAMP-stimulated protein kinase A activity in human fibrolamellar hepatocellular carcinoma. Pediatr Res. 2016;80:110–118. doi: 10.1038/pr.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engelholm L.H., Riaz A., Serra D., Dagnæs-Hansen F., Johansen J.V., Santoni-Rugiu E. CRISPR/Cas9 engineering of adult mouse liver demonstrates that the dnajb1-prkaca gene fusion is sufficient to induce tumors resembling fibrolamellar hepatocellular carcinoma. Gastroenterology. 2017;153:1662–1673.e10. doi: 10.1053/j.gastro.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graham R.P., Yeh M.M., Lam-Himlin D., Roberts L.R., Terracciano L., Cruise M.W. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Mod Pathol. 2018;31:141–149. doi: 10.1038/modpathol.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torbenson M. Review of the clinicopathologic features of fibrolamellar carcinoma. Adv Anat Pathol. 2007;14:217–223. doi: 10.1097/PAP.0b013e3180504913. [DOI] [PubMed] [Google Scholar]
- 91.Ganeshan D., Szklaruk J., Kundra V., Kaseb A., Rashid A., Elsayes K.M. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol. 2014;202:544–552. doi: 10.2214/AJR.13.11117. [DOI] [PubMed] [Google Scholar]
- 92.Lafaro K.J., Pawlik T.M. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma. 2015;2:151–157. doi: 10.2147/JHC.S75153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ichikawa T., Federle M.P., Grazioli L., Madariaga J., Nalesnik M., Marsh W. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology. 1999;213:352–361. doi: 10.1148/radiology.213.2.r99nv31352. [DOI] [PubMed] [Google Scholar]
- 94.Friedman A.C., Lichtenstein J.E., Goodman Z., Fishman E.K., Siegelman S.S., Dachman A.H. Fibrolamellar hepatocellular carcinoma. Radiology. 1985;157:583–587. doi: 10.1148/radiology.157.3.2997835. [DOI] [PubMed] [Google Scholar]
- 95.Palm V., Sheng R., Mayer P., Weiss K.-H., Springfeld C., Mehrabi A. Imaging features of fibrolamellar hepatocellular carcinoma in gadoxetic acid-enhanced MRI. Cancer Imaging. 2018;18:9. doi: 10.1186/s40644-018-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pérez-Guillermo M., Masgrau N.A., García-Solano J., Sola-Pérez J., de Agustín y de Agustín P. Cytologic aspect of fibrolamellar hepatocellular carcinoma in fine-needle aspirates. Diagn Cytopathol. 1999;21:180–187. doi: 10.1002/(sici)1097-0339(199909)21:3<180::aid-dc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 97.Abdul-Al H.M., Wang G., Makhlouf H.R., Goodman Z.D. Fibrolamellar hepatocellular carcinoma: an immunohistochemical comparison with conventional hepatocellular carcinoma. Int J Surg Pathol. 2010;18:313–318. doi: 10.1177/1066896910364229. [DOI] [PubMed] [Google Scholar]
- 98.Ross H.M., Daniel H.D.J., Vivekanandan P., Kannangai R., Yeh M.M., Wu T.-T. Fibrolamellar carcinomas are positive for CD68. Mod Pathol. 2011;24:390–395. doi: 10.1038/modpathol.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pinna A.D., Iwatsuki S., Lee R.G., Todo S., Madariaga J.R., Marsh J.W. Treatment of fibrolamellar hepatoma with subtotal hepatectomy or transplantation. Hepatology. 1997;26:877–883. doi: 10.1002/hep.510260412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atienza L.G., Berger J., Mei X., Shah M.B., Daily M.F., Grigorian A. Liver transplantation for fibrolamellar hepatocellular carcinoma: a national perspective. J Surg Oncol. 2017;115:319–323. doi: 10.1002/jso.24515. [DOI] [PubMed] [Google Scholar]
- 101.Ang C.S., Kelley R.K., Choti M.A., Cosgrove D.P., Chou J.F., Klimstra D. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3–9. [PMC free article] [PubMed] [Google Scholar]
- 102.Chakrabarti S., Tella S.H., Kommalapati A., Huffman B.M., Yadav S., Riaz I.B. Clinicopathological features and outcomes of fibrolamellar hepatocellular carcinoma. J Gastrointest Oncol. 2019;10:554–561. doi: 10.21037/jgo.2019.01.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abou-Alfa G.K., Mayer R., Venook A.P., O’Neill A.F., Beg M.S., LaQuaglia M. Phase II multicenter, open-label study of oral ENMD-2076 for the treatment of patients with advanced fibrolamellar carcinoma. Oncologist. 2020 doi: 10.1634/theoncologist.2020-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simon E.P., Freije C.A., Farber B.A., Lalazar G., Darcy D.G., Honeyman J.N. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U.S.A. 2015;112:E5916–E5925. doi: 10.1073/pnas.1424894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Averill A.M., Rehman H.T., Charles J.W., Dinh T.A., Danyal K., Verschraegen C.F. Inhibition of the chimeric DnaJ-PKAc enzyme by endogenous inhibitor proteins. J Cell. Biochem. 2019;120:13783–13791. doi: 10.1002/jcb.28651. [DOI] [PubMed] [Google Scholar]
- 106.Studer L.L., Selby D.M. Hepatic epithelioid hemangioendothelioma. Arch Pathol Lab Med. 2018;142:263–267. doi: 10.5858/arpa.2016-0171-RS. [DOI] [PubMed] [Google Scholar]
- 107.Lau K., Massad M., Pollak C., Rubin C., Yeh J., Wang J. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312–1318. doi: 10.1378/chest.11-0039. [DOI] [PubMed] [Google Scholar]
- 108.Mehrabi A., Kashfi A., Fonouni H., Schemmer P., Schmied B.M., Hallscheidt P. Primary malignant hepatic epithelioid hemangioendothelioma. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 109.Weiss S.W., Enzinger F.M. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 110.Sardaro A., Bardoscia L., Petruzzelli M.F., Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev. 2014;8:259. doi: 10.4081/oncol.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishak K.G., Sesterhenn I.A., Goodman Z.D., Rabin L., Stromeyer F.W. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839–852. doi: 10.1016/s0046-8177(84)80145-8. [DOI] [PubMed] [Google Scholar]
- 112.Rosenberg A., Agulnik M. Epithelioid hemangioendothelioma: update on diagnosis and treatment. Curr Treat Options Oncol. 2018;19:19. doi: 10.1007/s11864-018-0536-y. [DOI] [PubMed] [Google Scholar]
- 113.Tanas M.R., Sboner A., Oliveira A.M., Erickson-Johnson M.R., Hespelt J., Hanwright P.J. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002409. 98ra82. [DOI] [PubMed] [Google Scholar]
- 114.Tanas M.R., Ma S., Jadaan F.O., Ng C.K.Y., Weigelt B., Reis-Filho J.S. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene. 2016;35:929–938. doi: 10.1038/onc.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Errani C., Zhang L., Sung Y.S., Hajdu M., Singer S., Maki R.G. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Antonescu C.R., Le Loarer F., Mosquera J.-M., Sboner A., Zhang L., Chen C.-L. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel N.R., Salim A.A., Sayeed H., Sarabia S.F., Hollingsworth F., Warren M. Molecular characterization of epithelioid haemangioendotheliomas identifies novel WWTR1-CAMTA1 fusion variants. Histopathology. 2015;67:699–708. doi: 10.1111/his.12697. [DOI] [PubMed] [Google Scholar]
- 118.Doyle L.A., Fletcher C.D.M., Hornick J.L. Nuclear expression of CAMTA1 distinguishes epithelioid hemangioendothelioma from histologic mimics. Am J Surg Pathol. 2016;40:94–102. doi: 10.1097/PAS.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 119.Mulazzani L., Alvisi M. Imaging findings of hepatic epithelioid hemangioendothelioma and fibrolamellar hepatocellular carcinoma: a critical appraisal of current literature about imaging features of two rare liver cancers. Translational Cancer Res. 2018;8:S297–S310. doi: 10.21037/tcr.2018.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dong Y., Wang W.-P., Cantisani V., D’Onofrio M., Ignee A., Mulazzani L. Contrast-enhanced ultrasound of histologically proven hepatic epithelioid hemangioendothelioma. World J Gastroenterol. 2016;22:4741–4749. doi: 10.3748/wjg.v22.i19.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ganeshan D., Pickhardt P.J., Morani A.C., Javadi S., Lubner M.G., Elmohr M.M. Hepatic hemangioendothelioma: CT, MR, and FDG-PET-CT in 67 patients-a bi-institutional comprehensive cancer center review. Eur Radiol. 2020;30:2435–2442. doi: 10.1007/s00330-019-06637-3. [DOI] [PubMed] [Google Scholar]
- 122.Virarkar M., Saleh M., Diab R., Taggart M., Bhargava P., Bhosale P. Hepatic hemangioendothelioma: an update. World J Gastrointest Oncol. 2020;12:248–266. doi: 10.4251/wjgo.v12.i3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brahmbhatt M., Prenner S., Bittermann T. Liver transplantation for hepatic epithelioid hemangioendothelioma is facilitated by exception points with acceptable long-term outcomes. Transplantation. 2020;104:1187–1192. doi: 10.1097/TP.0000000000002982. [DOI] [PubMed] [Google Scholar]
- 124.Lerut J.P., Orlando G., Adam R., Schiavo M., Klempnauer J., Mirza D. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949–957. doi: 10.1097/SLA.0b013e31815c2a70. discussion 957. [DOI] [PubMed] [Google Scholar]
- 125.Lai Q., Feys E., Karam V., Adam R., Klempnauer J., Oliverius M. Hepatic epithelioid hemangioendothelioma and adult liver transplantation: proposal for a prognostic score based on the analysis of the ELTR-ELITA Registry. Transplantation. 2017;101:555–564. doi: 10.1097/TP.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 126.Konstantinidis I.T., Nota C., Jutric Z., Ituarte P., Chow W., Chu P. Primary liver sarcomas in the modern era: resection or transplantation? J Surg Oncol. 2018;117:886–891. doi: 10.1002/jso.24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abreu P., Gorgen A., Oldani G., Hibi T., Sapisochin G. Recent advances in liver transplantation for cancer: the future of transplant oncology. JHEP Rep. 2019;1:377–391. doi: 10.1016/j.jhepr.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chevreau C., Le Cesne A., Ray-Coquard I., Italiano A., Cioffi A., Isambert N. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO) Cancer. 2013;119:2639–2644. doi: 10.1002/cncr.28109. [DOI] [PubMed] [Google Scholar]
- 129.Sanduzzi-Zamparelli M., Rimola J., Montironi C., Nunes V., Avancini Ferreira Alves V., Sapena V. Hepatic epithelioid hemangioendothelioma: an international multicenter study. Dig Liver Dis. 2020;52:1041–1046. doi: 10.1016/j.dld.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 130.Gigante E., Lai Q., Lerut J.P., Nault J.-C. Hepatic haemangioendothelioma: a proteiform disease. Dig Liver Dis. 2020;52:1039–1040. doi: 10.1016/j.dld.2020.05.041. [DOI] [PubMed] [Google Scholar]
- 131.Thway K, Doyle LA, Fukayama M et Hornick JL. Angiosarcoma. The 2019 WHO Classification of Tumours of the Digestive System. Fifth Edition. Lyon (France) IARC. http://publication.iarc.fr//579.
- 132.Chaudhary P., Bhadana U., Singh R.a.K., Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol. 2015;41:1137–1143. doi: 10.1016/j.ejso.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 133.Kim H.R., Rha S.Y., Cheon S.H., Roh J.K., Park Y.N., Yoo N.C. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20:780–787. doi: 10.1093/annonc/mdn702. [DOI] [PubMed] [Google Scholar]
- 134.Falk H., Herbert J., Crowley S., Ishak K.G., Thomas L.B., Popper H. Epidemiology of hepatic angiosarcoma in the United States: 1964-1974. Environ Health Perspect. 1981;41:107–113. doi: 10.1289/ehp.8141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Popper H., Thomas L.B. Alterations of liver and spleen among workers exposed to vinyl chloride. Ann N Y Acad Sci. 1975;246:172–194. doi: 10.1111/j.1749-6632.1975.tb51093.x. [DOI] [PubMed] [Google Scholar]
- 136.Pickhardt P.J., Kitchin D., Lubner M.G., Ganeshan D.M., Bhalla S., Covey A.M. Primary hepatic angiosarcoma: multi-institutional comprehensive cancer centre review of multiphasic CT and MR imaging in 35 patients. Eur Radiol. 2015;25:315–322. doi: 10.1007/s00330-014-3442-0. [DOI] [PubMed] [Google Scholar]
- 137.Locker G.Y., Doroshow J.H., Zwelling L.A., Chabner B.A. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore) 1979;58:48–64. doi: 10.1097/00005792-197901000-00003. [DOI] [PubMed] [Google Scholar]
- 138.European Association for the Study of the Liver. Clinical Practice Guideline Panel: Chair. Panel Members. EASL Governing Board representative EASL clinical practice guideline: occupational liver diseases. J Hepatol. 2019;71:1022–1037. doi: 10.1016/j.jhep.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 139.Mundt K.A., Dell L.D., Crawford L., Gallagher A.E. Quantitative estimated exposure to vinyl chloride and risk of angiosarcoma of the liver and hepatocellular cancer in the US industry-wide vinyl chloride cohort: mortality update through 2013. Occup Environ Med. 2017;74:709–716. doi: 10.1136/oemed-2016-104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Przygodzki R.M., Finkelstein S.D., Keohavong P., Zhu D., Bakker A., Swalsky P.A. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest. 1997;76:153–159. [PubMed] [Google Scholar]
- 141.Hollstein M., Marion M.J., Lehman T., Welsh J., Harris C.C., Martel-Planche G. p53 mutations at A:T base pairs in angiosarcomas of vinyl chloride-exposed factory workers. Carcinogenesis. 1994;15:1–3. doi: 10.1093/carcin/15.1.1. [DOI] [PubMed] [Google Scholar]
- 142.Marks E.I., Pamarthy S., Dizon D., Birnbaum A., Yakirevich E., Safran H. ROS1-GOPC/FIG: a novel gene fusion in hepatic angiosarcoma. Oncotarget. 2019;10:245–251. doi: 10.18632/oncotarget.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zheng Y.-W., Zhang X.-W., Zhang J.-L., Hui Z.-Z., Du W.-J., Li R.-M. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906–911. doi: 10.1111/jgh.12506. [DOI] [PubMed] [Google Scholar]
- 144.Zeng D., Cheng J., Gong Z., Chen J., Long H., Zhu B. A pooled analysis of primary hepatic angiosarcoma. Jpn J Clin Oncol. 2020;50:556–567. doi: 10.1093/jjco/hyaa017. [DOI] [PubMed] [Google Scholar]
- 145.Thampy R., Elsayes K.M., Menias C.O., Pickhardt P.J., Kang H.C., Deshmukh S.P. Imaging features of rare mesenychmal liver tumours: beyond haemangiomas. Br J Radiol. 2017;90 doi: 10.1259/bjr.20170373. 20170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maluf D., Cotterell A., Clark B., Stravitz T., Kauffman H.M., Fisher R.A. Hepatic angiosarcoma and liver transplantation: case report and literature review. Transplant. Proc. 2005;37:2195–2199. doi: 10.1016/j.transproceed.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 147.Young R.J., Brown N.J., Reed M.W., Hughes D., Woll P.J. Angiosarcoma Lancet Oncol. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 148.Tripke V., Heinrich S., Huber T., Mittler J., Hoppe-Lotichius M., Straub B.K. Surgical therapy of primary hepatic angiosarcoma. BMC Surg. 2019;19:5. doi: 10.1186/s12893-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Orlando G., Adam R., Mirza D., Soderdahl G., Porte R., Paul A. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation—the European Liver Transplant Registry experience. Transplant J. 2013;95:872–877. doi: 10.1097/TP.0b013e318281b902. [DOI] [PubMed] [Google Scholar]
- 150.Tran Minh M., Mazzola A., Perdigao F., Charlotte F., Rousseau G., Conti F. Primary hepatic angiosarcoma and liver transplantation: radiological, surgical, histological findings and clinical outcome. Clin Res Hepatol Gastroenterol. 2018;42:17–23. doi: 10.1016/j.clinre.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 151.Casali P.G., Abecassis N., Aro H.T., Bauer S., Biagini R., Bielack S. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–iv67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 152.Penel N., Bui B.N., Bay J.-O., Cupissol D., Ray-Coquard I., Piperno-Neumann S. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 153.Zhou Y.-M., Sui C.-J., Zhang X.-F., Li B., Yang J.-M. Influence of cirrhosis on long-term prognosis after surgery in patients with combined hepatocellular-cholangiocarcinoma. BMC Gastroenterol. 2017;17:25. doi: 10.1186/s12876-017-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Okumura Y., Kohashi K., Tanaka Y., Kato M., Maehara Y., Ogawa Y. Activation of the Akt/mammalian target of rapamycin pathway in combined hepatocellular carcinoma and cholangiocarcinoma: significant correlation between p-4E-BP1 expression in cholangiocarcinoma component and prognosis. Virchows Arch. 2020;476:881–890. doi: 10.1007/s00428-019-02741-3. [DOI] [PubMed] [Google Scholar]
- 155.Cazals-Hatem D., Rebouissou S., Bioulac-Sage P., Bluteau O., Blanché H., Franco D. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41:292–298. doi: 10.1016/j.jhep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 156.Graham R.P., Yeh M.M., Lam-Himlin D., Roberts L.R., Terracciano L., Cruise M.W. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Mod Pathol. 2018;31:141–149. doi: 10.1038/modpathol.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flucke U., Vogels R.J.C., de Saint Aubain Somerhausen N., Creytens D.H., Riedl R.G., van Gorp J.M. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.