Abstract
Background
Persistent HIV infection of long-lived resting CD4 T cells, despite antiretroviral therapy (ART), remains a barrier to HIV cure. Women have a more robust type 1 interferon response during HIV infection relative to men, contributing to lower initial plasma viremia. As lower viremia during acute infection is associated with reduced frequency of latent HIV infection, we hypothesized that women on ART would have a lower frequency of latent HIV compared to men.
Methods
ART-suppressed, HIV seropositive women (n = 22) were matched 1:1 to 22 of 39 ART-suppressed men. We also compared the 22 women to all 39 men, adjusting for age and race as covariates. We measured the frequency of latent HIV using the quantitative viral outgrowth assay, the intact proviral DNA assay, and total HIV gag DNA. We also performed activation/exhaustion immunophenotyping on peripheral blood mononuclear cells and quantified interferon-stimulated gene (ISG) expression in CD4 T cells.
Results
We did not observe evident sex differences in the frequency of persistent HIV in resting CD4 T cells. Immunophenotyping and CD4 T-cell ISG expression analysis revealed marginal differences across the sexes.
Conclusions
Differences in HIV reservoir frequency and immune activation appear to be small across sexes during long-term suppressive therapy.
Keywords: HIV, reservoir, women, men, cure
Cross-sectional analysis of age- and race-matched antiretroviral-suppressed HIV seropositive participants demonstrated no evident sex differences in the frequency of inducible replication-competent HIV or intact HIV DNA within resting CD4 T cells. Marginal sex differences in immune activation markers were observed.
Human immunodeficiency virus (HIV) infection has claimed over 30 million lives and has an annual global incidence of approximately 2 million [1]. Antiretroviral therapy (ART) can quell viremia to undetectable levels and greatly extend lifespan; however, cessation of ART results in rebound of viremia within weeks in the majority of individuals due to latent replication-competent proviral DNA within CD4 T lymphocytes and possibly other cells [2–5]. Residual immune sequelae, the need for and the unknown effects of lifelong adherence to ART, and social stigma provide a strong rationale to develop approaches to eradicate or functionally cure HIV [6]. Successful protocols for HIV eradication or functional cure will require specific knowledge about the nature of the reservoir and the interplay of factors regulating the reservoir in all human populations. Women constitute one-half of people living with HIV; however, to date they are underrepresented in HIV cure studies [7, 8].
Differences in the acquisition rates and disease progression of HIV-1 infection between women and men have long been reported in epidemiological studies, reviewed in [9, 10]. These differences are likely due to both sociocultural (gender) and biological (sex) factors. The scope of this work focuses on the impact of biological sex on persistent HIV infection.
In both acute and chronic untreated infection, women have lower plasma viral loads than men [11–16]. Women also have higher T-cell activation and T-cell interferon-stimulated gene expression compared to men for a given viral load [17, 18]. However, despite lower viral loads, women progress to AIDS at a similar rate to men [11, 12, 15, 16].
Mechanistically, evidence suggests that this higher level of T-cell activation in women is mediated through sex-specific effects in plasmacytoid dendritic cells (pDCs) [17, 19]. pDCs are major producers of interferons and recognize HIV single-stranded RNA through Toll-like receptor 7 (TLR7). pDCs derived from women, compared with men, produced higher amounts of interferon-α in response to inactivated HIV-1, leading to greater CD8 T-cell activation in vitro [17]. Increased interferon responses, especially during chronic infection, may contribute to the fact that women progress to AIDS at similar rates to men despite lower viral loads [17, 20].
pDCs may be more responsive to HIV in women because the sex hormone β-estradiol can enhance the ability of pDCs to respond to TLR7 stimulation [21]. Sex hormones may also play a key role in modulating HIV replication directly, as β-estradiol inhibits HIV-1 gene expression by inducing the formation of a transcription-restrictive complex between β-catenin and estrogen receptor-α on the HIV promoter [22]. β-estradiol also blocks the activity of HIV latency reversal agents, possibly through the same mechanism [23]. Further, during untreated infection, HIV-1 RNA levels were shown to vary with the menstrual cycle: plasma viral loads fell from the early follicular phase to the midluteal phase of the menstrual cycle, concomitant with the physiological increase in β-estradiol [24].
Together, the β-estradiol–mediated negative modulation of HIV transcription and enhanced pDC interferon response likely contribute to a paradoxical reduction in plasma viremia in women but no decreased risk for progression to AIDS relative to men [9, 10, 12, 15, 16]. The impact of these sex differences observed during untreated infection on persistent HIV during therapy is unclear. In particular, the frequency of the reservoir and immune activation parameters in HIV-seropositive women on ART are understudied [25–28]. Further, we have previously demonstrated that in acute HIV infection in a cohort of men, the area under the viral load curve predicts the size of the reservoir and ART initiated during acute infection limits the size of the reservoir [29]. Therefore, given lower viral loads in untreated women, we predicted that women on ART would have reduced frequency of persistent HIV relative to men. To inform potential differences in persistent HIV frequency, we also measured immune activation/exhaustion markers in CD4/CD8 T cells, interferon-stimulated gene (ISG) expression in CD4 T cells, and estrogen receptor expression in CD4 T cells.
METHODS
Study Design and Human Subjects
Study participants (men and women) were recruited through the “University of North Carolina (UNC) Global HIV Prevention and Treatment Clinical Trials Unit”, the Women Interagency HIV Study (UNC and University of California San Francisco [UCSF] sites) and the UNC Center for AIDS Research HIV Clinical Cohort. This study was approved by the Biomedical Institutional Review Board of UNC and UCSF, and all participants provided informed consent. Participants were stably suppressed on ART (HIV-1 RNA <50 copies/mL) for at least 6 months prior to enrollment (Table 1) and had a CD4 cell count ≥ 300 cells/μL. Pregnant women were excluded from this study. Both pre- and postmenopausal women were included in the study and we did not exclude women using a systemic hormonal contraceptive at the time of enrollment (n = 2).
Table 1.
Matched Participant Clinical Characteristics
| Characteristic | Women | Matched Men | All Men |
|---|---|---|---|
| Sample size | 22 | 22 | 39 |
| Race/Ethnicity, No. (%) | |||
| African American | 15 (68) | 12 (55) | 16 (41) |
| Other | 1 (5) | 0 (0) | 0 (0) |
| Non-Hispanic white | 4 (18) | 10 (45) | 22 (56) |
| Hispanic white | 2 (9) | 0 (0) | 1 (3) |
| Race/Ethnicity, collapsed, No. (%) | |||
| African American | 15 (68) | 12 (55) | 16 (41) |
| White, white/Hispanic, or other | 7 (32) | 10 (45) | 23 (59) |
| Age, y, median (Q1, Q3) | 45.2 (39.6, 54.0) | 45.7 (34.3, 54.6) | 41.5 (29.2, 53.2) |
| Years on therapy, median (Q1, Q3) | 6.3 (3.7, 15.0) | 7.3 (4.9, 11.6) | 6.0 (2.5, 10.3) |
| Years suppressed, median (Q1, Q3) | 4.3 (2.6, 6.0) | 6.3 (3.5, 7.9) | 5.1 (2.3, 7.5) |
| Current CD4, cells/μL, median (Q1, Q3) | 883 (619, 998) | 728 (642, 847) | 737 (610, 847) |
| Pre-ART nadir CD4 | |||
| No. | 19 | 19 | 36 |
| No. missinga | 3 | 3 | 3 |
| Median, cells/μL (Q1, Q3) | 320 (216, 508) | 335 (173, 467) | 346 (185, 539) |
| ART initiation, No. (%) | |||
| Acute | 2 (9) | 2 (9) | 7 (18) |
| Chronic | 20 (91) | 20 (91) | 32 (82) |
Abbreviation: ART, antiretroviral therapy.
aWhen values were missing due to insufficient clinical information, the number of missing values is indicated.
Quantitative Viral Outgrowth Assay
Lymphocytes were obtained by continuous-flow leukapheresis and resting CD4 T cells (CD69−/CD25−/HLADR−) were isolated as previously described [30]. Recovery and quantification of replication-competent virus was performed as described elsewhere [29–31]. See Supplementary Methods for details.
Intact Proviral DNA and Total HIV Gag DNA Measurements
DNA was extracted from snap-frozen pellets of resting CD4 T cells using the QiaAmp DNA Mini kit (Qiagen). The intact proviral DNA assay and total HIV gag DNA assay were performed on resting CD4 T cells using digital droplet polymerase chain reaction (PCR) as previously described [32, 33]. A detailed description of the methodologies employed for this study are available in Supplementary Table 1 and Supplementary Figure 1.
Immunophenotyping
Cryopreserved peripheral blood mononuclear cells (PBMCs) were viably thawed and allowed to rest overnight in Iscove’s modified Dulbecco’s medium supplemented with 10% heat-inactivated fetal bovine serum and 1% PenStrep. Cells were washed in phosphate-buffered saline (PBS) and resuspended in an antibody staining cocktail for T cells with markers of T regulatory cells, activation, and exhaustion. Cells were subsequently washed in PBS and fixed in 3.2% paraformaldehyde prior to reading on an BD LSR Fortessa flow cytometer. Cytometry data was analyzed using FlowJo V10.6.1 using fluorescence minus 1 controls. Gating strategies and antibody information are available in Supplementary Table 2 and Supplementary Figure 4.
Gene Expression Analysis
Cryopreserved PBMCs were thawed and total CD4 T lymphocytes were isolated using magnetic negative selection (catalogue number 17952; Stemcell Technologies). RNA was extracted, cDNA was prepared, and gene expression was quantified using probe chemistry with normalization to 2 validated reference genes for lymphocytes [34]. See Supplementary Methods and Supplementary Table 3 for details.
Participant Matching and Statistical Analyses
In this cross-sectional study, we compared 5 different measures of the latent HIV reservoir, 7 gene expression measures, and 27 immunophenotyping measures between samples from women and men who were 18 years or older. Analyses were conducted both on (1) a 1:1 matched sample and (2) on a full unmatched sample with evaluable data using covariate adjustment. The geometric mean ratio (GMR, geometric mean women/geometric mean men) was the target estimand.
The 1:1 matched analysis is the prespecified primary approach. We matched on, or adjusted for, 2 factors: (1) years of age at time of leukapheresis and (2) race/ethnicity (collapsed to African-American [non-Hispanic] or non-African-American and/or Hispanic for matching feasibility). Reservoir, gene expression, and immunophenotyping measures were obtained for n = 22 women. Each woman was matched to a counterpart among a sample of n = 39 men. Nearest neighbor matching was used to select, for each woman participant, a male control with the smallest distance from the given woman participant with respect to the matching covariates. Matching was conducted in R version 3.5.3 using the matchit package, and results were replicated using the PSMATCH procedure in SAS version 9.4. This matching procedure did not require exact matches on either age or race but instead sought to minimize differences in both variables between matched pairs. While the study protocol lists 6 potential matching factors, ultimately we matched on only 2 factors to mitigate over-matching bias, for example adjusting away the impact of biological sex by matching on variables that could be impacted by biological sex and which also impact the outcomes. Detailed information regarding secondary unpaired analyses, data transformations, handling of censored values below the limit of detection, and reservoir measurement correlations is provided in the Supplementary Methods.
Exploratory Statistical Analyses: Adjusted Pairwise Associations
As an exploratory analysis, we examined adjusted, pairwise associations between the 3 sets of measures (reservoir, gene expression, and immunophenotyping) by biological sex, and evaluated whether these associations differ by biological sex. See Supplementary Methods for a detailed analysis approach.
RESULTS
No Evident Differences in Persistent HIV Frequency Across Sexes
We compared measures of HIV DNA and viral outgrowth in a cohort of n = 22 women and n = 39 men matched on age and race/ethnicity. Median years on ART, current CD4, nadir CD4, and treatment during chronic or acute infection were comparable across women and matched men (Table 1).
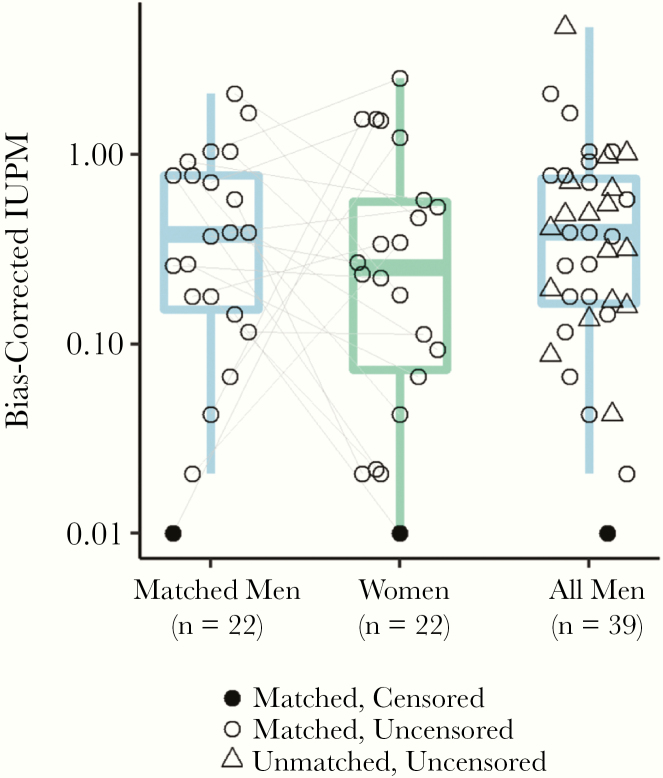
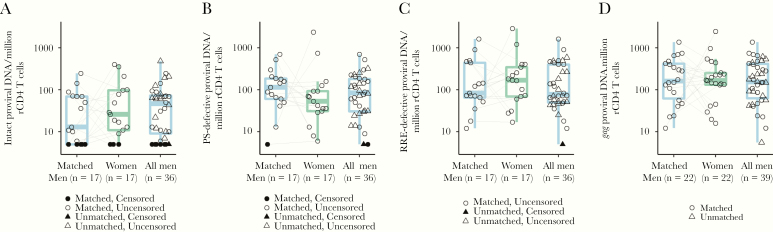
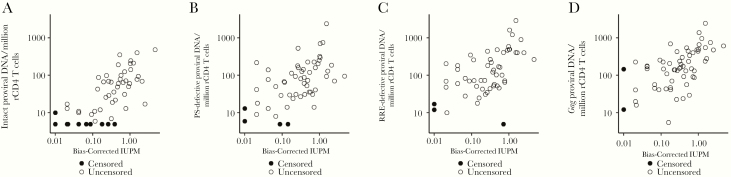
We did not detect evident differences across sexes in the frequency of replication-competent HIV in resting CD4 T cells as measured by the quantitative viral outgrowth assay (QVOA) (Figure 1). The frequency of intact and defective proviral DNA as measured by the intact proviral DNA assay using probes for the HIV rev response element and packaging signal also did not differ across sexes (Figure 2) [32]. Total HIV gag DNA frequency was similar across sexes (Figure 2). However, in both matched/paired and unpaired analyses, there was limited precision to detect an effect of biological sex on the frequency of persistent HIV due to the limited sample size of the cohort (Figure 2 and Supplementary Table 4). As previously reported [32, 35], there was a moderate correlation of HIV DNA measurements and QVOA measurements (Figure 3).
Figure 1.
Replication-competent reservoir measurements (quantitative viral outgrowth assay) across sexes. No evident differences for the frequency of replication-competent HIV in women for matched pairs (GMR, 0.72; 95% CI, .26–2.03; P = .54) or age- and race-adjusted unmatched analysis (GMR, 0.63; 95% CI, .28–1.41; P = .26). Connecting lines represent age- and race/ethnicity-matched participants. Abbreviations: CI, confidence interval; GMR, geometric mean ratio; HIV, human immunodeficiency virus; IUPM, Infectious Units Per Million rCD4 Cells.
Figure 2.
DNA reservoir measurements across sexes. Connecting lines represent age- and race-matched participants. Shapes with black fill represent instances where the frequency was below the limit of detection of the assay. A–C, Intact proviral DNA assay measurements. For 6 individuals there was an amplification failure for either the PS or RRE probe in the intact proviral DNA assay, likely due to proviral polymorphisms in primer/probe binding regions (Supplementary Figure 1). These individuals (or pairs, when an amplification failure occurred for 1 of 2 participants in a matched pair) were excluded from the analyses and the corresponding reduction in sample size is reflected below each graph. A, No evident difference in frequency of intact proviral HIV DNA in women for matched pairs (GMR, 2.09; 95% CI, .35–12.64) or age- and race/ethnicity-adjusted unmatched analysis (GMR, 0.95; 95% CI, .37–2.45). B, Similar frequency of PS-defective proviral across sexes in matched pairs (GMR, 0.68; 95% CI, .25–1.90) or age- and race/ethnicity-adjusted unmatched analysis (GMR, 0.77; 95% CI, 0.36–1.62). C, Similar frequency of RRE-defective proviral DNA across sexes in matched pairs (GMR, 1.30; 95% CI, .51–3.32) or age race/ethnicity adjusted unmatched analysis (GMR, 1.01; 95% CI, .49–2.07). D, Total gag HIV DNA measurements. No evident difference in frequency of total gag DNA across sexes in matched pairs (GMR, 1.00; 95% CI, .39–2.57) or age- and race/ethnicity-adjusted unmatched analysis (GMR, 1.10; 95% CI, .56–2.15). Abbreviations: CI, confidence interval; GMR, geometric mean ratio; HIV, human immunodeficiency virus; PS, packaging signal; RRE, rev response element.
Figure 3.
Correlation of QVOA and HIV DNA measures. There was a moderate correlation of HIV DNA measurements and QVOA measurements: (A) intact DNA ρ = 0.69 (95% CI, .54–.84); (B) PS-defective DNA (5′ deletion) ρ = 0.55 (95% CI, .36–.74); (C) RRE-defective DNA (3′ deletion or hypermutation) ρ = 0.55 (95% CI, .37–.74); (D) total gag DNA ρ = 0.57 (95% CI, .40–.75). Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IUPM, Infectious Units Per Million rCD4 Cells; PS, packaging signal; QVOA, quantitative viral outgrowth assay; RRE, rev response element.
As 11 of the 22 women in our cohort were postmenopausal at the time of study visit, we also compared the frequency of inducible persistent HIV measured by QVOA in pre- versus postmenopausal women given the potential for β-estradiol to suppress HIV expression [22–24]. We did not observe clear differences in QVOA measurements for pre- versus postmenopausal women (Supplementary Figure 2).
Increased Expression of ISG15 in ART-Suppressed Women Compared to Men
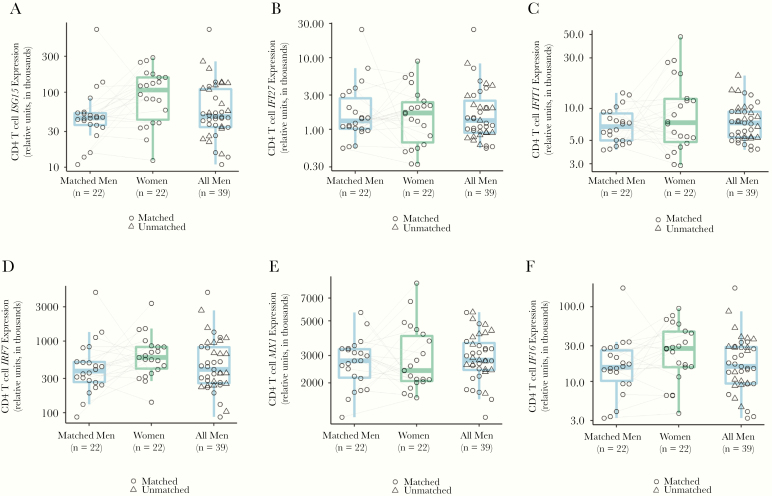
In order to determine if sex differences in ISG expression during untreated infection persisted in ART-suppressed individuals, we compared the expression of several ISGs (MX1, IFIT1, IFI27, IRF7, IFI6, and ISG15) in CD4 T cells from matched ART-suppressed HIV infected men and women. ISGs were selected based on their known upregulation in CD4 T cells during untreated HIV infection and/or known sex-differential expression in CD4 T cells during untreated HIV infection [18, 36]. We observed higher (GMR = 1.85, P = .02 for matched/paired analysis) expression of ISG15 in women compared to men (Figure 4). There was also a trend toward higher expression of IRF7 and IFI6 in CD4+ T cells of women compared to men (Figure 4 and Supplementary Table 5).
Figure 4.
Higher expression of ISG15 in CD4 T cells from women. A, Increased ISG gene expression in women: matched pairs GMR, 1.85 (95% CI, 1.10–3.12); age- and race/ethnicity-adjusted unpaired analysis GMR, 1.83; 95% CI, 1.12–3.02. B–F, Trends toward higher expression of IRF7 (D) and IFI6 (F) were observed in women. Abbreviations: CI, confidence interval; GMR, geometric mean ratio; ISG, interferon-stimulated gene.
Similar Estrogen Receptor-α Expression in ART-Suppressed Women Compared to Men
As β-estradiol has been reported to have a suppressive effect on HIV expression [22–24], we also examined gene expression levels of estrogen receptor-α, the predominant form expressed in CD4 T cells [37]. Consistent with other studies in HIV-seronegative and -seropositive individuals, estrogen receptor-α expression within CD4 T cells was similar in men and women (Supplementary Figure 3 and Supplementary Table 5) [26, 37].
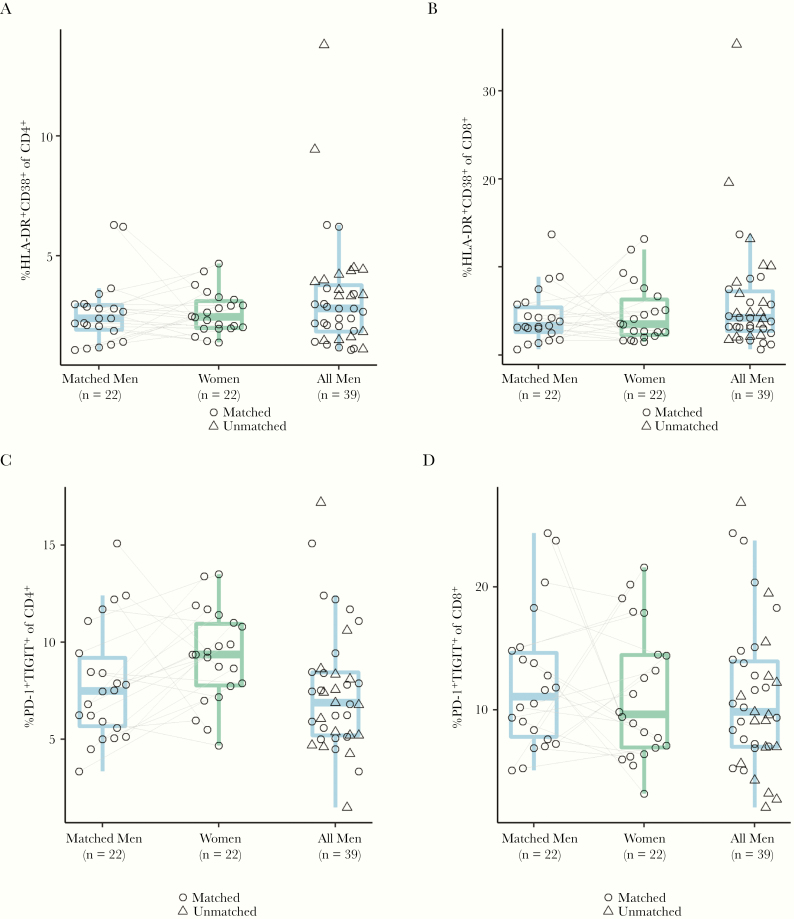
Similar Immune Activation or Exhaustion Markers on CD4 and CD8 T Cells in ART-Suppressed Women Compared to Men
During untreated infection, women have higher expression of activation markers (HLA-DR+CD38+) on CD8 T cells for a given viral load [17]. Therefore, we performed flow cytometry on PBMCs in order to determine the levels of markers of immune activation (HLA-DR, CD38) and exhaustion (programmed cell death protein 1 [PD-1], and T-cell immunoreceptor with Ig and ITIM domains [TIGIT]) on total and memory CD4 and CD8 T cell populations (Supplementary Figure 4). However, we did not detect clear differences in any activation or exhaustion marker expression level across sexes, although there was a trend toward higher PD1+TIGIT+ levels on total CD4 T cells from women (Figure 5 and Supplementary Table 6). We also compared the frequency of CD4 T regulatory cells, defined as CD127loCD25hiCD4+ across sexes and found no evident differences (Supplementary Table 6).
Figure 5.
CD4/CD8 immune activation or exhaustion markers across sexes. A, Percentage of HLA-DR+CD38+ CD4 T cells was similar across sexes (matched pairs mean difference = 0.00; 95% CI, −.74 to .74). B, No evident difference in percentage of HLA-DR+CD38+ CD8 T cells across sexes (matched pairs mean difference = 0.42; 95% CI, −1.9 to 2.73). C, No evident difference in percentage of PD-1+TIGIT+ CD4 T cells across sexes (matched pairs mean difference = 1.40; 95% CI, −.18 to 2.99). D, No evident difference in percentage of PD-1+TIGIT+ CD8 T cells across sexes (matched pairs mean difference = −0.89; 95% CI, −4.23 to 2.45). Abbreviation: CI, confidence interval; PD-1, programmed cell death protein-1.
Exploratory Analysis of Association Between ISG Expression, Immune Activation/Exhaustion, and Reservoir Measurements
There are mixed reports of associations of immune activation measures with reservoir size [38–43], and interferon responses may be associated with decline of HIV DNA [44–47]. Therefore, as an exploratory analysis, we examined adjusted, pairwise associations between the 3 sets of measures (HIV reservoir, ISG/estrogen receptor-α expression, and immune activation/exhaustion markers) by biological sex, and evaluated whether these associations differed by biological sex. We did not identify any pairwise associations that were significant at the α = .05 level after applying a false-discovery rate adjustment (Supplementary Table 7 and Supplementary Table 8). Notably, there was no apparent relationship between peripheral CD4 or CD8 T cell immune activation (CD38+HLA-DR+) or immune exhaustion markers (PD1+TIGIT+) and any measure of persistent HIV frequency in this cohort (estimated slopes close to 0; Supplementary Table 8).
DISCUSSION
Sex differences in persistent HIV infection are understudied, particular in cure research. There is strong rationale to understand potential sex differences in persistence HIV infection as insight into sex differences may be informative for design of cure trials or anti-latency therapies.
Here, we observed no evident sex differences in the frequency of the HIV latent reservoir; however, our estimate is imprecise as it results from a limited sample of 22 women. We observed higher expression of ISGs in CD4 T cells from women compared to men, and no apparent differences in immune activation or exhaustion markers across sexes.
Two cross-sectional studies showed that women were more likely to have a lower frequency of total HIV DNA in PBMCs compared to men, providing the first evidence of potential sex differences in persistent HIV infection [25, 28]. More recently, however, Scully and colleagues found no evident difference in integrated or total HIV DNA frequency in CD4 T cells in a cohort of men and women matched 1:1 based upon duration of viral suppression, absolute CD4 T-cell count, and nadir CD4 count [26]. In our analysis, we matched 1:1 on only age and ethnicity/race in order to avoid attenuating the effect of biological sex on persistent HIV frequency by matching out factors that are potentially on the causal pathway. Nonetheless, our finding of no evident difference in total HIV DNA across sexes aligns with that of Scully and colleagues [26]. The discrepancy of a lack of sex-based difference in HIV DNA frequency in isolated CD4 T cells [26] versus a sex effect in PBMCs [25, 28] may be explained by differences in lymphocyte percentage of PBMCs across sexes [48], a small effect size, and/or low precision in smaller studies (n = 22 pairs in this study, n = 26 pairs in [26]).
It is worth noting that the majority of HIV DNA is defective and measurement of HIV DNA with single-probe assays does not accurately quantify virus that is likely to cause viral rebound upon cessation of ART [5]. To better understand if there are differences in the frequency of intact proviruses across sexes, we performed the intact proviral DNA assay [32]. This assay measures proviral DNA that contains both an intact packaging signal and rev response element. This excludes over 95% of proviruses with defects detectable by near-full–length genome sequencing, although it still counts some proviruses with small defects outside of the 2 measured regions as intact [32]. This provides a much more accurate upper limit on the frequency of replication-competent HIV DNA than single-probe assays and may capture noninducible proviruses that single-stimulation QVOA assays are unable to measure [32]. Using this assay, we found no evident differences in intact HIV DNA across sexes.
In addition to understanding sex differences in HIV DNA frequency, we also endeavored to assess differences in inducible latent HIV frequency across sexes. Here, we measured inducible HIV using QVOA, which detects replication-competent HIV that is inducible from 1 round of mitogenic T-cell activation [5]. In agreement with a comparison of the QVOA across sexes by Prodger and colleagues in Ugandan and American cohorts, we found no evident sex differences in QVOA measurements [27]. Interestingly, Prodger and colleagues detected a trend toward lower QVOA measurements in Ugandan women compared to men, but not in American women compared to men [27]. Other sex-based comparisons of inducible HIV RNA have been performed using the Tat/rev induced limiting dilution assay (TILDA) and envelope detection of in vitro transcription sequencing (EDITS) assays. However, these studies of inducible HIV RNA have mixed results [23, 26]. Taken together, results from this study and others [25–28] suggest that any sex-based difference in persistent HIV frequency is likely marginal and difficult to detect in small cohort studies. The extent to which each reservoir assay measures intact, defective, and/or inducible persistent HIV may also contribute to discrepant results across small cohort studies.
To inform potential differences in reservoir frequency, we also measured immune parameters that have been demonstrated to show sex differences in untreated HIV infection, including CD8 T-cell activation markers and interferon-stimulated genes [17, 18]. In concordance with data in untreated HIV infection, we observed a higher level of ISG15 expression in CD4 T cells from women compared to men [18]. It remains to be resolved whether this increased level of ISG15 expression in CD4 T cells is mediated solely by biological sex, or if HIV infection plays a mediating role. Given the lower level of both (1) CD4 T-cell HIV RNA expression and (2) low-level viremia in women on long-term therapy [26], it seems unlikely that increased ISG15 expression in women would be due to higher residual HIV antigen burden. As ISG15 is thought to inhibit HIV budding, this may be an important sex difference to consider in trials of anti-latency therapeutics [49].
Given the differences in immune activation across sexes in untreated HIV infection [17], we also assessed levels of CD4 and CD8 T-cell activation and exhaustion markers. Interestingly, we observed no evident differences in immune activation/exhaustion marker expression in CD4/CD8 T cells across sexes, whereas Scully and colleagues observed higher immune activation (HLA-DR, CD38) and exhaustion (PD-1) markers on CD4 and CD8 T cells from men [26]. There are several potential reasons for this difference, which are not mutually exclusive. The effect size of sex appears to be small [26], so differences may be masked by sample size or different analysis approaches. Another possible explanation for the discrepant results is that participants in our study were suppressed for longer than in the study by Scully and colleagues (median 4.3 vs 2.8 years for women and 6.3 vs 3.3 years for men, respectively [26]) and the sex difference in residual immune activation and exhaustion may have declined with longer duration of virologic suppression.
There are mixed reports of associations of immune activation measures with reservoir size in the literature [38–43], and interferon responses may be associated with decline of HIV DNA [44–47]. Therefore, as an exploratory analysis, we examined adjusted, pairwise associations between the 3 sets of measures (HIV reservoir, ISG/ estrogen receptor-α expression, and immune activation/exhaustion markers) by biological sex, and also evaluated whether these associations differ by biological sex. We did not find any noteworthy relationships between these parameters. Notably, we did not observe a relationship between peripheral blood CD4/CD8 immune activation (CD38+ HLA-DR+) or immune exhaustion markers (PD1+TIGIT+) and any measure of persistent HIV frequency in this cohort. This is consistent with a large longitudinal study of HIV reservoir frequency and immune activation that found no association between peripheral blood immune activation markers and HIV persistence beyond the first year of ART [43].
There are several limitations to our study. First, our sample size was limited due to difficulties obtaining stable venous access for leukapheresis procedures in several women in our cohort. This limited our statistical power to detect small differences in reservoir frequencies, gene expression, and immunophenotypes. In addition, our study did not exclude menopausal women (11 of 22 women in the cohort) or women taking systemic hormonal contraceptives (2 of 22 women in the cohort). While this reflects the general population, it may have masked potential sex differences that occur, for example, only in premenopausal women. However, our finding of no evident sex difference in the frequency of persistent HIV aligns with the findings of Scully and colleagues who studied premenopausal women not taking systemic hormonal contraception [26]. As β-estradiol may play a suppressive role in HIV expression, we also compared the frequency of inducible persistent HIV measured by QVOA in pre- versus postmenopausal women [22–24]. Although the sample size is limited, we did not observe clear differences in inducible replication-competent HIV for pre- versus postmenopausal women in this cross-sectional analysis. Future longitudinal studies of the inducible HIV reservoir in women across reproductive stages may be more informative.
Taken together, this and other studies suggest that differences in HIV reservoir frequency and immune activation may be small across sexes during long-term suppressive therapy, and challenging to detect in small cohort studies [23, 25–28]. However, sex differences are still important to consider in HIV cure research trials. In particular, the suppressive effect of β-estradiol on HIV expression may confound the interpretation of antilatency therapeutic trials [23, 26] and sex differences in interferon responses to HIV may have implication for latency clearance [17, 18]. Future work in this space should further investigate the mechanistic underpinnings of these differences and their implication for antilatency therapeutic trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the participants who made this study possible; A. Edmonds for clinical data compilation support; F. Prince, Y. Park, J. Cohen, M. Holden, and the staff of the UNC Blood Bank, UNC CTRC, and Blood Centers of the Pacific for clinical support; and M. Gilleskie for help with graphics development.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH (grant numbers UM1AI126619 to D. M. M., R01AI134363 to N. M. A., F30AI145588 to S. D. F., P30-AI050410 to J. J. E. and K. R. M., and U01‐HL146194 to A. A. A.); and the National Center for Advancing Translational Sciences, NIH (grant number UL1TR002489). Clinical data for some participants were collected by the Women’s Interagency HIV Study (now the MACS/WIHS Combined Cohort Study [MWCCS]); MWCCS principal investigators, centers, and NIH grant numbers: Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub, Data Analysis and Coordination Center (U01‐HL146193); Bradley Aouizerat and Phyllis Tien, Connie Wofsy Women’s HIV Study, Northern California CRS (U01‐HL146242); Roger Detels, Los Angeles CRS (U01‐HL146333); and A. A. A., UNC CRS. The MWCCS is supported by the National Heart, Lung, and Blood Institute, with additional support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Human Genome Research Institute, National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, National Institute of Diabetes and Digestive and Kidney Diseases, University of California San Francisco CTSA (grant number UL1‐ TR000004) and University of North Carolina CFAR.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997; 94:13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278:1291–5. [DOI] [PubMed] [Google Scholar]
- 4. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 5. Falcinelli SD, Ceriani C, Margolis DM, Archin NM. New frontiers in measuring and characterizing the HIV reservoir. Front Microbiol 2019; 10:2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214(suppl 2):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr 2016; 71:181–8. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO). Summary of the global HIV epidemic. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 9. Griesbeck M, Scully E, Altfeld M. Sex and gender differences in HIV-1 infection. Clin Sci (Lond) 2016; 130:1435–51. [DOI] [PubMed] [Google Scholar]
- 10. Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209(suppl 3):S86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180:666–72. [DOI] [PubMed] [Google Scholar]
- 12. Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011; 203:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 2002; 35:313–22. [DOI] [PubMed] [Google Scholar]
- 14. Napravnik S, Poole C, Thomas JC, Eron JJ Jr. Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr 2002; 31:11–9. [DOI] [PubMed] [Google Scholar]
- 15. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344:720–5. [DOI] [PubMed] [Google Scholar]
- 16. Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352:1510–4. [DOI] [PubMed] [Google Scholar]
- 17. Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang JJ, Woods M, Lindsay RJ, et al. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis 2013; 208:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ziegler SM, Beisel C, Sutter K, et al. Human pDCs display sex-specific differences in type I interferon subtypes and interferon α/β receptor expression. Eur J Immunol 2017; 47:251–6. [DOI] [PubMed] [Google Scholar]
- 20. Utay NS, Douek DC. Interferons and HIV infection: the good, the bad, and the ugly. Pathog Immun 2016; 1:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seillet C, Laffont S, Trémollières F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 2012; 119:454–64. [DOI] [PubMed] [Google Scholar]
- 22. Szotek EL, Narasipura SD, Al-Harthi L. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor α on the HIV promoter to suppress HIV transcription. Virology 2013; 443:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Das B, Dobrowolski C, Luttge B, et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018; 115:E7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenblatt RM, Ameli N, Grant RM, Bacchetti P, Taylor RN. Impact of the ovulatory cycle on virologic and immunologic markers in HIV-infected women. J Infect Dis 2000; 181:82–90. [DOI] [PubMed] [Google Scholar]
- 25. Cuzin L, Pugliese P, Sauné K, et al. ; Dat’AIDS Study Group Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS 2015; 29:1665–71. [DOI] [PubMed] [Google Scholar]
- 26. Scully EP, Gandhi M, Johnston R, et al. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J Infect Dis 2019; 219:1084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prodger JL, Lai J, Reynolds SJ, et al. Reduced frequency of cells latently infected with replication-competent human immunodeficiency virus-1 in virally suppressed individuals living in Rakai, Uganda. Clin Infect Dis 2017; 65:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fourati S, Flandre P, Calin R, et al. Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother 2014; 69:753–6. [DOI] [PubMed] [Google Scholar]
- 29. Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A 2012; 109:9523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keedy KS, Archin NM, Gates AT, Espeseth A, Hazuda DJ, Margolis DM. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J Virol 2009; 83:4749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trumble IM, Allmon AG, Archin NM, et al. SLDAssay: a software package and web tool for analyzing limiting dilution assays. J Immunol Methods 2017; 450:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gay CL, Kuruc JD, Falcinelli SD, et al. Assessing the impact of AGS-004, a dendritic cell-based immunotherapy, and vorinostat on persistent HIV-1 Infection. Sci Rep 2020; 10:5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ledderose C, Heyn J, Limbeck E, Kreth S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res Notes 2011; 4:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013; 9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sedaghat AR, German J, Teslovich TM, et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol 2008; 82:1870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett 2005; 97:107–13. [DOI] [PubMed] [Google Scholar]
- 38. Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis 2013; 208:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cockerham LR, Siliciano JD, Sinclair E, et al. CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One 2014; 9:e110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med 2010; 7:e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poizot-Martin I, Faucher O, Obry-Roguet V, et al. Lack of correlation between the size of HIV proviral DNA reservoir and the level of immune activation in HIV-infected patients with a sustained undetectable HIV viral load for 10 years. J Clin Virol 2013; 57:351–5. [DOI] [PubMed] [Google Scholar]
- 42. Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olesen R, Vigano S, Rasmussen TA, et al. Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with panobinostat. J Virol 2015; 89:10176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun H, Buzon MJ, Shaw A, et al. Hepatitis C therapy with interferon-α and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis 2014; 209:1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Azzoni L, Foulkes AS, Papasavvas E, et al. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis 2013; 207:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiao YM, Weng WJ, Gao QS, et al. Hepatitis C therapy with interferon-α and ribavirin reduces the CD4 cell count and the total, 2LTR circular and integrated HIV-1 DNA in HIV/HCV co-infected patients. Antiviral Res 2015; 118:118–22. [DOI] [PubMed] [Google Scholar]
- 48. Chen Y, Zhang Y, Zhao G, et al. Difference in leukocyte composition between women before and after menopausal age, and distinct sexual dimorphism. PLoS One 2016; 11:e0162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morales DJ, Lenschow DJ. The antiviral activities of ISG15. J Mol Biol 2013; 425:4995–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.