Abstract
Background
Limited evidence indicates greater female-to-male (F–M) transmission of genital infection with human papillomavirus (HPV) relative to male-to-female (M–F). We verified the hypothesis of a differential transmission rate in couple-based studies by conducting a systematic review and meta-analysis.
Methods
We searched MEDLINE, EMBASE, Scopus, and Cochrane Library databases for studies published until December 2019. We calculated pooled estimates of F–M and M–F transmission rates and their rate differences per 100 person-months, with 95% confidence intervals (CI), using a random-effects model. We counted occurrences of directionality preponderance for each HPV type.
Results
We identified 7 eligible studies published between 2008 and 2019, providing data for 752 couples. Pooled estimates for F–M and M–F transmission rates were 3.01 (95% CI, 1.19–7.64; I2 = 97%) and 1.60 (95% CI, 0.86–2.98; I2 = 89%), respectively. The overall rate difference was 0.61 (95% CI, −0.27 to 1.49; I2 = 75%). Three studies provided rates by sex and HPV genotype; 2 favored a preponderance of F–M and 1 favored M–F transmission.
Conclusions
There was slight evidence for a differential transmission rate favoring higher F–M than M–F transmission with substantial statistical heterogeneity across studies.
Keywords: human papillomavirus, HPV transmission, HPV infection, heterosexual couples, systematic review, meta-analysis
There is limited published evidence supporting higher female-to-male than male-to-female human papillomavirus transmission. There is significant heterogeneity among studies; however, future transmission investigations should consider factors such as age and recency of relationships as key variables.
Human papillomavirus (HPV) is the most common sexually transmitted viral infection worldwide [1]. Persistent infection with oncogenic types, mostly HPVs 16 and 18, is associated with the development of cancer at multiple anatomic sites in men and women [2]. As of 2008, 100% of cervical, 88% of anal, 70% of vaginal, 50% of penile, and 43% of vulvar cancers were associated with HPV infections, globally [3]. HPV is highly transmissible via genital-to-genital transmission [4], while hand-to-genital transmission is less likely [5].
Most of the literature on genital HPV infection is comprised of individual-based epidemiological studies that measured HPV prevalence and incidence rates, via multiple follow-up visits on the same study subjects. However, these studies—by design—do not include information about HPV infection in the individuals’ partner(s). Few studies have determined HPV infection in the dyad, that is the pair of individuals in a relationship. Such couple-based studies have the opportunity to measure HPV transmission rates. For example, if the male in a monogamous relationship tested positive for HPV-16 and his female partner was HPV-16 negative at enrolment, subsequent HPV-16 positivity in the female would provide evidence of a transmission event from the male to the female within the dyad. Thus, unlike individual-based HPV studies, couple-based investigations permit the examination of transmission dynamics between partners.
The “EUROGIN 2014 Roadmap: Differences in HPV infection natural history, transmission, and HPV-related cancer incidence by gender and anatomic site of infection” [6] briefly discussed 5 heterosexual couple-based longitudinal studies [7–11] and suggested greater rates of female-to-male (F–M) compared to male-to-female (M–F) transmission. However, formal meta-analytic procedures to assess differences in between-gender directionality of HPV transmission have not been performed.
The current systematic review and meta-analysis aimed to assess the evidence for differences in between-gender directionality of transmission of genital-to-genital αHPV infections (ie, mucosotropic ones) in heterosexual couples.
METHODS
Search Strategy and Selection Criteria
Following PRISMA guidelines [12], 2 authors (R. B. and K. W.) developed the search strategy and independently searched MEDLINE, EMBASE, Scopus, and the Cochrane Library for studies published from the databases’ earliest coverage until 1 December 2019. The earliest database coverage year is 1946 for MEDLINE, 1947 for EMBASE, 1970 for Scopus, and 1996 for the Cochrane Library. No language or publication year restrictions were applied but molecular testing methods for HPV genotyping appropriate for large-scale epidemiologic investigations were not available before the mid-1980s. The following combination of keywords and MeSH terms were used: “HPV,” “papillomavirus infections,” “papillomaviridae,” “transmission,” “heterosexuality,” “couples,” and “sexual partners” (Supplementary Table 1). In addition, ProQuest was searched for pertinent dissertations, and the 2018 EUROGIN and International Papillomavirus Conference proceedings were reviewed for recent unpublished abstracts relevant to this review. We registered our protocol on the International Prospective Register of Systematic Reviews (PROSPERO; identifier CRD42020134491).
Studies were eligible for inclusion if (1) the population included heterosexual couples aged 18 years and over, (2) genital samples were collected, and (3) transmission rates of αHPV types were reported. We excluded individual-based studies and review articles, studies unrelated to HPV transmission, or those based on cross-sectional data. In the event of multiple publications reporting transmission rates using the same study population, the most recent publication containing the largest sample size was selected.
Rayyan QCRI, a web-based application for systematic reviews, was used for primary and secondary screening. The primary screen of titles and abstracts was independently done in triplicate (by R. B., A. M., and K. W.). Articles in disagreements between screeners were included for secondary screening (ie, full-text review). All authors agreed upon the final selection of studies; 2 researchers (E. L. F. and M. Z.) resolved disagreement on article eligibility. Backward and forward citation tracking was applied to the selected studies to identify additional relevant publications.
Data Extraction
Data, extracted by R. B. and independently validated by both A. M. and K. W., included the following: characteristics of study participants (age, relationship status, recency of relationship, circumcision status, etc.), publication year, study sample size, analysis sample size, length of follow-up, proportion of monogamous couples, instructions prior to visits, sample collection method, sampling interval, DNA extraction method, HPV genotyping method (including primers used), HPV prevalence, method used to calculate transmission rates, number of transmission episodes, person-time, as well as overall and type-specific HPV transmission rates. Minor differences in HPV type taxonomy were observed. Most notably, IS39 and HPV-82 were previously classified as separate types. However, IS39 has been reclassified as a subtype of HPV-82 and thus its status as an independent HPV type was abolished [13]. Taking this into consideration, we recounted the HPV types detected in each study.
Statistical Analysis
If person-time was not reported, it was determined via back calculations, dividing the reported episodes by transmission rate. To examine the directionality of transmission, separate pooled estimates for F–M and M–F transmission rates as well as rate differences between the 2 were calculated via a random-effects model using R (version 3.5.1). Specifically, metafor and meta packages were used to meta-analyze the data using a generalized linear mixed model and calculate the corresponding 95% confidence intervals (CI) following a Poisson distribution. For the pooled rate-differences, an inverse variance method was used. The forest function was used to generate forest plots for transmission rates and rate differences. A sensitivity analysis was performed by removing each study individually and examining the robustness of transmission rate pooled estimates and their heterogeneity. The ggplot2 package was used to create the scatterplot for the heterogeneity sensitivity analysis. In this analysis, we removed each study at a time to assess the effect on resulting I2 value (ie, the impact of each study on heterogeneity). We used the same process for our sensitivity analysis with transmission rates. For the transmission rate difference, an additional, exploratory sensitivity analysis consisted of excluding studies with wide CI and observing the change in pooled estimate.
In studies that reported incidence and/or transmission rates by sex and HPV genotypes, we identified and counted occurrences of directionality preponderance (F–M or M–F) for each genotype. For example, reported HPV-16 transmission rates of 3.0 and 2.0 for the F–M and M–F directionalities, respectively, would be 1 count favoring F–M transmission. We considered the incidence rates in males and females to infer directionality under the assumption that HPV transmission occurred between study partners. Transmission rates by Alphapapillomavirus subgenera were also calculated by summing type-specific HPV rates corresponding to HPV types in each subgenus group, assuming that all type-specific HPV transmission episodes were exclusively independent of each other. Subgenera groupings include subgenus 1 (HPVs 6, 7, 11, 32, 40, 42, 43, 44, 54, 74, and 91; primarily types that cause at most benign lesions), subgenus 2 (HPVs 16, 18, 26, 30, 31, 33, 34, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, and 82; possible, probable, or proven carcinogenic types), and subgenus 3 (HPVs 2, 3, 10, 27, 29, 57, 61, 62, 71, 72, 81, 83, 84, 87, 89, 90, and 94; commensal mucosotropic HPV types) [13, 14].
RESULTS
Study Selection and Characteristics
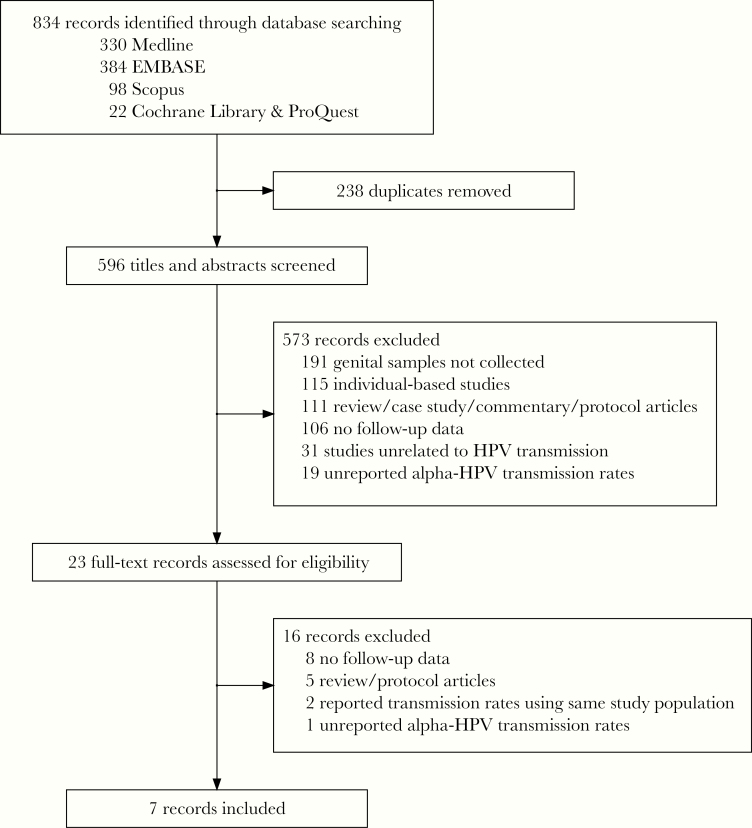
Figure 1 summarizes the process of selecting eligible studies. Overall, our search strategy identified 834 potential articles. After removing 238 duplicates, 596 articles were initially screened based on their title and abstract. Of these, the full text of 23 articles was used for secondary screening which resulted in 7 eligible studies. These reported data from a total of 1173 couples, but we only included in the current transmission analysis data on 752 couples who had discordant HPV infections.
Figure 1.
Flow chart of study selection process. The identification, selection of eligible studies, and reasons for exclusion are summarized. Searching 2018 conference proceedings (EUROGIN and International Papillomavirus Conference) yielded no additional articles.
A detailed description of the selected studies is provided in Table 1. All were cohort studies published between 2008 and 2019 and conducted in Canada, United States, China, and South Africa. Participants’ ages ranged from 18 to 70 years. Only 1 study [7] considered recency of the relationship, by study design, limiting recruitment to couples dating less than 6 months before enrollment. The remaining studies either included couples with a longer-term relationship or did not provide information about relationship length. Some studies instructed participants to refrain from sexual activity 24–48 hours prior to clinic visits [7, 9, 15], whereas 2 others [8, 11] did not report if any instructions were provided, 1 study [16] gave participants no instructions regarding sexual activity, and 1 provided mixed instructions for participants to abstain or engage in sexual activity prior to study visit [10]. Sampling and genotyping information are in Supplementary Table 2. Five studies used the same primer set [7–11] PGMY09/11 to detect 36 HPV types, whereas 2 used different primer sets to detect 46 [16] (SPF1/GP6+) and 16 [15] (GP51/61 primers) HPV types.
Table 1.
Characteristics of Studies Included in a Systematic Review and Meta-analysis on the Directionality of Genital HPV Infection Transmission
| Author, y | Geographical Location (Study Period) | Baseline (Analysis) Sample Size | Follow-Up Length, mo | Age, y Mean (Range) | Relationship Status | Relationship Length, mo | Monogamy | Instructions Prior to Visit | Circumcision, % |
|---|---|---|---|---|---|---|---|---|---|
| Hernandez et al, 2008 [8] | Hawaii, USA (Feb 2005–Nov 2006) | 38 (25) couples | Mean, 7.5 | Women, 26 (18–57) Men, 28 (18–59) | Dating (48%), married (8%), separateda (12%), cohabitating (32%) | Mean, 24.8b Median, 12.4b | 100% of couples | NR | 80 |
| Burchell et al, 2011 [7] | Montreal, Canada (2005–2010) | 308 (179) couples | Median, 5.5 (range 1.8–15.5) | Women, 21.5 Men, 23.9 | Dating | Mean, 4c | 65% (86/133) of female and 74% (66/89) of male partnersd | Abstain from oral, vaginal, or anal sex for 24 h | 57 |
| Mbulawa et al, 2013 [11] | Cape Town, South Africa (2006–2009) | 486 (not reported) couples | 24 | Women, 35 (18–66) Men, 38 (19–67) | Partner | Median, 40.8 | NR | NR | 96.6e |
| Widdice et al, 2013 [10] | California, USA (Feb 2006–Jul 2007) | 25 (25) couples | 1.5 | Women, 22.6 Men, 25.5 | Partner | Median Women, 32 | 92% of couples | Have vaginal intercourse 24 h before visit 2 | 64 |
| (IQR, 9–49) Men, 26 (IQR, 8.5–42) | Abstain from all sexual interaction before visit 3 Engage in normal sexual behaviors for remaining visits (4–6) | ||||||||
| Nyitray et al, 2014 [9] | Tampa, USA (2006–2010) | 137 (65) couples | Median, 25 | Women,f 33 (18–70) Men,f 33 (18–70) | Partner (mix of dating, married, and living together) | Median, 3.5 years | 83% of men and women (6 mo prior to enrollment) | Abstain from sexual activity for 48 h | NR |
| Liu et al, 2015 [16] | Anyang, China (2009–2013) | 874 (296) couples | Median men, 35.7 women, 30.5 | Women, 44 (39–52.5) Men, 44 (39–54) | Married | NRg | NR | Authors reported no sexual activity instructions provided to participants | NR |
| Su et al, 2019 [15] | Liuzhou, China (May 2014–Mar 2016) | 390 (97) couples | Median, 12.5 | Median Women,h 40 (32–46) Men,h 42 (35–49) | Married | NRi | 96.5% of men and 97.9 % of women (y prior to enrollment) | Abstain from sexual activity for 48 h | NR |
Abbreviations: HPV, human papillomavirus; IQR, interquartile range; NR, not reported.
aSeparated includes widowed and divorced.
bAuthor provided us with this information via personal communication.
cFor HPV type discordant couples (n = 179).
dStudy estimates were based on both the ongoing nature of partnerships and discordant HPV infections among couples.
eAssumed to be 96.6%. Circumcision was reported to be 3.4% in 1 study [11] but 2 other publications by the same team and using the same study population reported it to be greater than 95% [17, 18].
fBased on 99 couples who were analyzed for incidence. The 65 couples constitute a subset of these 99 couples.
gVia personal communication, the authors informed us that relationship length information was not collected in their questionnaire. However, given that most study couples reported having only 1 lifetime partner and a median age at first pregnancy of 23 years, the authors suggest that the median relationship duration should be more than 20 years for study couples.
hBased on 340 couples.
iVia personal communication, the authors informed us that they did not collect relationship duration information for the study couples.
HPV Prevalence and Transmission Rates
Five studies [7, 8, 10, 11, 15] reported HPV baseline prevalence. Nyitray et al [9] reported period prevalence during 24 months for 99 couples. For 3 studies [7, 10, 11], HPV prevalence data were obtained from previous publications [18–20] based on the same study population. Liu et al [16] did not report prevalence data, with no other publication found for the same study population that reported on prevalence. HPV prevalence in women ranged from 15.9% to 84%, and in men 9.7% to 76% (Table 2). It was the same in men and women in 2 studies [7, 11], higher in men than women in another 2 [8, 9], and higher in women than men in 2 other studies [10, 15]. Ranges of transmission rates per 100 person-months were 0.71–9.23 for M–F and 0.56–21.35 for F–M (Table 2). Five studies reported greater F–M transmission than M–F [7–11]. Conversely, 2 studies found M–F transmission to exceed F–M [15, 16].
Table 2.
Prevalence of HPV Infection in Men and Women and HPV Transmission Rates Per 100 Person-Months
| Authors, y | Prevalence (%) | F–M Transmission | M–F Transmission | |||
|---|---|---|---|---|---|---|
| Women | Men | No. of Events | Rate (95% CI) | No. of Events | Rate (95% CI) | |
| Hernandez et al, 2008 [8] | 60a | 68a | 20 | 17.4 (10.6–25.8) | 5 | 4.9 (16–10.0) |
| Burchell et al, 2011 [7] | 56b | 56b | 41 | 4.0 (3.0–5.5) | 46 | 3.5 (2.7–4.5) |
| Mbulawa et al, 2013 [11] | 58c | 58c | 66 | 2.80 (2.03–3.86) | 42 | 1.17 (.82–1.67) |
| Widdice et al, 2013 [10] | 84d | 76d | 6 | 21.35 (7.8–46.48) | 2 | 9.23 (1.12–33.34) |
| Nyitray et al, 2014 [9]e | 68 | 74 | 17 | 1.23 (.71–1.96) | 10 | 0.73 (.35–1.35) |
| Liu et al, 2015 [16] | NR | NR | 9 | 0.56 (.30–1.16) | 24 | 0.71 (.48–1.09) |
| Su et al, 2019 [15] | 16 | 10 | 9 | 1.13 (.59–2.17) | 4 | 1.15 (.43–3.07) |
Abbreviations: CI, confidence interval; F–M, female to male; HPV, human papillomavirus; M–F, male to female; NR, not reported.
aManually calculated using data from Table 1 in the Hernandez et al study [8].
bPrevalence estimates obtained from a previous study [19] by the same authors using the same study population.
cPrevalence estimates obtained from a previous study [18] by the same authors using the same study population.
dPrevalence estimates obtained from Table 2 in previous study [20] by the same authors using the same study population.
ePrevalence estimates based on 99 couples (period prevalence). Transmission rates based on 65 couples.
Meta-Analysis
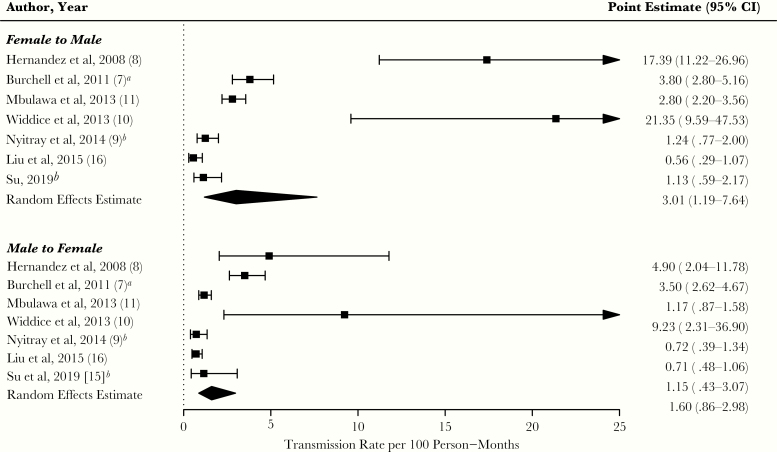
For 2 studies [9, 16], we calculated person-time using the authors’ estimates of infection counts and rates. The pooled estimate (per 100 person-months) for F–M transmission was 3.01 (95% CI, 1.19–7.64; I2 = 97%, P < .01), whereas that for M–F transmission was 1.60 (95% CI, 0.86–2.98; I2 = 89%, P < .01) (Figure 2).
Figure 2.
Forest plot of human papillomavirus transmission rates per 100 person-months for each study, by directionality. Transmission rates and random effects pooled estimates are provided by female-to-male and male-to-female directionality. The diamond edges represent the pooled estimate and corresponding 95% confidence interval (CI): top and bottom edges represent the exact point estimate, while left and right edges represent, respectively, the lower and upper bound of the 95% CI. A right-facing arrow on the upper bound of the CI indicates that it exceeded the value of 25. The I2 statistics for female-to-male and male-to-female directionality were 97% (P < .01) and 89% (P < .01), respectively.aReported data (rate and CI) in original study differ from the meta-analysis estimate due to the use of Poisson regression in the current analysis to account for multiple observations per couple in the original study. Our estimate was based on dividing events over time.
bFemale-to-male and male-to-female transmission rates were estimated in metafor by back-calculating person time (dividing events by transmission rate reported in the original study).
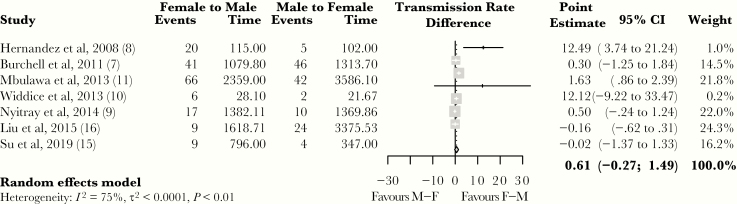
Figure 3 shows the transmission rate differences. The overall, pooled rate difference was 0.61 infections (95% CI, −0.27 to 1.49; I2 = 75%) per 100 person-months.
Figure 3.
Forest plot of the rate difference between female-to-male and male-to-female human papillomavirus transmission rates per 100 person-months for each study. Transmission rate differences and a random effects pooled estimate are provided. The diamond edges represent the pooled estimate and corresponding 95% confidence interval (CI): top and bottom edges represent the exact point estimate, while left and right edges represent, respectively, the lower and upper bound of the 95% CI. Positive and negative point estimates favor female-to-male (F–M) and male-to-female (M–F) transmission, respectively, whereas a 0 indicates no difference in transmission rate. The size of the blocks indicates the weight assigned to each study to determine the pooled estimate, with bigger blocks corresponding to greater weights.
Sensitivity Analyses
The sensitivity analysis for transmission rates (HPV infections per 100 person-months) ranged from 2.23 to 3.99 for F–M directionality and from 1.28 to 1.90 for M–F directionality. Supplementary Figure 1 presents a scatter plot of the heterogeneity (I2) upon removing individual studies. In terms of F–M transmission rate, I2 ranged from 95.9% to 97.3% whereas that for M–F transmission rate ranged from 79.6% to 91.9%.
Excluding 2 studies [8, 10], with the highest transmission rates, from the analysis resulted in a pooled estimate of transmission rate difference of 0.48 HPV infections per 100 person-months (95% CI, −0.28 to 1.23; I2 = 75%) (Supplementary Figure 2).
HPV Type-Specific Counts and Subgenus-Specific Rates
The study by Liu et al [16] was the only one to provide type-specific HPV transmission rates by between-gender directionality. However, we extended the data from that study by including the studies by Nyitray et al [9] and Mbulawa et al [11], which provided type-specific incidence rates by gender. For the latter study, we used data from a previous study by the authors [18], which presented incidence rates by sex within the same study population. We assumed that the magnitude of the incidence rate could serve as an indicator for the directionality of between-gender transmission. Except for these 3 studies, we could not find other papers reporting on type-specific incidence or transmission rates by sex.
HPV type-specific F–M and M–F transmission rates for the above 3 studies are shown in Supplementary Table 3. For each study, we compared type-specific rates to assess whether the preponderance of transmission was M–F or F–M, or was similar between genders. Type-specific rates in Supplementary Table 3 were then converted into gender directionality preponderance counts and shown in Table 3. Out of 46 HPV types detected by Liu et al [16], a total of 14 counts favored M–F transmission, 6 counts favored F–M transmission, and equal directionality was observed for 26 counts. Corresponding values were 16, 11, and 9 in the Nyitray et al study [9], and 29, 6, and 1 in the study by Mbulawa et al [18].
Table 3.
Overall Summary and Preponderancea of the Incidence or Transmission of HPV Genotype-Specific Ratesb
| Comparisons Between Male and Female Type-Specific Rates | Nyitray et al, 2014 [9]c | Mbulawa et al, 2012 [18]c | Liu et al, 2015 [16]d |
|---|---|---|---|
| Genotypes not tested | 16 | 16 | 6 |
| Genotypes with equal rates | 9 | 1 | 26 |
| Genotypes with M–F > F–M transmission ratesa | 11 | 6 | 14 |
| Genotypes with F–M > M–F transmission ratesa | 16 | 29 | 6 |
| Total No. genotypes | 52 | 52 | 52 |
Abbreviations: F–M, female-to-male; HPV, human papillomavirus; M–F, male-to-female.
aPreponderance was defined as the counts of type-specific rates favoring 1 directionality.
bRefer to Supplementary Table 3 for type-specific rates used to determine the overall counts.
cIncidence rates in males and females were used to infer directionality under the assumption that HPV transmission occurred between study partners. For example, a higher female HPV incidence rate would infer higher M-to-F transmission and vice-versa.
dTransmission rates were used.
Type-specific rates in Supplementary Table 3 were combined to generate aggregate Alphapapillomavirus subgenus-specific incidence/transmission rates by sex/directionality, as shown in Table 4. Higher incidence rates were observed in men compared to women across all subgenera for the studies by Nyitray et al [9] and Mbulawa et al [18], suggesting a higher tendency for F–M transmission. For the study by Liu et al [16], M–F transmission rates were higher than F–M for subgenus groups 1 and 2, but not subgenus 3.
Table 4.
Incidence or Transmission Rates (per 100 Person-Months) Summed According to HPV Subgenus Groups
| Subgeneraa | Nyitray et al, 2014 [9] | Mbulawa et al, 2012 [18] | Liu et al, 2015 [16] | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | M–F | F–M | |
| Subgenus 1 | 1.12 | 0.93 | 0.94 | 0.62 | 18.58 | 2.70 |
| Subgenus 2 | 3.04 | 2.73 | 5.77 | 3.39 | 45.94 | 4.50 |
| Subgenus 3 | 1.78 | 1.70 | 4.05 | 2.46 | 3.03 | 18.87 |
Abbreviations: F–M, female-to-male; HPV, human papillomavirus; M–F, male-to-female.
aHPV types were categorized based on subgenus groups with the assumption that all HPV genotypes are exclusively independent from each other. Subgenus 1 included HPVs 6, 7, 11, 32, 40, 42, 43, 44, 54, 74, and 91. Subgenus 2 included HPVs 16, 18, 26, 30, 31, 33, 34, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, and 82. Subgenus 3 included HPVs 2, 3, 10, 27, 29, 57, 61, 62, 71, 72, 81, 83, 84, 87, 89, 90, and 94. Not tested and not reported in Supplementary Table 3 were considered as 0.
DISCUSSION
To our knowledge, this systematic review and meta-analysis is the first to examine genital-to-genital αHPV transmission dynamics between heterosexual partners. In addition to the 5 publications [7–11] included in the recent descriptive review [6], we identified 2 more [15, 16]. Based on all 7 studies including 1173 couples, we found a higher F–M than M–F HPV transmission rate. The pooled rate difference slightly favored a F–M directionality, albeit not statistically significant. By removing 2 studies [8, 10], the point estimates were attenuated but still favored F–M being higher than M–F transmission, notwithstanding the high heterogeneity observed among studies. Preponderance estimates considering rates for specific HPV genotypes also supported F–M transmission being higher than M–F [9, 11]. When HPV types were examined as combined species within subgenera, incidence rates were higher in men than women for all subgenera [9, 11]. Under our ad hoc assumption that the difference in magnitude of the incidence rate may suggest the directionality in transmission, females transmitted a greater number of infections to males, thus favoring a F–M transmission directionality. However, 1 study’s [16] preponderance estimates and aggregated subgenus 1 and 2 transmission rates favored M–F transmission.
A major challenge in this review was the large statistical heterogeneity among studies. This was not surprising, in view of large variation concerning study design and participants’ characteristics (eg, unit of analysis, age, recency of relationship, sampling interval, and circumcision status). Stratified analyses by these important factors could explain the sources of heterogeneity but the number of studies was too low to permit such analyses. Nonetheless, our objective was to quantify HPV transmission rates and infer directionality based on these studies, even though the pooled estimates might not be generalizable to other populations.
The mean age of participants varied considerably among studies, ranging from 21.5 to 44 years [9, 11, 15, 16]. HPV infections are very common in young women [21], with a peak in prevalence between ages 20 and 25, after which it declines, then plateaus at approximately the age of 35, and peaks again from 40 to 45 years [22]. Conversely, HPV prevalence in men appears to vary little across all age groups [10, 23]. As such, considering populations with different age structures might contribute to the heterogeneity of measuring HPV transmission, especially when the age of women varies. Furthermore, an older study population is more likely to be comprised of couples who have been in more committed relationships (ie, marriage) for longer periods. A longer relationship duration allows for more HPV transmission opportunities to have already occurred between couples before the study begins. Likewise, couples in recently formed relationships would have had less time for transmission to occur, and thus capturing HPV transmission would be more likely. Unsurprisingly, the studies with the oldest study population had some of the lowest transmission rates [15, 16] whereas the study with the youngest study population had the highest transmission rates with over 25 couples included [7]. Therefore, recently formed young couples would be an ideal population to study HPV transmission [4] as it may reflect exposure to HPV at the onset of sexual activity [24]. However, most studies [8–11, 15, 16] were not based on recent relationships and some exclusively contained married couples [15, 16].
Another factor that might have contributed to the observed high heterogeneity relates to the discrepancy among studies in calculating person-time and number of transmission events, which could have resulted in different estimates. Two studies [8, 10] used “person” as their unit of analysis and, interestingly, reported the highest transmission rates. Conversely, all other studies used “infection” as their unit of analysis [7, 9, 15, 16], except for 1 [11] in which it was not clear how events and person-time were calculated. The advantage of using “infection” over “person” as the unit of analysis is the ability to measure subsequent infections within an individual. For example, consider a female infected with HPVs 16 and 18 and her male partner being infection-free at baseline. At the first follow-up visit, 2 months later, the female’s infection status remains unchanged while the male is infected with HPV-16. At the third visit, 4 months after study enrollment, the male becomes infected with HPV-18, while still testing positive for HPV-16 and his female partner also still testing positive for HPVs 16 and 18. By using “infection” as the unit of analysis, the overall F–M transmission rate would be 0.33 infections per month (2 infections in 6 months), whereas using “person” as the unit of analysis would yield an overall F–M transmission rate of 0.5 infections per month (1 infection in 2 months).
In relation to specimen collection, the sampling intervals ranged from days [10] to biannual visits [9, 11, 15, 16] with 2 studies [7, 8] in between this range. The sampling interval in Hernandez et al [8] aligns well with the 6- to 12-week period required for desquamation and infection of a previously unexposed individual [25]. With shorter sampling interval, it is questionable whether the detected infections represent new infection or previous infection. As such, a shorter sampling interval could potentially lead to an overestimation of the number of new infections whereas this could be underestimated with a longer interval [10]. Furthermore, carriage of HPV DNA from an infected partner can lead to transmission misclassification [26]. Contamination from a partner is most likely to have occurred in the study [10] instructing participants to have sexual intercourse before one of the study visits. Apart from this study, only 3 [7, 9, 15] instructed participants to abstain from sexual activities before the visit. Even with proper instructions, partner contamination is possible if participants disobey studies’ instructions to abstain from sexual activity. Thus, reasonable sampling interval and abstaining from any kind of sexual activity can be incorporated in future studies for optimal study design to measure HPV transmission.
Four studies [7, 8, 10, 11] reported the proportion of circumcised men, ranging from 57% to 96.6%. Uncircumcised men are at higher risk for genital HPV infection, but this association has not been consistently supported [8]. It has been hypothesized that the foreskin’s retraction during intercourse exposes the inner mucosal surface to HPV leading to an increased risk of infection [8]. If this holds true, uncircumcised men may experience more transmission events leading to a greater F–M transmission. The proportion of circumcised men varied by almost 40% and could be a contributing factor to the heterogeneity in studies examining transmission dynamics. Additionally, differences in anatomical site sampling may contribute to variation in measured transmission. For instance, in men, sampling of multiple genital sites rather than just the glans penis could enhance HPV detection. Su et al [15] sampled the glans penis but not the scrotum and they reported the second lowest F–M transmission rates. Therefore, differences in sampling of anatomical sites should also be further investigated as to how influential they are in determining transmission.
Some of the strengths of our meta-analysis include a comprehensive search strategy, detailed data extraction methods, use of random effects modeling technique, and examination of preponderance and rates by subgenera, as additional analytical strategies. On the other hand, the small number of identified studies constrained further analysis; sources of heterogeneity could not be adequately addressed by conducting stratified analysis or meta-regression to assess the impact of main study variables on transmission rates. Moreover, not all studies reported complete monogamy amongst their study participants. It is possible that some incident infections could have been acquired through sexual activity with another partner. As additional limitations, not all studies instructed participants to refrain from sexual intercourse prior to sample collection, which may have increased false positives via HPV deposition from partner. Only 1 study [16] reported type-specific HPV transmission by directionality. Presenting this information would enable future research to evaluate the extent of a differential HPV transmission hypothesis at the type-specific level.
Understanding HPV transmission dynamics is critical as the World Health Organization endeavors to accelerate the elimination of cervical cancer globally with clear goals and targets for the 2020–2030 decade [27]. To attain this, vaccination will continue to play an increasingly important role and this needs to be assessed in future couple-based studies examining HPV transmission. Most of the studies included herein were conducted before the vaccine era. However, 1 of these studies has since provided evidence supporting the impact of HPV vaccination on reducing transmission [28]. Furthermore, our search strategy identified an ongoing couple-based randomized controlled trial examining the reduction of HPV transmission via vaccination over multiple visits [29]. The multifactorial design of this study by which couples with discordant vaccination regimens can be evaluated would shed light on HPV transmission dynamics. Additionally, more couple-based cohort studies are needed for a better understanding of the directionality of HPV transmission.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Drs Talía Malagón and Farzin Khosrow-Khavar for their much appreciated input on the statistical analysis; and Drs Brenda Hernandez, Mengfei Liu, and Ting Wu (on behalf of Dr Yingying Su) for providing additional information on the couples’ relationship durations.
Author contributions. E. L. F. conceived the study question. R. B. and K. W. carried out the literature search. R. B., A. M., and K. W. performed the primary and secondary screening. R. B. and A. M. conducted the data analysis and led writing of the manuscript under supervision of M. Z. and E. L. F. All authors contributed to data interpretation and critical revision of the manuscript.
Financial support. This work was supported by the Canadian Institutes of Health Research (grant number FDN-143347 Foundation Grant to E. L. F.).
Potential conflicts of interest. E. F. reports personal fees from Roche and Merck outside the submitted work. E. F. and M. Z. have a patent “Methylation Markers in Cervical Cancer” pending. A. M. reports a grant from Canadian Institutes of Health Research and McGill Faculty of Medicine Internal Studentship Award (jointly funded by Gershman Memorial Fellowship and the Dr John A. Lundie Research Fellowship), outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: HPV Prevention and Control Board Meeting, Antwerp, Belgium, 14–15 November 2019; European Research Organisation on Genital Infection and Neoplasia 2019, Monte Carlo, Monaco, 4–7 December 2019; and International Papillomavirus Conference, Barcelona, Spain, 20–24 July 2020 (virtual presentation).
References
- 1. Veldhuijzen NJ, Snijders PJ, Reiss P, Meijer CJ, van de Wijgert JH. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect Dis 2010; 10:862–74. [DOI] [PubMed] [Google Scholar]
- 2. Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008; 110(suppl 2):S4–7. [DOI] [PubMed] [Google Scholar]
- 3. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13:607–15. [DOI] [PubMed] [Google Scholar]
- 4. Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine 2006; 24(suppl 3):S3/52–61. [DOI] [PubMed] [Google Scholar]
- 5. Malagón T, Louvanto K, Wissing M, et al. Hand-to-genital and genital-to-genital transmission of human papillomaviruses between male and female sexual partners (HITCH): a prospective cohort study. Lancet Infect Dis 2019; 19:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015; 136:2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burchell AN, Coutlée F, Tellier PP, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis 2011; 204:1723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis 2008; 14:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nyitray AG, Lin HY, Fulp WJ, et al. The role of monogamy and duration of heterosexual relationships in human papillomavirus transmission. J Infect Dis 2014; 209:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 2013; 207:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mbulawa ZZ, Johnson LF, Marais DJ, Coetzee D, Williamson AL. The impact of human immunodeficiency virus on human papillomavirus transmission in heterosexually active couples. J Infect 2013; 67:51–8. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62:1006–12. [DOI] [PubMed] [Google Scholar]
- 13. de Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324:17–27. [DOI] [PubMed] [Google Scholar]
- 14. Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005; 337:76–84. [DOI] [PubMed] [Google Scholar]
- 15. Su Y, Wei F, Huang X, et al. Prevalence, concordance and transmission of human papillomavirus infection among heterosexual couples in Liuzhou, China: an observational perspective study. J Infect Dis 2019; 220:980–9. [DOI] [PubMed] [Google Scholar]
- 16. Liu M, He Z, Zhang C, et al. Transmission of genital human papillomavirus infection in couples: a population-based cohort study in rural China. Sci Rep 2015; 5:10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mbulawa ZZ, Coetzee D, Marais DJ, et al. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J Infect Dis 2009; 199:1514–24. [DOI] [PubMed] [Google Scholar]
- 18. Mbulawa ZZ, Marais DJ, Johnson LF, Coetzee D, Williamson AL. Impact of human immunodeficiency virus on the natural history of human papillomavirus genital infection in South African men and women. J Infect Dis 2012; 206:15–27. [DOI] [PubMed] [Google Scholar]
- 19. Burchell AN, Tellier PP, Hanley J, Coutlée F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis 2010; 37:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Widdice LE, Breland DJ, Jonte J, et al. Human papillomavirus concordance in heterosexual couples. J Adolesc Health 2010; 47:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol 2018; 47:2–13. [DOI] [PubMed] [Google Scholar]
- 22. Ingles DJ, Lin HY, Fulp WJ, et al. An analysis of HPV infection incidence and clearance by genotype and age in men: The HPV Infection in Men (HIM) study. Papillomavirus Res 2015; 1:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The Human Papillomavirus Infection in Men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 2008; 17:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis 2009; 200:1059–67. [DOI] [PubMed] [Google Scholar]
- 25. Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev 2012; 25:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malagón T, Burchell AN, El-Zein M, et al. ; HITCH Study Group Estimating HPV DNA deposition between sexual partners using HPV concordance, Y chromosome DNA detection, and self-reported sexual behaviors. J Infect Dis 2017; 216:1210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Accelerating the elimination of cervical cancer as a global public health problem, 2019. http://apps.who.int/gb/ebwha/pdf_files/EB144/B144_CONF1-en.pdf. Accessed 8 June 2020.
- 28. Wissing MD, Burchell AN, El-Zein M, Tellier PP, Coutlée F, Franco EL. Vaccination of young women decreases human papillomavirus transmission in heterosexual couples: findings from the HITCH cohort study. Cancer Epidemiol Biomarkers Prev 2019; 28:1825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franco E. Transmission reduction and prevention with HPV vaccination (TRAP-HPV) study. ClinicalTrials.gov identifier: NCT01824537, 2013. https://clinicaltrials.gov/ct2/show/NCT01824537. Accessed 8 June 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.