Abstract
Background
The innate immune system recalls a challenge to adapt to a secondary challenge, a phenomenon called trained immunity. Training involves cellular metabolic, epigenetic and functional reprogramming, but how broadly trained immunity protects from infections is unknown. For the first time, we addressed whether trained immunity provides protection in a large panel of preclinical models of infections.
Methods
Mice were trained and subjected to systemic infections, peritonitis, enteritis, and pneumonia induced by Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Citrobacter rodentium, and Pseudomonas aeruginosa. Bacteria, cytokines, leukocytes, and hematopoietic precursors were quantified in blood, bone marrow, and organs. The role of monocytes/macrophages, granulocytes, and interleukin 1 signaling was investigated using depletion or blocking approaches.
Results
Induction of trained immunity protected mice in all preclinical models, including when training and infection were initiated in distant organs. Trained immunity increased bone marrow hematopoietic progenitors, blood Ly6Chigh inflammatory monocytes and granulocytes, and sustained blood antimicrobial responses. Monocytes/macrophages and interleukin 1 signaling were required to protect trained mice from listeriosis. Trained mice were efficiently protected from peritonitis and listeriosis for up to 5 weeks.
Conclusions
Trained immunity confers broad-spectrum protection against lethal bacterial infections. These observations support the development of trained immunity-based strategies to improve host defenses.
Keywords: innate immunity, infection, sepsis, trained immunity, peritonitis, Listeria, pneumonia, monocyte/macrophage, neutrophil, stem cell
We used preclinical models to demonstrate that trained immunity confers broad-spectrum protection against bacterial infections. Trained immunity increased myeloid progenitors and circulating inflammatory monocytes and neutrophils, and depletion or neutralization of monocytes/macrophages and interleukin 1 signaling impaired trained immunity-mediated protection.
Innate immune cells express pattern recognition receptors specific for microbial-associated and danger-associated molecular patterns that are released by stressed or injured cells. The interaction of pattern recognition receptors with microbial- or danger-associated molecular patterns activates intracellular signaling pathways that coordinate metabolic adaptation, epigenetic changes, and gene expression. The cellular and soluble mediators mobilized on infection regulate the development of the inflammatory response, the establishment of antimicrobial cellular and humoral responses, and the restoration of homeostasis once the pathogen has been contained or eradicated. Dysfunctions in these processes may have dramatic consequences for the infected host, as observed in patients with sepsis [1–5].
It has long been thought that immunological memory was restricted to antigen-specific memory and a privilege of the adaptive immune system carried by lymphocytes. However, the description of systemic acquired resistance in plants, specific memory in invertebrates, antigen-specific memory by natural killer cells, and heterologous protection conferred by BCG, smallpox, and measles vaccines suggested the existence of a form of innate immune memory [6–13]. The term trained immunity was proposed to reflect the fact that the innate immune system recalls or adapts to a first challenge to mount a robust response to a secondary challenge by a similar or dissimilar microbial stimulus [14, 15]. The concept of innate immune training was posed by showing that a nonlethal challenge by Candida albicans improved the innate immune response of mice [16].
The molecular mechanisms underlying trained immunity include metabolic, epigenetic, and functional reprogramming of bone marrow myeloid precursors and innate immune cells. β-glucan, a fungal cell wall compound commonly used to study trained immunity, is detected by monocytes/macrophages through the dectin 1 receptor. Dectin 1 triggering activates a PI3K (phosphoinositol 3-kinase)/AKT/mTOR (mammalian target of rapamycin)/HIF-1α (hypoxia-inducible factor-1α) pathway that induces a metabolic shift toward aerobic glycolysis, increases glutaminolysis that replenishes the tricarboxylic acid cycle, activates the cholesterol synthesis pathway, and blocks the itaconate pathway [16–21]. As a consequence, metabolites such as fumarate, succinate, and mevalonate accumulate and act as cofactors of epigenetic modifiers and as amplifiers of trained immunity [19, 22]. β-glucan, C. albicans, and BCG vaccine induce genome-wide epigenetic changes, including monomethylation and trimethylation of histone (H) 3 lysine (K) 4 and acetylation of H3K27 at promoters and enhancers of genes associated with metabolic, immune, and host defense pathways [16, 23, 24]. Hence, trained monocytes/macrophages produce increased levels of cytokines (tumor necrosis factor [TNF], interleukin 1β [IL-1β], and interleukin 6 [IL-6]) when challenged with microbial compounds [16, 19, 24, 25].
Whether the induction of trained immunity confers a wide-ranging advantage during infections is unknown. Therefore, we questioned to which extent trained immunity protects from heterologous infections and at anatomic sites distant from the priming training site. Our results showed that a unique training scheme potently protected mice from clinically relevant pathogens inoculated through diverse routes to induce peritonitis, systemic infections, enteritis and pneumonia. Trained immunity was particularly efficient at protecting mice from lethal listeriosis, which was dependent on monocytes/macrophages and interleukin 1 (IL-1) signaling.
MATERIALS AND METHODS
Products used in this study are described in Supplementary Table 1.
Ethics Statement
Animal experimentation was approved by the Service des Affaires Vétérinaires, Direction Générale de l’Agriculture, de la Viticulture et des Affaires Vétérinaires, état de Vaud (Epalinges, Switzerland) under authorizations 876.9 and 877.9 and performed according to Swiss and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Mice, Cells, and Bacteria
C57BL/6J (wild-type, MyD88−/−, and Toll-like receptor [TLR] 2−/−), and BALB/cByJ female mice (Charles River Laboratories) were 8–10 weeks old. Mice were housed under specific pathogen-free conditions (license VD-H04). Mice were free of mouse hepatitis and norovirus. Bone marrow cells were cultured in Roswell Park Memorial Institute medium [26], supplemented with 50 IU/mL macrophage colony-stimulating factor (M-CSF) (ImmunoTools) to generate bone marrow–derived macrophages (BMDMs). BMDMs were trained as described elsewhere [18, 23]. Bone marrow cells were cultured for 24 hours with 10 μg/mL zymosan and M-CSF, washed, cultured 6 days in fresh medium containing M-CSF, detached, enumerated, and seeded (2 × 106 cells/mL) in 96-well plates.
Peritoneal cells obtained by a peritoneal lavage were plated at 105 cells/well in 96-well plate in 100 μL of Roswell Park Memorial Institute, washed after 4 hours, and stimulated for 24 hours. Listeria monocytogenes 10403S, methicillin-resistant Staphylococcus aureus AW7, and Escherichia coli O18 were grown in brain-heart infusion broth, Citrobacter rodentium DBS100 in LB Broth Miller, Pseudomonas aeruginosa PAO1 in LB Broth Lennox, and C. albicans 5102 in yeast extract–peptone-dextrose [27–30]. Heat inactivation was performed for 2 hours at 70°C.
Flow Cytometry
Cells were collected and stained as described in the Supplementary Methods, using antibodies described in Supplementary Table 1 [31, 32]. Data were acquired using an Attune NxT Flow Cytometer (Thermo Fisher Scientific) and analyzed using FlowJo_V10_CL software (FlowJo). Gating strategies are presented in Supplementary Figure 1.
Whole-Blood Bactericidal Assay and Cytokine Production
The whole-blood assay is described in the Supplementary Methods. Cytokines were quantified by means of enzyme-linked immunosorbent assay, a ProCarta kit (Invitrogen), and a Bio-Plex 200 system (Bio-Rad) [33].
Isolation of Bone Marrow Monocytes and Chromatin Immunoprecipitation
The isolation of bone marrow monocytes and chromatin immunoprecipitation were performed as described in the Supplementary Methods [34].
In Vivo Models
Age-matched female mice were randomly divided into groups. Mice were injected intraperitoneally with 1 mg of zymosan or heat-killed C. albicans or intravenously with 0.1 mg of zymosan before bacterial challenge. Staphylococcal sepsis was also tested in BALB/cByJ mice. Enteritis was induced in TLR2−/− mice deprived of food for 8 hours before bacterial challenge. The role of monocytes/macrophages was assessed using mice injected intraperitoneally with 200 µL of clodronate or phosphate-buffered saline liposomes (LIPOSOMA research) 7 and 4 days before infection. The role of polymorphonuclear neutrophils (PMNs) was assessed using mice injected intraperitoneally with 100 µg of 1A8 anti-Ly6G monoclonal antibody (mAb) or 2A3 immunoglobulin G2a isotype control mAb (Bio X Cell) 6 days, 3 days, and 1 day before infection.
Cell depletion (≥95% and ≥60% for monocytes/macrophages and PMNs, respectively) was evaluated on the day of infection by means of flow cytometry. Depletion in trained mice reduced PMNs to levels similar to those measured in isotype control mAb-treated untrained mice (mean [standard deviation], 3.7 [1.2] vs 2.7 [0.7] × 106 PMNs/mL; n = 8; P > .05). To assess the role of IL-1 signaling, mice were injected intraperitoneally with 500 µg of anakinra (Kineret; Sobi) 6, 5, 4, and 3 days before infection. Body weight, severity score, and survival were recorded at least once daily [35].
Statistical Analyses
Graphics represent data obtained from individual mice, or boxes with minimum-to-maximum whiskers. Data were analyzed for normal distribution and homogeneity of variances and compared with the appropriate parametric (2-tailed unpaired Student t ) or nonparametric (2-tailed Mann-Whitney) statistical test. The false discovery rate was controlled, when necessary. The Kaplan-Meier method was used for building survival curves, and differences were analyzed using the log-rank sum test. Analyses were performed using PRISM version 8.0.1 software (GraphPad Software). P values were 2 sided.
RESULTS
Impact of Trained Immunity on E. coli Peritonitis
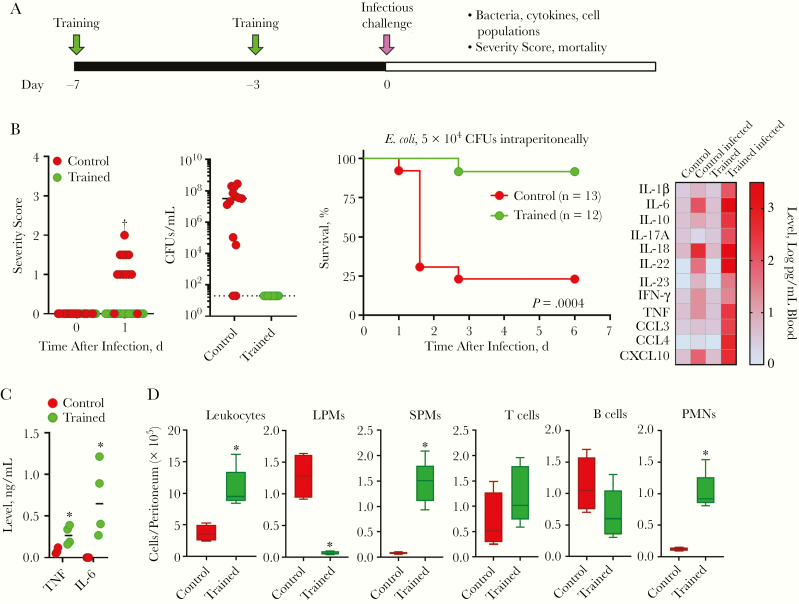
C57BL/6J mice were trained with zymosan, a cell wall preparation rich in β-glucan, given intraperitoneally 7 and 3 days before infection [18] unless specified otherwise (Figure 1A). In a first model, control and trained mice were challenged intraperitoneally with E. coli. Trained mice coped much better with peritonitis than control mice, showing lower severity scores, absence of E. coli dissemination in the blood, and improved survival rates (92% vs 23% survival; P < .001) (Figure 1B). Cytokine levels were increased in the blood of trained mice (Figure 1B), likely reflecting diffusion from the peritoneal cavity in which trained cells responded massively to E. coli.
Figure 1.
Trained immunity protects from Escherichia coli peritonitis. A, Experimental model to study protection from infection mediated by trained immunity. Unless specified otherwise, training was induced by 2 intraperitoneal injections of 1 mg zymosan, performed 4 days apart (at −7 and −3 days) using C57BL/6J mice. B, Control and trained mice were challenged intraperitoneally with 104 colony-forming units (CFUs) of E. coli. Severity score and survival were recorded. Blood was collected 18 hours after infection to quantify bacteria (Dashed line indicates lower limit of detectio) and cytokines using Luminex technology (n = 6 mice per group). C, Tumor necrosis factor (TNF) and interleukin 6 (IL-6) production by peritoneal cells from control and trained mice exposed ex vivo for 6 hours (TNF) or 24 hours (IL-6) to 10 ng/mL lipopolysaccharide. D, Leukocytes in the peritoneal cavity of control and trained mice before infection (n = 4–5 mice). *P ≤ .05; †P ≤ .001. Abbreviations: CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; IFN, interferon; IL-1β (etc), interleukin 1β (etc); LPMs, large peritoneal macrophages; PMNs, polymorphonuclear neutrophils; SPMs, small peritoneal macrophages; TNF, tumor necrosis factor.
Supporting this assumption, peritoneal cells from trained mice produced high levels of TNF and IL-6 in response to lipopolysaccharide (LPS) stimulation (Figure 1C). Moreover, the peritoneal cavity of trained mice contained more leukocytes, among which were more phagocytes (Figure 1D). PMNs were increased 8.2-fold, and there was a shift in the macrophage population. In control mice, the peritoneal cavity contained mainly homeostatic large peritoneal macrophages (LPMs) that virtually disappeared in trained mice at the expense of inflammatory and bactericidal small peritoneal macrophages (SPMs).
Impact of Trained Immunity on Systemic Staphylococcal and Listeria Infections
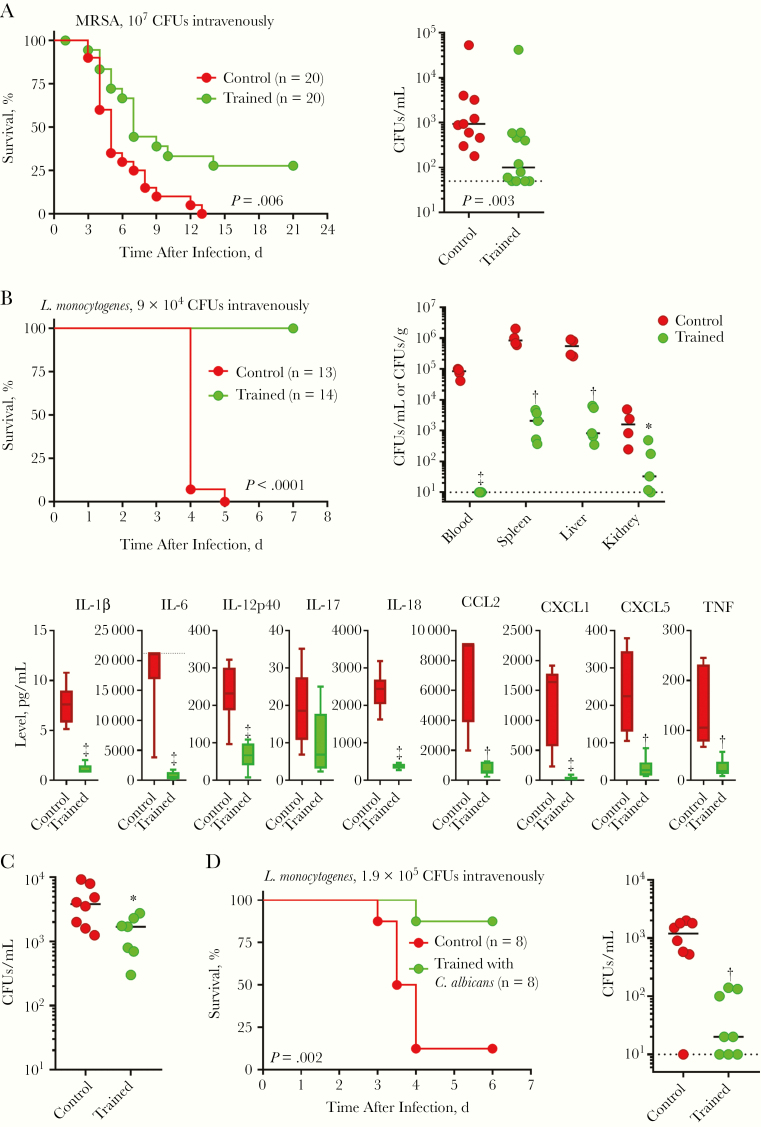
To explore whether trained immunity protected from systemic infections, C57BL/6J mice were injected intravenously with methicillin-resistant S. aureus. Trained mice survived better than control mice (31% vs 0% survival; P = .006) and had 10-fold less bacteria in blood 2 days after infection (Figure 2A). Very similar results were obtained using BALB/cByJ mice (Supplementary Figure 2A), and subsequent experiments were all performed using C57BL/6J mice.
Figure 2.
Trained immunity protects from systemic staphylococcal and Listeria infections. A, Control and trained mice were challenged intravenously with 107 colony-forming units (CFUs) of methicillin-resistant Staphylococcus aureus (MRSA). Survival was recorded. Blood was collected 2 days after infection to quantify bacteria. B, Control and trained mice were challenged intravenously with 9 × 104 CFUs of Listeria monocytogenes. Survival was recorded. Blood, spleen, liver, and kidney were collected 3 days after infection to quantify bacteria. Dashed line indicates lower limit of detection. Blood was collected 2 days after infection to quantify cytokines using Luminex technology (n = 6 mice per group). Dashed line indicates upper limit of detection. C, Control mice and mice trained with zymosan intravenously (0.1 mg at day −7) were infected intravenously with 105 CFUs of L. monocytogenes. Blood was collected 2 days after infection to quantify bacteria. D, Control mice and mice trained with heat-killed Candida albicans were infected intravenously with 1.9 × 105 CFUs of L. monocytogenes. Survival was recorded, and blood was collected 2 days after infection to quantify bacteria. *P ≤ .05; †P ≤ .01; ‡P ≤ .001. Abbreviations: CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; IL-1β (etc), interleukin 1β (etc); TNF, tumor necrosis factor.
Mice were challenged intravenously with a lethal dose of L. monocytogenes. Most strikingly, all trained mice survived infection, whereas all control mice died within 5 days (Figure 2B). Bacteria were not detected in blood collected from trained mice 2 and 3 days after infection, whereas up to 105 colony-forming units/mL were measured in the circulation of control mice (Figure 2B and SupplementaryFigure 2B). Trained mice had 2–3-log lower counts of L. monocytogenes in spleen, liver, and kidney (Figure 2B), and L. monocytogenes was undetectable in organs collected from mice surviving infection for 1–2 months.
Confirming an efficient control of bacterial burden in trained mice, cytokines and chemokines were detected at much lower concentrations in blood collected 3 days after infection (Figure 2B). Training mice with zymosan given intravenously as a single dose 7 days before infection also efficiently reduced bacterial burden, suggesting that training can be induced through diverse routes (Figure 2C). Because training was demonstrated by challenging mice with heat-killed C. albicans [16], we questioned whether a similar approach would protect from listeriosis. Mice trained with heat-killed C. albicans were powerfully protected from lethal listeriosis (P = .002) and had greatly reduced bacteremia (Figure 2D). Thus, trained immunity efficiently protected mice from systemic bacterial infections.
Impact of Trained Immunity on Enteritis and Pneumonia
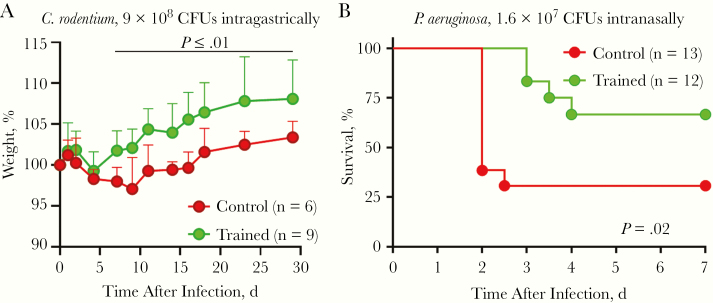
To extend the panel of microorganisms and routes of inoculation tested, we developed models of enteritis and pneumonia. Enteritis induced by C. rodentium was established in TLR2−/− mice, because the bacteria are cleared quickly in immunocompetent animals. All mice lost weight and had some diarrhea, but none died. Trained and control mice recovered their initial weights 7 and 18 days after infection, respectively. Trained mice showed improved weight from day 7 to day 30 (P < .01) (Figure 3A). Pneumonia was induced by an intranasal challenge with P. aeruginosa. The survival of trained mice was largely improved (P = .02) (Figure 3B). Overall, trained immunity protected mice in all the preclinical models tested, suggesting the enhancement of broad mechanisms of defense.
Figure 3.
Trained immunity protects from enteritis and pneumonia. A, Weight of control and trained mice challenged intragastrically with 9 × 108 colony-forming units (CFUs) of Citrobacter rodentium. B, Survival of control and trained mice challenged intranasally with 1.6 × 107 CFUs of Pseudomonas aeruginosa.
Impact of Trained Immunity on Blood Antimicrobial Activity and Myelopoiesis
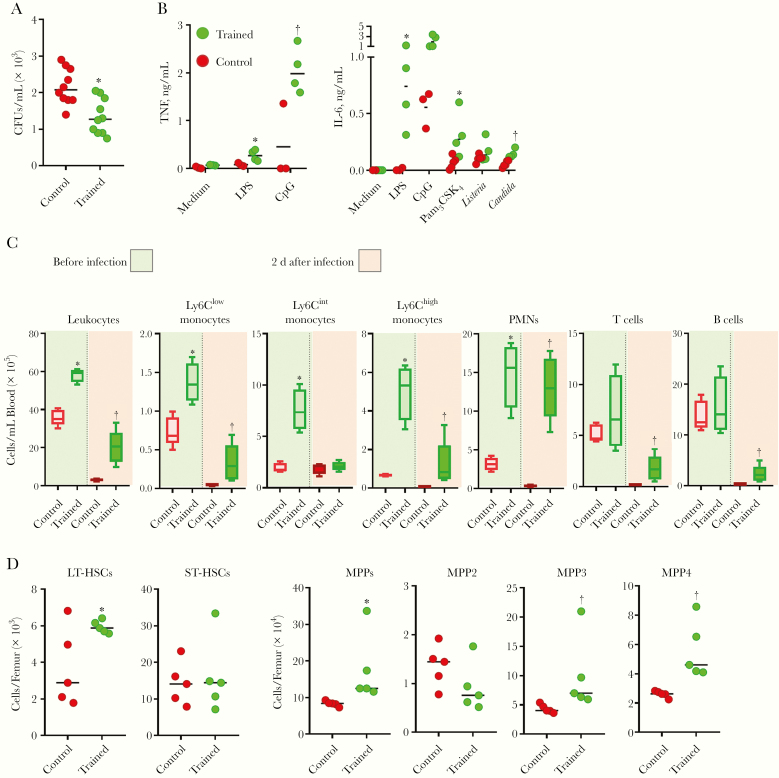
Trained immunity protected mice remarkably well from systemic listeriosis (92 of 96 trained mice survived vs 0 of 74 control mice; n = 5 experiments). We reasoned that blood should provide an efficient barrier against L. monocytogenes burden. Ex vivo, the blood of trained mice limited the growth of L. monocytogenes better than that of control mice (Figure 4A), and it was more reactive to microbial products, as shown by increased production of TNF and IL-6 in response to LPS, CpG, Pam3CSK4, L. monocytogenes and C. albicans (Figure 4B).
Figure 4.
Trained immunity increases blood antimicrobial activity and stimulates myelopoiesis. A, Bacteria in whole blood from control and trained mice exposed ex vivo for 2 hours to 1.2 × 103 colony-forming units (CFUs)/mL of Listeria monocytogenes. B, Tumor necrosis factor (TNF) and interleukin 6 (IL-6) production by whole blood from control and trained mice exposed ex vivo for 6 hours (TNF) or 24 hours (IL-6) to 10 ng/mL lipopolysaccharide (LPS), 10 μmol/L CpG, 100 ng/mL Pam3CSK4, 107 heat-killed L. monocytogenes, and 10 mg/mL heat-killed Candida albicans. C, Leukocytes in blood collected from control and trained mice before infection (left part) and 2 days after an intravenous challenge with 1.1 × 105 CFUs of L. monocytogenes (right part) (n = 5 mice per group). D, Long-term and short-term hematopoietic stem cells (LT-HSCs and ST-HSCs), multipotent progenitors (MPPs), MPP2, MPP3, and MPP4 in the bone marrow of control and trained mice. *P ≤ .05; †P ≤ .01. Abbreviation: PMNs, polymorphonuclear neutrophils.
We then quantified leukocytes in control and trained mice, using blood collected before and 2 days after infection with L. monocytogenes (Figure 4C). Training increased leukocyte counts 1.6 fold, reflecting more Ly6Chigh, Ly6Cint, and Ly6Clow monocytes (inflammatory, intermediate, and nonclassic monocytes) and PMNs (7.6-, 3.9-, 1.9-, and 4.7-fold increase, respectively, vs control; P < .05). In trained mice, Ly6Chigh and Ly6Cint monocytes expressed more CD11b/Itgam (2.5- and 1.8-fold higher mean fluorescence intensity), indicative of a primed/activated phenotype (Supplementary Figure 3). Conversely, PMNs expressed lower CD11b (2.5 lower mean fluorescence intensity), which is associated with an immature status [36]. The absolute numbers of T and B lymphocytes were not affected.
L. monocytogenes induced a massive depletion of leukocytes 2 days after infection, which was less pronounced in trained mice (2.9- vs 11.5-fold decrease in trained vs control mice) (Figure 4C). Ly6Chigh and Ly6Clow monocytes were more preserved in trained mice. PMNs were rather stable in trained mice, but they decreased 9.9-fold in control mice. T cells and B cells decreased 4–7-fold in trained mice, but 40-fold in control mice. The relative preservation of blood leukocytes suggested an enhanced hematopoiesis in trained mice. Indeed, the bone marrow of trained mice contained more long-term hematopoietic stem cells and more multipotent progenitors (MPPs), including more myeloid-biased MPP3 and lymphoid-biased MPP4 (Figure 4D).
Role of Monocytes/Macrophages and IL-1 Signaling
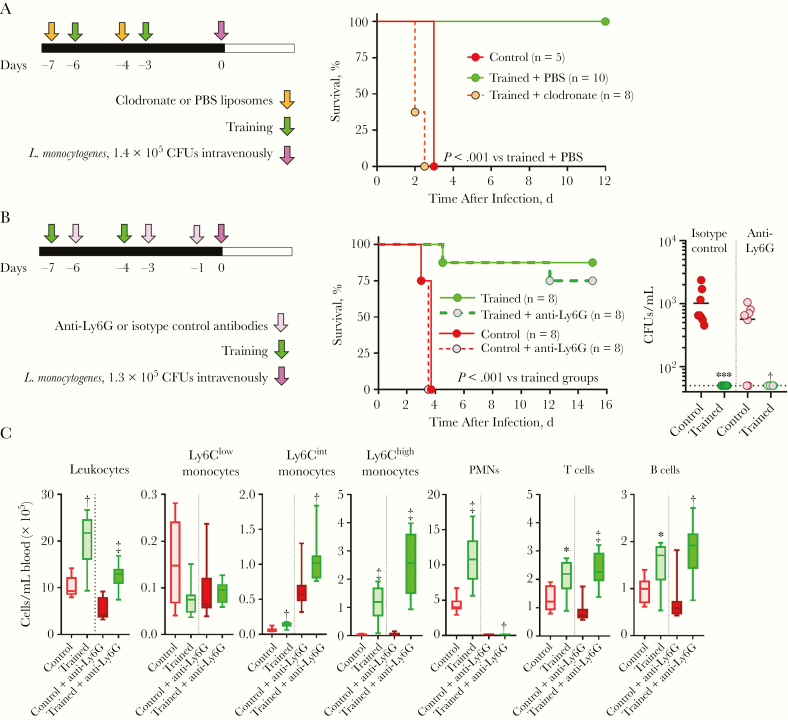
We analyzed the impact of depleting monocytes/macrophages by treating mice with clodronate liposomes during induction of training. Clodronate treatment fully abolished the protection conferred by training (Figure 5A). Because PMNs were also increased during training, we tested the impact of PMN depletion using anti-Ly6G mAb. In both trained and control mice, the depletion of PMNs modified neither survival nor bacteremia (Figure 5B). Two days after infection, PMNs were still fully depleted from the blood of anti-Ly6G mAb-treated mice, whether trained mice or control mice (Figure 5C). These observations suggested that monocytes are central effector cells for the protection against listeriosis conferred by trained immunity.
Figure 5.
Monocytes/macrophages are essential to protect trained mice from listeriosis. A, Experimental model to study the role of monocytes/macrophages in trained immunity (left) and survival of mice treated with clodronate or phosphate-buffered saline (PBS) liposomes and challenged intravenously with 1.4 × 105 colony-forming units (CFUs) of Listeria monocytogenes (right). B, Experimental model to study the role of polymorphonuclear neutrophils (PMNs) in trained immunity (left). Survival of mice treated with anti-Ly6G or isotype control antibody and challenged intravenously with 1.3 × 105 CFUs of L. monocytogenes (middle) and bacteria in blood collected 18 hours after infection (right). C, Leukocytes in blood collected 2 days after infection with L. monocytogenes (n = 8 mice per group.) *P ≤ .01; †P ≤ .01; ‡P ≤ .001.
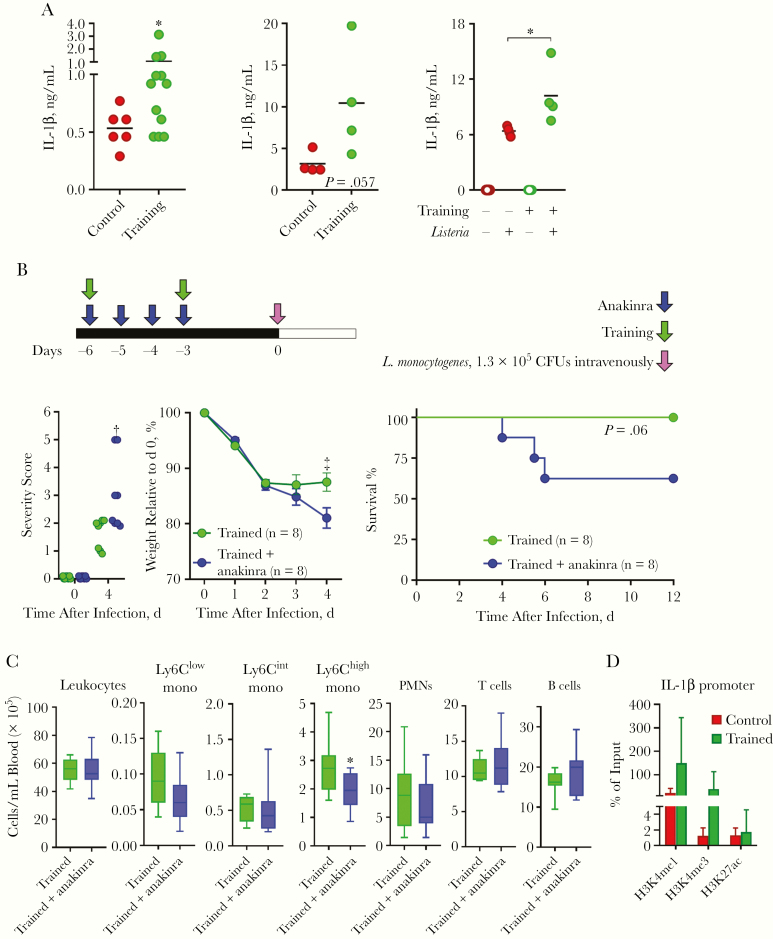
We tested the contribution of IL-1β/IL-1 signaling, because IL-1β has been proposed to play a role in trained immunity [37]. IL-1β was detected at higher concentrations in blood from trained mice (Figure 6A). Blood from trained mice showed a trend toward producing higher levels of IL-1β on exposure to L. monocytogenes, and BMDMs trained in vitro with zymosan produced higher levels of IL-1β in response to L. monocytogenes (Figure 6A).
Figure 6.
Interleukin 1 (IL-1) signaling participates to protect trained mice from listeriosis. A, Interleukin 1β (IL-1β) concentrations in blood collected from control and trained mice at day 0 (left), in whole blood from control and trained mice exposed ex vivo for 6 hours to 108 heat-killed Listeria monocytogenes (middle), and in cell culture supernatants of control and trained bone marrow–derived macrophages cultured for 24 hours with or without 1 live L. monocytogenes per cell (right). B, Experimental model to study the role of IL-1 signaling in trained immunity (top). Severity score, weight loss, and survival (bottom) of mice trained with or without anakinra treatment and challenged intravenously with 1.3 × 105 colony-forming units (CFUs) of L. monocytogenes (n = 8 mice per group). C, Leukocytes in blood collected just before infection from mice trained with or without anakinra (n = 9–10 mice per group). D, H3K4me1, H3K4me3 and H3K27ac signal at the Il1b promoter of bone marrow monocytes isolated from mice trained or not 3 weeks earlier were quantified by chromatin immunoprecipitation followed by real-time polymerase chain reaction and expressed as percentage of input (n = 4 mice per group). *P ≤ .05; †P ≤ .01; ‡P ≤ .001. Abbreviation: PMNs, polymorphonuclear neutrophils.
Because MyD88 is the adaptor signaling molecule downstream interleukin 1R, we quantified leukocytes as a surrogate of IL-1 signaling-mediated training in MyD88−/− mice. None of the changes observed in trained wild-type mice were detected in trained MyD88−/− mice, which behaved similarly to untrained MyD88−/− mice (Supplementary Figure 4). The role of IL-1 signaling was tested in mice treated daily for 4 days with recombinant IL-1 receptor antagonist (anakinra) during the induction of trained immunity (Figure 6B-C). Severity score and weight loss (P < .01 and P < .001, respectively) were increased in anakinra-treated mice. Accordingly, 3 of 8 anakinra-treated trained mice died of listeriosis, whereas all trained mice survived infection (P = .06). The partial effect on mortality rate was related to a partial (1.4-fold) reduction in Ly6Chigh monocytes (P = .02) (Figure 6C). These data supported the assumption that IL-1 signaling is involved in the antilisterial activity conferred by trained immunity. Interestingly, chromatin immunoprecipitation assays performed on monocytes isolated 3 weeks after the induction of training revealed increased marks associated with active chromatin (H3K4me1 and H3K4me3, Figure 6D) and trained immunity [16, 23, 24].
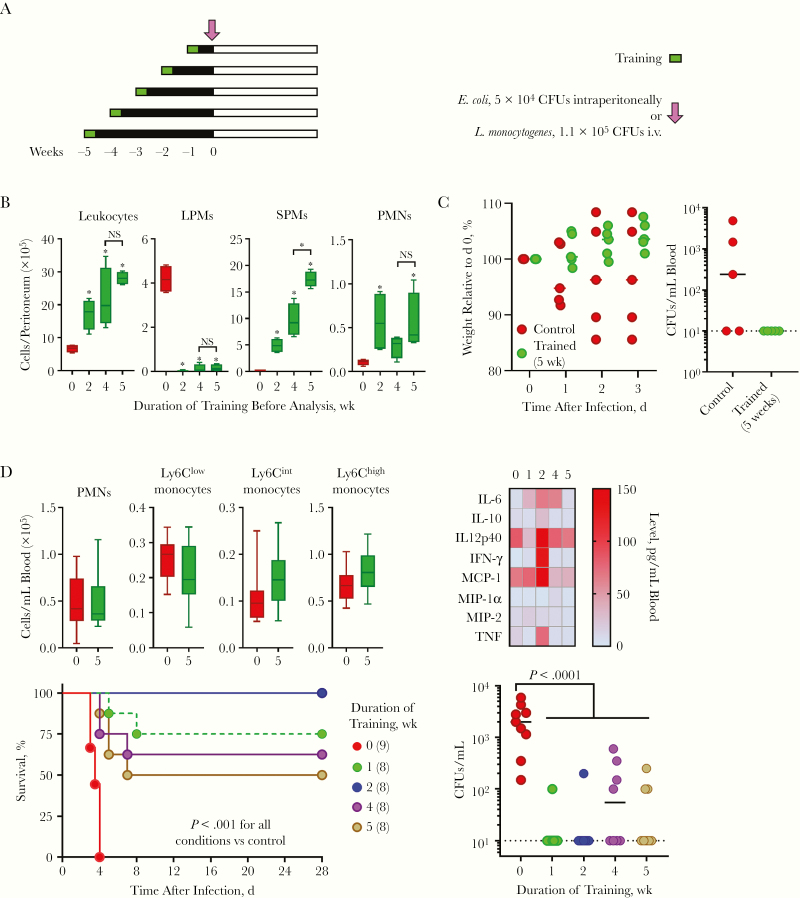
Length of Protection Conferred by Trained Immunity
The length of protection conferred by trained immunity against lethal bacterial infections is unknown. To start filling that gap, we analyzed mice trained up to 5 weeks earlier (Figure 7A). In the peritoneal cavity, leukocytes steadily increased from 2 to 5 weeks after training, reaching a maximum after 5 weeks (Figure 7B). The decreased number of LPMs remained drastic at all time points, whereas the number of SPMs increased 2–5 weeks after training. PMNs reached a maximum value 2 week after training and remained stable for up to 5 weeks. In line with these observations, mice trained 5 weeks earlier were protected from E. coli peritonitis, as shown by reduced weight loss and bacterial dissemination into the blood compared with untrained mice (Figure 7C). Blood cytokine levels increased mainly 1–2 weeks after training, and returned to baseline levels 5 weeks after challenge (Figure 7D). Moreover, blood leukocyte counts were back to normal 5 weeks after training (Figure 7D). Impressively, mice trained 1, 2, 4, or 5 weeks earlier were all protected from listeriosis, in term of both survival and bacteremia (Figure 7D).
Figure 7.
Trained immunity protects from peritonitis and listeriosis for at least 5 weeks. A, Experimental model to study the effects of training over time. B, Leukocytes in the peritoneal cavity of mice trained 0, 2, 4, and 5 weeks earlier (n = 5 mice per group). C, Weight loss and bacteria in blood collected 2 days after an intraperitoneal challenge with 5 × 104 colony-forming units (CFUs) of Escherichia coli. D, Leukocytes (n = 8–10) and cytokines (n = 6) in blood of mice trained 0, 1, 2, 4 and 5 weeks earlier (top). Survival and bacteria in blood collected 2 days after infection of mice challenged intravenously with 1.1 × 105 CFUs of Listeria monocytogenes). *P ≤ .05. Abbreviations: IFN, interferon; IL-6 (etc), interleukin 6 (etc); LPMs, large peritoneal macrophages; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NS, not significant; PMNs, polymorphonuclear neutrophils; SPMs, small peritoneal macrophages; TNF, tumor necrosis factor.
DISCUSSION
We report the first broad analysis of the impact of trained immunity on bacterial infections. Trained immunity protected mice from a large panel of clinically relevant bacterial pathogens inoculated systemically and locally to induce peritonitis, enteritis, and pneumonia.
In all models, bacterial dissemination was controlled. In the peritonitis model, this results from a massive accumulation of PMNs and SPMs in the peritoneal cavity. At baseline, SPMs represent <10% of peritoneal macrophages, which are composed mainly of self-maintaining LPMs. Local inflammation triggers the migration of LPMs to the omentum, where these cells produce growth factors and chemokines that stimulate myelopoiesis and induce the influx of PMNs and inflammatory monocytes, which are precursors of SPMs [38]. Hence, LPMs and SPMs play key roles as initiator and effector cells of trained immunity when training and infection occur in the peritoneum.
Stimulation of myelopoiesis greatly increased blood leukocyte counts, above all those of PMNs and Ly6Chigh inflammatory monocytes exhibiting a primed/activated phenotype. Changes in peripheral blood gave an indubitable advantage to trained mice during systemic infections, as demonstrated in models of listeriosis and staphylococcal infection. The picture was rather unexpected in the model of listeriosis, because almost all trained mice survived a challenge equivalent to 10–20 times the LD100. Results of cell depletion experiments suggested that monocytes/macrophages were the main drivers of protection. This is in agreement with the fact that inflammatory monocytes were essential whereas PMNs were dispensable for clearing bacteria during the early and late phases of systemic infection by L. monocytogenes [39]. Circulating inflammatory monocytes migrated to foci of infection in liver and spleen to give rise to TNF- and inducible nitric oxide synthase–producing dendritic cells and monocyte-derived macrophages that replenished Kupffer cells dying through necroptosis. This helped enhance antibacterial immunity and restore tissue integrity [40, 41].
Trained immunity protected mice from enteritis and pneumonia, pointing to distant effects. Intraperitoneal injections of LPS induced epigenetic reprogramming of brain resident macrophages and modulated neuropathology in models of Alzheimer disease and stroke [42]. The broad effects of trained immunity during infections may result from the action of soluble mediators that directly or indirectly stimulate the response of intestinal, airway, and lung parenchyma cells or resident immune cells. For example, adenoviral infection improved the activity of self-renewing memory alveolar macrophages that promoted neutrophilia and protected from Streptococcus pneumoniae lung infection [43].
Bone marrow hematopoietic stem and progenitor cells adapted to acute and chronic peripheral inflammation and infection through cell-extrinsic and cell-intrinsic mechanisms, increasing proliferation and skewing toward the myeloid lineage to provide activated innate immune cells [44–46]. Our training protocol increased the number of hematopoietic stem cells and MPPs, accounting for higher leukocyte counts before and during infection. In the same vein, the adoptive transfer in naive mice of long-term hematopoietic stem cells or bone marrow cells collected from mice trained with β-glucan and BCG vaccine increased the proportion of blood Gr1+CD11b+ myeloid cells and protected from pulmonary tuberculosis [25, 44]. We are performing experiments to delineate the length of protection conferred by trained immunity. This information will be valuable for preclinical and clinical development of trained immunity-based therapeutics.
The diversity of the models of infection tested supports wide effects of trained immunity. Work will be required to establish whether trained immunity protects from additional bacterial, fungal and viral infections. Trained immunity is most typically induced with β-glucan, which promotes T-helper (Th) 1/Th17 proinflammatory responses essential to fight bacteria, viruses, and fungi. However, training might be tipped toward Th2 immune responses beneficial during parasitic infections [47].
IL-1β has gained attention as a possible hub regulating trained immunity [24, 37, 44–46]. Uninfected trained mice expressed increased blood levels of IL-1β, and treatment with anakinra compromised trained immunity, indicating that IL-1 signaling played a role. Trained peripheral blood mononuclear cells produced higher levels of IL-1β, and IL-1β itself fueled human monocytes to produce higher levels of cytokines on stimulation [17]. In vivo, BCG vaccine–induced IL-1β production was correlated with the capacity to control viremia in healthy subjects challenged with yellow fever vaccine [24]. IL-1 family members can affect trained immunity through manifold mechanisms [37]. Interestingly, training mice with β-glucan sustained IL-1β signaling that promoted glycolytic activity and proliferation of hematopoietic stem and progenitor cells [44]. This aspect is highly relevant for listeriosis, which affects blood leukocytes and constrains vigorous myelopoiesis. Inflammasomes control IL-1β secretion and are likely to be involved in trained immunity. Supporting this hypothesis, feeding Ldr−/− atherosclerotic mice a Western diet induced an oxidized low-density lipoprotein/NOD-like receptor family, pyrin domain containing 3 (NLRP3)/IL-1 axis, leading to the establishment of trained immunity [45].
Induction of trained immunity is an attractive approach to increase vaccine efficacy and resistance to pathogens. Training with β-glucan counteracted endotoxin-mediated immune tolerance associated with poor outcome in sepsis [3, 4, 21, 48]. However, immunotherapies may have doubled-edge sword effects. Low-grade inflammation sustained by trained immunity may be involved in the pathophysiology of chronic and autoinflammatory disorders [49]. Monocytes from patients with hyperimmunoglobulin D syndrome have a trained phenotype [22] and Western diet feeding induced trained immunity [45]. Interfering with the sensing of training inducers, IL-1 signaling, and inflammation, metabolic and epigenetic changes may be exploited to avoid pathogenic processes linked to trained immunity. For instance, trained immunity induced by helminth products provided an anti-inflammatory environment, attenuating the development of experimental autoimmune encephalomyelitis [50].
In summary, induction of trained immunity remodeled bone marrow and blood cellular compartments, providing efficient barriers against bacterial infections. Protection was remarkably broad when considering the pathogens and sites of infection tested. These data support the development of trained immunity-based strategies to improve the efficacy of vaccines and host defenses against infections, and they may give clues about the pathological processes underlying inflammatory and autoimmune disorders.
Supplementary Material
Notes
Author contributions. E. C., T. H., and D. L. R. performed the in vitro experiments. E. C., T. H., C. T., F. A., and D. L. R. performed the in vivo experiments. T. R. conceived the project. M. G. N. provided protocols and discussed the project. E. C. and T. R. designed the experiments and wrote the article. All authors discussed the results and revised the article.
Financial support. This work was supported by the Swiss National Science Foundation (grants 149511 and 173123 to T. R.), the Société Académique Vaudoise (scholarship to T. H.), and the European Sepsis Academy Horizon 2020 Marie Skłodowska-Curie Action: Innovative Training Network (grant 676129 to C. T. and F. A.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Savva A, Roger T. Targeting Toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol 2013; 4:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 2017; 17:407–20. [DOI] [PubMed] [Google Scholar]
- 4. Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol 2018; 14:121–37. [DOI] [PubMed] [Google Scholar]
- 5. Ciarlo E, Savva A, Roger T. Epigenetics in sepsis: targeting histone deacetylases. Int J Antimicrob Agents 2013; 42(suppl):S8–12. [DOI] [PubMed] [Google Scholar]
- 6. Kurtz J, Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature 2003; 425:37–8. [DOI] [PubMed] [Google Scholar]
- 7. Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol 2006; 16:1206–10. [DOI] [PubMed] [Google Scholar]
- 8. Kachroo A, Robin GP. Systemic signaling during plant defense. Curr Opin Plant Biol 2013; 16:527–33. [DOI] [PubMed] [Google Scholar]
- 9. Goodridge HS, Ahmed SS, Curtis N, et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol 2016; 16:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farber DL, Netea MG, Radbruch A, Rajewsky K, Zinkernagel RM. Immunological memory: lessons from the past and a look to the future. Nat Rev Immunol 2016; 16:124–8. [DOI] [PubMed] [Google Scholar]
- 11. de Bree LCJ, Koeken VACM, Joosten LAB, et al. Non-specific effects of vaccines: Current evidence and potential implications. Semin Immunol 2018; 39:35–43. [DOI] [PubMed] [Google Scholar]
- 12. Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 2019; 25:13–26. [DOI] [PubMed] [Google Scholar]
- 13. Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol 2016; 16:112–23. [DOI] [PubMed] [Google Scholar]
- 14. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011; 9:355–61. [DOI] [PubMed] [Google Scholar]
- 15. Netea MG, Joosten LA, Latz E, et al. Trained immunity: a program of innate immune memory in health and disease. Science 2016; 352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quintin J, Saeed S, Martens JHA, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012; 12:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ifrim DC, Joosten LA, Kullberg BJ, et al. Candida albicans primes TLR cytokine responses through a dectin-1/Raf-1-mediated pathway. J Immunol 2013; 190:4129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014; 345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 2016; 24:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saz-Leal P, Del Fresno C, Brandi P, et al. Targeting SHIP-1 in myeloid cells enhances trained immunity and boosts response to infection. Cell Rep 2018; 25:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dominguez-Andres J, Novakovic B, Li Y, et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab 2019; 29:211–220 e5. [DOI] [PubMed] [Google Scholar]
- 22. Bekkering S, Arts RJW, Novakovic B, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 2018; 172:135–146 e9. [DOI] [PubMed] [Google Scholar]
- 23. Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014; 345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018; 23:89–100 e5. [DOI] [PubMed] [Google Scholar]
- 25. Kaufmann E, Sanz J, Dunn JL, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018; 172:176–190 e19. [DOI] [PubMed] [Google Scholar]
- 26. Ciarlo E, Roger T. Screening the impact of sirtuin inhibitors on inflammatory and innate immune responses of macrophages and in a mouse model of endotoxic shock. Methods Mol Biol 2016; 1436:313–34. [DOI] [PubMed] [Google Scholar]
- 27. Roger T, Froidevaux C, Le Roy D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A 2009; 106:2348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meller S, Di Domizio J, Voo KS, et al. TH17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol 2015; 16:970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roger T, Schneider A, Weier M, et al. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proc Natl Acad Sci U S A 2016; 113:E997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Savva A, Brouwer MC, Roger T, et al. Functional polymorphisms of macrophage migration inhibitory factor as predictors of morbidity and mortality of pneumococcal meningitis. Proc Natl Acad Sci U S A 2016; 113:3597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heinonen T, Ciarlo E, Le Roy D, Roger T. Impact of the dual deletion of the mitochondrial sirtuins SIRT3 and SIRT5 on anti-microbial host defenses. Front Immunol 2019; 10:2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heinonen T, Ciarlo E, Rigoni E, Regina J, Le Roy D, Roger T. Dual deletion of the sirtuins SIRT2 and SIRT3 impacts on metabolism and inflammatory responses of macrophages and protects from endotoxemia. Front Immunol 2019; 10:2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciarlo E, Heinonen T, Herderschee J, et al. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep 2016; 6:37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roger T, Ding X, Chanson AL, Renner P, Calandra T. Regulation of constitutive and microbial pathogen-induced human macrophage migration inhibitory factor (MIF) gene expression. Eur J Immunol 2007; 37:3509–21. [DOI] [PubMed] [Google Scholar]
- 35. Roger T, Delaloye J, Chanson AL, Giddey M, Le Roy D, Calandra T. Macrophage migration inhibitory factor deficiency is associated with impaired killing of gram-negative bacteria by macrophages and increased susceptibility to Klebsiella pneumoniae sepsis. J Infect Dis 2013; 207:331–9. [DOI] [PubMed] [Google Scholar]
- 36. Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 2016; 127:2173–81. [DOI] [PubMed] [Google Scholar]
- 37. Moorlag SJCFM, Röring RJ, Joosten LAB, Netea MG. The role of the interleukin-1 family in trained immunity. Immunol Rev 2018; 281:28–39. [DOI] [PubMed] [Google Scholar]
- 38. Buscher K, Wang H, Zhang X, et al. Protection from septic peritonitis by rapid neutrophil recruitment through omental high endothelial venules. Nat Commun 2016; 7:10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi C, Hohl TM, Leiner I, Equinda MJ, Fan X, Pamer EG. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J Immunol 2011; 187:5293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003; 19:59–70. [DOI] [PubMed] [Google Scholar]
- 41. Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 2015; 42:145–58. [DOI] [PubMed] [Google Scholar]
- 42. Wendeln AC, Degenhardt K, Kaurani L, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018; 556:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yao Y, Jeyanathan M, Haddadi S, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 2018; 175:1634–1650 e17. [DOI] [PubMed] [Google Scholar]
- 44. Mitroulis I, Ruppova K, Wang B, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018; 172:147–161 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christ A, Gunther P, Lauterbach MAR, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018; 172:162–175 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol 2019; 20:802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yasuda K, Adachi T, Koida A, Nakanishi K. Nematode-infected mice acquire resistance to subsequent infection with unrelated nematode by inducing highly responsive group 2 innate lymphoid cells in the lung. Front Immunol 2018; 9:2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheng SC, Scicluna BP, Arts RJ, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 2016; 17:406–13. [DOI] [PubMed] [Google Scholar]
- 49. Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov 2019; 18:553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quinn SM, Cunningham K, Raverdeau M, et al. Anti-inflammatory trained immunity mediated by helminth products attenuates the induction of T cell-mediated autoimmune disease. Front Immunol 2019; 10:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.