Abstract
Co-expression of human epidermal growth factor receptor-2 (HER2) and hormone receptor (HR) predicted worse prognosis in early breast cancer before trastuzumab was developed. We aimed to investigate whether HER2 positivity was still associated with worse outcome in high-risk estrogen receptor (ER) positive patients treated with trastuzumab and chemotherapy. In the present study, 227 ER+/HER2+ patients treated with trastuzumab and chemotherapy (HER2-pos-T group) and 1097 ER+/HER2-patients treated with chemotherapy alone (HER2-neg group) during 2009 and 2015 were retrospectively enrolled for the comparison of disease-free survival (DFS) and overall survival (OS). At a median follow-up of 59 months, 174 DFS events and 69 deaths were observed. The estimated 5-year DFS rate was 94.2% in the HER2-pos-T group and 87.4% in the HER2-neg group (Log-rank P = 0.014). HER2-pos-T group was associated with significantly better DFS in multivariate analysis (HR 0.38, 95% CI: 0.22–0.67, Log-rank P = 0.001). The estimated 5-year OS rates for the two groups were 97.2% and 95.7%, respectively (Log-rank P = 0.183). In multivariable analysis, patients in the HER2-pos-T group had significantly better OS compared with those in the HER2-neg group (HR 0.40, 95% CI: 0.17–0.95, Log-rank P = 0.037). We concluded that high-risk ER+/HER2+ breast cancer patients treated with chemotherapy and trastuzumab had superior prognosis compared with ER+/HER2-patients. Therefore, HER2 positivity itself may not be considered as an unfavorable factor for ER + patients in the era of trastuzumab.
Keywords: Breast neoplasms, Prognosis, Receptor, ErbB-2, Receptors, Estrogen, Trastuzumab
Highlights
-
•
ER+/HER2+ early breast cancer patients treated with trastuzumab-based chemotherapy had superior prognosis.
-
•
ER+/HER2+ early breast cancer patients had distinct patterns of relapse or death from those of ER+/HER2-patients.
-
•
HER2 positivity itself may not be considered as an unfavorable factor for ER + patients in the era of trastuzumab.
1. Introduction
Human epidermal growth factor receptor-2 (HER2) is amplified or overexpressed in approximately 15%–25% of newly diagnosed early breast cancers, and about 50% of these cases concurrently express hormone receptor (HR) [[1], [2], [3]]. Previous studies indicated that co-expression of HR and HER2 was associated with more aggressive tumor biological behaviors and worse disease outcomes compared with HR+/HER2-tumors [[4], [5], [6], [7]]. Thus, HR+/HER2+ patients tended to be treated with escalation endocrine therapy and chemotherapy before the advent of anti-HER2 drugs.
In the past decade, anti-HER2 monoclonal antibodies or tyrosine kinase inhibitors have dramatically improved the prognosis of patients with HER2+ breast cancers [[8], [9], [10]]. However, there are limited data evaluating the prognosis of HR+/HER2+ patients compared with HR+/HER2-patients in the era of HER2-targeted therapy. The present study was designed to investigate whether HER2 positivity was associated with worse outcome in high-risk estrogen receptor (ER) positive patients treated with trastuzumab and chemotherapy.
2. Materials and methods
Patients. We retrospectively analyzed early breast cancer patients operated at XXX Hospital between January 2009 and December 2015. Clinicopathological, treatment, and follow-up data for the patients were retrieved from XXX Database. ER+/HER2+ patients treated with trastuzumab and chemotherapy (HER2-pos-T group) and ER+/HER2-patients treated with chemotherapy alone (HER2-neg group) were included for the further analysis. The main exclusion criteria were as following [1]: neo-adjuvant therapy [2], ductal carcinoma in situ [3], ER- [4], missing of clinical or pathological information [5], no chemotherapy, and [6] HER2+, no trastuzumab.
Histopathological assessments. Tumor pathology was independently assessed and reviewed by two pathology specialists from the Department of Pathology, XXX Hospital, consistent with our previous reports [11,12]. In brief, ER positivity was defined as at least 1% tumor cells with nuclear staining in immunohistochemistry (IHC) [13]. HER2 status was first evaluated by IHC and, for HER2 2+ tumors, further examined by fluorescence in situ hybridization (FISH) according to the ASCO/CAP guidelines [[14], [15], [16]]. HER2 positivity was defined as IHC 3+ or FISH +. ER-/PR + patients were not included in the study.
Follow-up. All patients were followed up by outpatient visit or call every 3 months during the first 2 years after surgery, every 6 months between the 3rd and 5th years, then annually until death [11,12]. Disease-free survival (DFS) was defined as the time period from the date of surgery to the date of the following events: locoregional recurrence, contralateral breast cancer, secondary non-breast malignancy, distant metastasis at any site, and death. Overall survival (OS) was computed from the date of surgery to the date of death of any cause. Last follow-up was conducted in June 2019.
Statistical analysis. Chi-square test and Fisher’s exact test, if necessary, were used to compare the descriptive characteristics of categorical variables between these two groups. Factors with P < 0.05 were further tested in multivariate logistic regression model. DFS and OS rates for the two groups were estimated using Kaplan-Meier method and compared via Log-rank test. Multivariate cox proportional hazards regression analysis was performed to investigate independent prognostic factors in ER+/HER2+ patients treated with trastuzumab-based chemotherapy. Annual hazard rate curves were smoothed using ggplot function by R project, version 3.6.2 (R Foundation for Statistical Computing). All the other statistical procedures were performed with SPSS software, version 22.0 (SPSS Company, Chicago, IL). Two side P < 0.05 was considered statistically significant.
3. Results
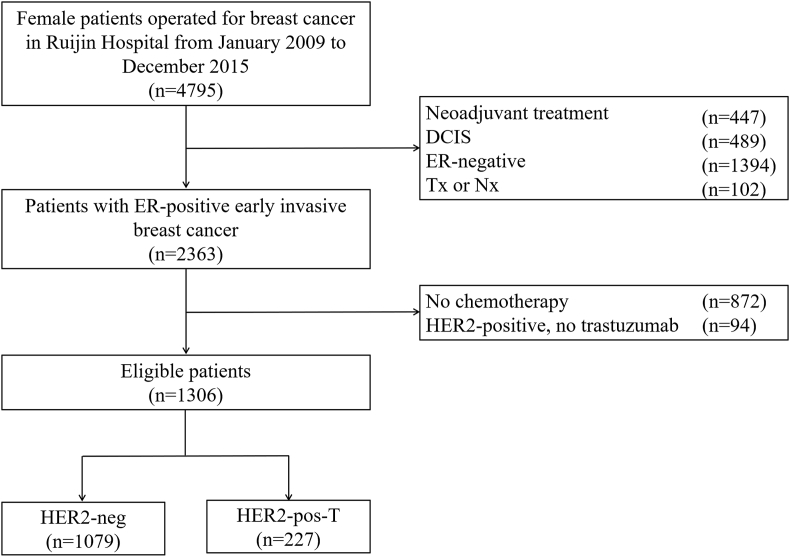
Baseline patient characteristics. A total of 4795 female breast cancer patients underwent surgical operation at XXX Hospital between January 2009 and December 2015. As shown in Fig. 1, 1306 cases were included in the present analysis. As shown in Table 1, the median age was 52 (Interquartile range [IQR] 45–60) years, and 635 (48.6%) patients were pre/peri-menopausal at diagnosis. There were 839 (64.4%) patients with tumors > 2.0 cm and 688 (52.7%) patients with positive axillary lymph nodes (ALNs). Overall, anthracycline- or taxane-based chemotherapy regimens were applied in 150 (11.6%) and 405 (31.2%) patients, respectively. A total of 691 (53.2%) patients received chemotherapy regimens containing both anthracycline and taxane.
Fig. 1.
Flowchart of the 1306 patients included in the study. Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; Tx, primary tumor cannot be assessed; Nx, lymph nodes cannot be assessed; HER2, human epidermal growth factor receptor 2.
Table 1.
Tumor and patient characteristics stratified by different groups.
| Characteristics | Total n = 1306 (%) | HER2-neg n = 1079 (%) | HER2-pos-T n = 227 (%) | P value |
|---|---|---|---|---|
| Age (y/o) | 0.541 | |||
| <35 | 53 (4.1) | 43 (4.0) | 10 (4.4) | |
| 35–50 | 525 (40.2) | 427 (39.6) | 98 (43.2) | |
| >50 | 728 (55.7) | 609 (56.4) | 119 (52.4) | |
| Menstrual status | 0.089 | |||
| Pre/Peri- | 635 (48.6) | 513 (47.5) | 122 (53.7) | |
| Post- | 671 (51.4) | 566 (52.5) | 105 (46.3) | |
| Family history | 0.788 | |||
| No | 1238 (94.8) | 1022 (94.7) | 216 (95.2) | |
| Yes | 68 (5.2) | 57 (5.3) | 11 (4.8) | |
| Breast surgery | 0.015 | |||
| Mastectomy | 944 (72.3) | 765 (70.9) | 179 (78.9) | |
| BCS | 362 (27.7) | 314 (29.1) | 48 (21.1) | |
| Histology type | 0.696 | |||
| IDC | 1216 (93.1) | 1006 (93.2) | 210 (92.5) | |
| Non-IDC | 90 (6.9) | 73 (6.8) | 17 (7.5) | |
| Tumor size | 0.039 | |||
| ≤2.0 cm | 464 (35.6) | 397 (36.9) | 67 (29.6) | |
| >2.0 cm | 839 (64.4) | 680 (63.1) | 159 (70.4) | |
| ALN status | 0.001 | |||
| Negative | 618 (47.3) | 487 (45.1) | 131 (57.7) | |
| Positive | 688 (52.7) | 592 (54.9) | 96 (42.3) | |
| Histological grade | <0.001 | |||
| Ⅰ/Ⅱ | 707 (58.2) | 622 (62.1) | 85 (40.1) | |
| Ⅲ | 507 (41.8) | 380 (37.9) | 127 (59.9) | |
| LVI | 0.171 | |||
| No | 1218 (93.3) | 1011 (93.7) | 207 (91.2) | |
| Yes | 88 (6.7) | 68 (6.3) | 20 (8.8) | |
| ER | <0.001 | |||
| <10% | 59 (4.5) | 35 (3.2) | 24 (10.6) | |
| 10–50% | 240 (18.4) | 178 (16.5) | 62 (27.3) | |
| >50% | 1007 (77.1) | 866 (80.3) | 141 (62.1) | |
| PR | <0.001 | |||
| <20% | 663 (50.8) | 513 (47.5) | 150 (66.1) | |
| ≥20% | 643 (49.2) | 566 (52.5) | 77 (33.9) | |
| Ki-67 | <0.001 | |||
| <14% | 424 (32.5) | 394 (36.5) | 30 (13.2) | |
| ≥14% | 882 (67.5) | 685 (63.5) | 197 (86.8) | |
| Endocrine therapy | 0.739 | |||
| No | 80 (6.1) | 65 (6.0) | 15 (6.6) | |
| Yes | 1226 (93.9) | 1014 (94.0) | 212 (93.4) | |
| Chemotherapy | <0.001 | |||
| A | 150 (11.6) | 149 (13.9) | 1 (0.4) | |
| T | 405 (31.2) | 393 (36.7) | 12 (5.3) | |
| A-T | 632 (48.7) | 448 (41.8) | 184 (81.8) | |
| A + T | 59 (4.5) | 56 (5.2) | 3 (1.3) | |
| Others | 51 (3.9) | 26 (2.4) | 25 (11.1) |
Abbreviations: HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ALN: axillary lymph node; LVI, lymph-vascular invasion; ER, estrogen receptor; PR, progesterone receptor; A: anthracycline; T: taxane; y/o, years old.
Clinicopathological features of ER+/HER2+ patients treated with trastuzumab-based chemotherapy. Among eligible patients, 227 (17.4%) patients were categorized into the HER2-pos-T group, and the remaining 1079 (82.6%) cases were in the HER2-neg group. As shown in Table 1, tumor size, ALN status, histological grade, ER level, PR level, and Ki-67 level were significantly different between the two groups (P < 0.05). There were no statistically significant differences in terms of age, menstrual status, family history and LVI status in univariate analysis (P > 0.05). In multivariate logistic regression analysis, the overall distribution of menstrual status, ALN status, histological grade, ER level, PR level, and Ki-67 level had significant differences between these two groups (P < 0.05, Supplementary Table S1).
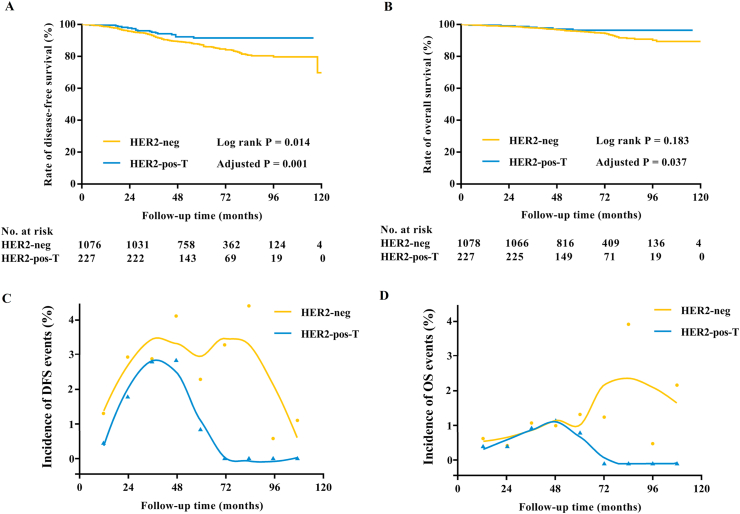
Prognosis of ER+/HER2+ patients treated with trastuzumab-based chemotherapy. As shown in Table 2, there were 174 DFS events at a median follow-up of 59 (IQR 44–78) months. The DFS was statistically significantly different between the two groups, with the estimated disease-free probability at 5 years being 87.4% for ER+/HER2-patients and 94.2% for ER+/HER2+ patients (Log-rank P = 0.014, Fig. 2A). Tumor size, ALN status, histological grade, ER level, and PR level affected DFS in univariate analysis (Log-rank P < 0.05, Supplementary Table S2). As shown in Table 3, multivariable analysis showed that tumor >2.0 cm (HR 1.58, 95% CI: 1.09–2.29, Log-rank P = 0.015), positive ALN (HR 1.47, 95% CI: 1.05–2.05, Log-rank P = 0.025), and grade III (HR 1.54, 95% CI: 1.11–2.14, Log-rank P = 0.010) were independently associated with worse DFS. Particularly, patients in the HER2-pos-T group had significantly better DFS than those in the HER2-neg group (HR 0.38, 95% CI: 0.22–0.67, Log-rank P = 0.001, Table 3).
Table 2.
Details of DFS and OS events stratified by different groups.
| Overall n = 1305 (%) | HER2-neg n = 1078 (%) | HER2-pos-T n = 227 (%) | |
|---|---|---|---|
| DFS events | |||
| No recurrence | 1131 (86.6) | 921 (85.4) | 210 (92.5) |
| Local-regional recurrence | 28 (2.1) | 25 (2.3) | 3 (1.3) |
| Contralateral breast cancer | 14 (1.1) | 12 (1.1) | 2 (0.9) |
| Second non-breast malignancy | 23 (1.8) | 22 (2.0) | 1 (0.4) |
| Distant metastasis | 78 (6.0) | 72 (6.7) | 6 (2.6) |
| Death without recurrence | 31 (2.4) | 26 (2.4) | 5 (2.2) |
| OS events | |||
| Alive | 1237 (94.7) | 1017 (94.3) | 220 (96.9) |
| Death of any cause | 69 (5.3) | 62 (5.7) | 7 (3.1) |
| Death with recurrence | 38 (2.9) | 36 (2.3) | 2 (0.9) |
| Death without recurrence | 31 (2.4) | 26 (2.4) | 5 (2.2) |
Abbreviations: HER2, human epidermal growth factor receptor 2; DFS: disease-free survival; OS: overall survival.
Fig. 2.
Survival analysis in the whole population. (A) The estimated 5-year DFS rate was 87.4% for HER2-neg patients and 94.2% for HER2-pos patients treated with trastuzumab. HER2-pos-T was associated with significantly better DFS both in univariate analysis (Log-rank P = 0.014) and multivariate analysis (HR 0.38, 95% CI: 0.22–0.67, Log-rank P = 0.001). (B) The estimated 5-year OS rate was 95.7% in the HER2-neg group and 97.2% in the HER2-pos-T group (Log-rank P = 0.183). HER2-pos-T was associated with significantly better OS (HR 0.40, 95% CI: 0.17–0.95, Log-rank P = 0.037) after adjusting menstrual status, histological type, tumor size, ALN status, histological grade, ER, and PR. (C) Estimated and smoothed annual incidence of DFS events in the HER2-neg group and HER2-pos-T group ∗. (D) Estimated and smoothed annual incidence of OS events in the HER2-neg group and HER2-pos-T group ∗. ∗ In C/D panels, dots and triangles represent estimated annual incidence of DFS/OS events in the HER2-neg group and HER2-pos-T group, respectively.
Table 3.
Multivariate analysis of prognostic factors associated with DFS and OS.
| Characteristics | DFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Menstrual status (Post- vs. Pre/Peri-) | – | – | 1.77 (1.01–3.11) | 0.047 |
| Histology type (non-IDC vs. IDC) | ∞ (∞-∞) | 0.950 | ∞ (∞-∞) | 0.949 |
| Tumor size (>2.0 cm vs. ≤ 2.0 cm) | 1.58 (1.09–2.29) | 0.015 | 1.97 (1.03–3.75) | 0.040 |
| ALN status (Positive vs. Negative) | 1.47 (1.05–2.05) | 0.025 | 1.85 (1.06–3.24) | 0.031 |
| Histological grade (Ⅲ vs. Ⅰ/Ⅱ) | 1.54 (1.11–2.14) | 0.010 | 1.90 (1.10–3.26) | 0.021 |
| ER | 0.005 | <0.001 | ||
| 10–49% vs. < 10% | 1.00 (0.49–2.01) | 0.84 (0.35–2.02) | ||
| ≥50% vs. < 10% | 0.56 (0.28–1.11) | 0.28 (0.13–0.70) | ||
| PR (≥20% vs. < 20%) | 0.75 (0.53–1.06) | 0.099 | 0.50 (0.26–0.95) | 0.036 |
| Group (HER2-pos-T vs. HER2-neg) | 0.38 (0.22–0.67) | 0.001 | 0.40 (0.17–0.95) | 0.037 |
Abbreviations: HER2, human epidermal growth factor receptor 2; ALN: axillary lymph node; ER, estrogen receptor; PR, progesterone receptor; y/o, years old.
A total of 69 patients died during the follow-up period, of which 62 were in the HER2-neg group and 7 were in the HER2-pos-T group (Table 2). Among these patients, 26 (41.9%) in the HER2-neg group and 5 (71.4%) in the HER2-pos-T group didn’t experience prior tumor recurrence. No statistically obvious OS difference was observed between these two groups (Log-rank P = 0.183, Fig. 2B). The estimated 5-year OS rates were 95.7% and 97.2% in the HER2-neg group and the HER2-pos-T group, respectively. Menstrual status, tumor size, ALN status, histological grade, ER level, and PR level were related to OS in univariate analysis (Log-rank P < 0.05, Supplementary Table S2). In multivariable analysis (Table 3), post-menopause (HR 1.77, 95% CI: 1.01–3.11, Log-rank P = 0.047), tumor > 2.0 cm (HR 1.97, 95% CI: 1.03–3.75, Log-rank P = 0.040), positive ALN (HR 1.85, 95% CI: 1.06–3.24, Log-rank P = 0.031), and grade III (HR 1.90, 95% CI: 1.10–3.26, Log-rank P = 0.021) were associated with worse OS, while ER >50% (HR 0.28, 95% CI: 0.12–0.70, Log-rank P = 0.006) was related to better OS. More importantly, patients in the HER2-pos-T group had significantly improved OS than those in the HER2-neg group after adjusting these clinicopathological factors (HR 0.40, 95% CI: 0.17–0.95, Log-rank P = 0.037, Table 3).
The estimated annual incidence rates of DFS and OS events are shown in Fig. 2. For ER+/HER2-patients, relapse and death occurred steadily throughout the follow-up period and the hazard curve for DFS exhibited a typical double-peaked pattern. The peaks occurred at 3 years and 6–7 years after surgery. By contrast, DFS events were less common and confined to the first 5 years for ER+/HER2+ patients, with a peak at the 3-year time point.
As with DFS, patients with ER+/HER2+ disease had a lower estimated annual risk for OS events compared to ER+/HER2-cases. For ER+/HER2-patients, the annual hazard for death remained elevated during the follow-up period and two peaks were recorded at 4 years and 6–7 years, respectively. For ER+/HER2+ patients, however, the annual hazard for death decreased sharply beyond 5 years, which was similar to ER+/HER2-patients during the first 5 years.
Prognostic effect was tested for patients in certain subgroups including age, menstrual status, tumor size, ALN status, tumor grade, ER level, PR level, and Ki-67 level. With relatively small patient numbers, there seemed to be no significant interactions (P > 0.05, Supplementary Figs. S1 and S2).
4. Discussion
Previous studies showed that HR+/HER2+ patients had distinct clinical profiles and prognosis compared to those with HR+/HER2-disease [[4], [5], [6], [7]]. In our cohort, ER+/HER2+ patients treated with trastuzumab and chemotherapy had more unfavorable prognostic factors but significantly better disease outcome than ER+/HER2-patients receiving chemotherapy alone, indicating that HER2-positivity itself may not be considered as an unfavorable factor for ER + patients treated with chemotherapy in the era of trastuzumab.
HER2+ breast cancer accounts for 15–25% of all breast cancers and about 50% of HER2+ tumors also express hormone receptor [2,4,5]. Patients with HR+/HER2+ tumors exhibit more aggressive clinic-pathological characteristics and worse prognosis compared to those with HR+/HER2-tumors [6,7]. Consistent with previous reports, our results, recruiting patients all treated with chemotherapy, showed that ER+/HER2+ patients were significantly associated with higher percentage of pre/peri-menopause, higher tumor grade, and higher Ki-67 expression level compared with ER+/HER2-patients. Moreover, ER+/HER2+ patients had lower ER and PR expression levels in the present study, indicating that ER+/HER2+ patients in our cohort had unfavorable prognostic factors compared with those in the ER+/HER2-group.
To our knowledge, this is the first study evaluating the prognostic role of HER2 on high-risk ER + patients who receive adjuvant chemotherapy in the era of trastuzumab. Previously, two trials that assessed the efficacy of trastuzumab for HER2+ patients prospectively included a parallel observational cohort in which HER2-patients received same chemotherapy [17,18]. In the FinHer study, HER2+ women treated with trastuzumab and chemotherapy had comparable 3-year rate of survival free from distant recurrence to HER2-patients (HR 1.09, 95% CI 0.52–2.29, Log-rank P = 0.82) [17]. Similarly, the 5-year results of the NOAH trial showed no survival difference between these two groups (58% versus [vs.] 61%, respectively) [18]. Moreover, there were studies evaluating the prognostic value of HER2 status and trastuzumab for women with metastatic breast cancer, which showed that patients with HER2+ disease who received trastuzumab had improved prognosis compared to HER2-patients [[19], [20], [21]]. For example, an observational study found that HR+/HER2+ breast cancer patients treated with trastuzumab had longer survival after metastasis than HR+/HER2-patients (HR 0.64, 95% CI 0.45–0.92, Log-rank P = 0.02) [19]. However, with the clinic-pathological difference between HER2+ and HER2-tumors, the actual prognostic efficacy of HER2 status remained unknown. According to our results, ER+/HER2+ patients treated with trastuzumab had significantly better 5-year DFS and OS than those with ER+/HER2-tumors after adjusting baseline clinic-pathological factors, indicating that HER2 positivity itself should no longer be considered as an adverse factor for ER + patients if they were treated with adjuvant trastuzumab and chemotherapy.
Patients with HR + early breast cancers continue to be at risk for recurrence many years after the initial diagnosis and treatment including endocrine therapy [22]. The risk of late relapse in HR+/HER2-breast cancer is well defined, but the risk in HR+/HER2+ patients treated with adjuvant trastuzumab-based chemotherapy remains largely unknown [23]. Recently, Chumsri S and coworkers demonstrated persistent benefit of adjuvant trastuzumab treatment for HER2+ patients in the NCCTG N9831 and the NSABP B31 [23]. From 5 to 10 years after surgery, the cumulative incidence rate of relapse or death in HR+/HER2+ patients receiving trastuzumab and chemotherapy was 8.6% and the annualized rate was estimated to be 0.33–2.52% [23]. Similar to this observation, no recurrence or death beyond 5 years were observed for patients in the HER2-pos-T group in our cohort with relatively short follow up period, indicating that HR+/HER2+ patients treated with chemotherapy and trastuzumab have favorable long-term prognosis compared with HR+/HER2-patients, which may guide further clinical treatment decision making.
For high-risk pre-menopausal women with HR+/HER2-breast cancer, ovarian function suppression (OFS) in addition to tamoxifen or its combination with an aromatase inhibitor has improved the disease outcome [[24], [25], [26], [27]]. By contrast, the benefit of OFS and optimal endocrine therapy for HR+/HER2+ patients remain unknown with clinical trials exhibiting heterogeneity between OFS treatment benefit and HER2 status [26,28,29]. This could result from the different patient, disease stage, or chemotherapy usage between these trials [28]. Notably, 69% and 53% of HER2+ patients in the SOFT and TEXT trials received trastuzumab-based chemotherapy [26]. In the present study, no obvious DFS or OS differences were identified between ER+/HER2+ and ER+/HER2-patients in the pre/peri-menopausal cohort. Moreover, treatment-related amenorrhea (TRA) was found to be associated with improved DFS (HR 0.58, 95% CI 0.45–0.76) and OS (HR 0.63, 95% CI 0.40–0.99) in pre-menopausal HR+/HER2+ breast cancer patients from the ALTTO trial [30], which might explain the subgroup analysis of the SOFT trial (n = 236) showing that adding OFS to tamoxifen seemed to be of particular benefit in HER2+ patients (interaction P = 0.04). Based on above issues, HER2 positivity itself should not be considered as an indicator for OFS usage in the presence of trastuzumab and chemotherapy.
Pertuzumab was reported to increase the rate of invasive DFS among HER2+ early breast cancer patients when it is added to trastuzumab and chemotherapy by the 3-year result of Aphinity trial (HR 0.81, 95% CI 0.66–1.00, Log-rank P = 0.045), which was homogenous among HR+ and HR-subgroups (interaction P = 0.54) [31]. Recently, SABCS updated the interim analysis of this trial, where benefit of dual HER2 blockade with pertuzumab and trastuzumab retained in the trial population [32]. And for HR+/HER2+ patients, 6-year rate of invasive DFS was 91.2% in the pertuzumab group and 88.2% in the placebo group (HR 0.73, 95% CI 0.59–0.92), revealing a remarkable prognosis improvement among this subgroup [32]. Moreover, phase III randomized studies such as MONARCH-E, PENELOPE-B, PALLAS are evaluating the potential for CDK4/6 inhibitors to enhance standard adjuvant endocrine therapy in early node positive HR+/HER2-breast cancer patients who are at high risk of disease recurrence [33]. These therapies, separately or together, will further change the prognosis of early HR + breast cancers.
Our study directly compared the disease outcome of ER+/HER2+ breast cancer patients treated with trastuzumab and those patients with ER+/HER2-tumors receiving chemotherapy alone in a large cohort. There were also some limitations. This was a single-center retrospective study and selection bias was not avoidable. For example, our results showed that the percentage of positive ALN in HER2-patients was higher than that in the HER2+ patients (54.9% vs. 42.3%), which contrasted with previous reports and may be explained by that all enrolled HER2-patients in our cohort received chemotherapy. Although this study enrolled large number of patients, event rates were still relatively low, especially in the HER2+ cohort. In addition, only 91 of the 1306 patients received OFS, making it difficult to evaluate the predictive value of HER2 status for OFS benefit. Third, the follow-up period was still too short to evaluate the risk of late disease recurrence for patients with ER+/HER2+ tumor. Moreover, information about duration of endocrine therapy was not yet available, which needed further study.
In conclusion, we found that ER+/HER2+ patients had more unfavorable clinic-pathological features than those patients with ER+/HER2-tumors treated with chemotherapy. More importantly, ER+/HER2+ patients treated with trastuzumab and chemotherapy had superior disease outcome compared with ER+/HER2-patients, indicating that HER2 positivity itself may not be considered as a risk factor for ER + patients in the era of trastuzumab, which warrants further clinical investigation.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number: 81772797). Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172007); Ruijin Hospital, Shanghai Jiao Tong University School of Medicine-"Guangci Excellent Youth Training Program” (GCQN-2017-A18). All these financial sponsors had no role in the study design, data collection, analysis or interpretation.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Prof. Jian Cao and Ms. Yidong Du for their work in the establishment and management of our database. We also appreciate the multidisciplinary team members and breast cancer specialized nurses for their assistance to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.10.002.
Contributor Information
Xiaosong Chen, Email: chenxiaosong0156@hotmail.com.
Kunwei Shen, Email: kwshen@medmail.com.cn.
Ethics approval and consent to participate
This article does not contain any studies with human participants performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patient consent for publication
Not applicable.
Availability of data and materials
All the datasets generated and/or analyzed during the present study are included in this published article.
Authors’ contributions
XC and KS conceived the study and revised the manuscript. SL verified the data, performed the statistical analysis and drafted the manuscript. JW, OH, JH, LZ, WC, and YL were responsible for data collection. All authors read and approved the final manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hayes D.F. HER2 and breast cancer - a phenomenal success story. N Engl J Med. 2019;381:1284–1286. doi: 10.1056/NEJMcibr1909386. [DOI] [PubMed] [Google Scholar]
- 2.King C.R., Kraus M.H., Aaronson S.A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 3.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Ryden L., Landberg G., Stal O., Nordenskjold B., Ferno M., Bendahl P.O. HER2 status in hormone receptor positive premenopausal primary breast cancer adds prognostic, but not tamoxifen treatment predictive, information. Breast Canc Res Treat. 2008;109:351–357. doi: 10.1007/s10549-007-9660-2. [DOI] [PubMed] [Google Scholar]
- 5.Montemurro F., Di Cosimo S., Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol. 2013;24:2715–2724. doi: 10.1093/annonc/mdt287. [DOI] [PubMed] [Google Scholar]
- 6.Blows F.M., Driver K.E., Schmidt M.K., Broeks A., van Leeuwen F.E., Wesseling J., Cheang M.C., Gelmon K., Nielsen T.O. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000279. e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K., Quan J., Yang J., Chen Z. The potential markers of endocrine resistance among HR+/HER2+ breast cancer patients. Clin Transl Oncol. 2020;22(4):576–584. doi: 10.1007/s12094-019-02163-2. [DOI] [PubMed] [Google Scholar]
- 8.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M., Goldhirsch A., de Azambuja E., Castro G., Jr., Untch M., Smith I. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez E.A., Romond E.H., Suman V.J., Jeong J.H., Sledge G., Geyer C.E., Jr., Martino S., Rastogi P., Gralow J. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slamon D., Eiermann W., Robert N., Pienkowski T., Martin M., Press M., Mackey J., Glaspy J., Chan A. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Sun L., Mao Y., Zhu S., Wu J., Huang O., Li Y., Chen W., Wang J. Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Canc. 2013;13:390. doi: 10.1186/1471-2407-13-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong Y., Chen X., Fei X., Lin L., Wu J., Huang O., He J., Zhu L., Chen W. Can breast cancer patients with HER2 dual-equivocal tumours be managed as HER2-negative disease? Eur J Canc. 2018;89:9–18. doi: 10.1016/j.ejca.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Hammond M.E., Hayes D.F., Dowsett M., Allred D.C., Hagerty K.L., Badve S., Fitzgibbons P.L., Francis G., Goldstein N.S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., Dowsett M., Fitzgibbons P.L., Hanna W.M. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 15.Wolff A.C., Hammond M.E., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M., Bilous M. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 16.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., Bilous M., Ellis I.O., Fitzgibbons P. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 17.Joensuu H., Kellokumpu-Lehtinen P.L., Bono P., Alanko T., Kataja V., Asola R., Utriainen T., Kokko R., Hemminki A. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 18.Gianni L., Eiermann W., Semiglazov V., Lluch A., Tjulandin S., Zambetti M., Moliterni A., Vazquez F., Byakhov M.J. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 19.Lobbezoo D.J., van Kampen R.J., Voogd A.C., Dercksen M.W., van den Berkmortel F., Smilde T.J., van de Wouw A.J., Peters F.P., van Riel J.M. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Canc Res Treat. 2013;141:507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 20.Dawood S., Broglio K., Buzdar A.U., Hortobagyi G.N., Giordano S.H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planchat E., Durando X., Abrial C., Thivat E., Mouret-Reynier M.A., Ferriere J.P., Pomel C., Kwiatkowski F., Chollet P. Prognostic value of initial tumor parameters after metastatic relapse. Canc Invest. 2011;29:635–643. doi: 10.3109/07357907.2011.621911. [DOI] [PubMed] [Google Scholar]
- 22.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P., Peto R., Pritchard K.I., Bergh J. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chumsri S., Li Z., Serie D.J., Mashadi-Hossein A., Colon-Otero G., Song N., Pogue-Geile K.L., Gavin P.G., Paik S. Incidence of late relapses in patients with HER2-positive breast cancer receiving adjuvant trastuzumab: combined analysis of NCCTG N9831 (alliance) and NRG oncology/NSABP B-31. J Clin Oncol. 2019;37:3425–3435. doi: 10.1200/JCO.19.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis P.A., Regan M.M., Fleming G.F., Lang I., Ciruelos E., Bellet M., Bonnefoi H.R., Climent M.A., Da Prada G.A. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagani O., Regan M.M., Walley B.A., Fleming G.F., Colleoni M., Lang I., Gomez H.L., Tondini C., Burstein H.J. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis P.A., Pagani O., Fleming G.F., Walley B.A., Colleoni M., Lang I., Gomez H.L., Tondini C., Ciruelos E. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagani O., Francis P.A., Fleming G.F., Walley B.A., Viale G., Colleoni M., Lang I., Gomez H.L., Tondini C. Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: results from TEXT and SOFT. J Clin Oncol. 2019 doi: 10.1200/JCO.18.01967. JCO1801967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regan M.M., Fleming G.F., Walley B., Francis P.A., Pagani O. Adjuvant systemic treatment of premenopausal women with hormone receptor-positive early breast cancer: lights and shadows. J Clin Oncol. 2019;37:862–866. doi: 10.1200/JCO.18.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrone F., De Laurentiis M., De Placido S., Orditura M., Cinieri S., Riccardi F., Ribecco A.S., Putzu C., Del Mastro L. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Canc. 2019;118:178–186. doi: 10.1016/j.ejca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Lambertini M., Campbell C., Bines J., Korde L.A., Izquierdo M., Fumagalli D., Del Mastro L., Ignatiadis M., Pritchard K. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst. 2019;111:86–94. doi: 10.1093/jnci/djy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Minckwitz G., Procter M., De Azambuja E., Zardavas D., Benyunes M., Viale G., Suter T., Arahmani A., Rouchet N. Adjuvant pertuzumab and trastuzumab in early her2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccart M., Procter M., Fumagalli D., de Azambuja E., Clark E., Ewer M.S., Restuccia E., Jerusalem G., Dent S. SABCS; San Antonio, Texas, USA: 2019. Interim overall survival analysis of APHINITY (BIG 4-11): a randomized multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer. Presented at 2019. Abstract GS3-04. [Google Scholar]
- 33.Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in hormone receptor-positive early breast cancer: preliminary results and ongoing studies. Breast Cancer. 2018;25:506–516. doi: 10.1007/s12282-018-0864-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the datasets generated and/or analyzed during the present study are included in this published article.