Abstract
Based on the PACIFIC study, the standard care of unresectable locally advanced non-small cell lung cancer (LA-NSCLC) shifted from concurrent chemo-radiotherapy (CCRT) alone to CCRT followed by durvalumab consolidation in 2017. In the era of immunotherapy, two kinds of therapeutic drugs are involved in the management of LA-NSCLC: chemotherapeutics and anti-PD-1/PD-L1 agents. However, the best choices of systematic chemotherapy, immunotherapy, and treatment schedule remain controversial. The immune modulation effects of chemotherapy, as well as the potential immunosuppressive impact of pretreatment medications, should be taken into consideration. Indeed, chemotherapeutics are double-edged swords to immunotherapy, with both stimulatory and suppressive effects on the immune system. Moreover, low-dose chemotherapy is reported to enhance anti-tumor immune responses with reduced toxicities. As for glucocorticoids, there is no consensus about its exact impact on the efficacy of immunotherapy. In addition, the timing of anti-PD-1/PD-L1 agent related to CCRT has three modes: induction, concurrent, and consolidation therapy. Although CCRT followed by durvalumab consolidation is the standard of care, the best sequence of immunotherapy and chemo-radiotherapy is still under debate. Furthermore, the efficacy and toxicity of various PD-1/PD-L1 inhibitors should be compared, especially in the background of CCRT. In this review, we will summarize the detailed knowledge about chemotherapeutics and anti-PD-1/PD-L1 axis agents, and discuss the potential implications in designing novel, effective treatment strategies for LA-NSCLC.
Keywords: Immunotherapy, lung cancer, chemotherapy, stage III, locally advanced
Introduction
The paradigm of treatment for unresectable locally advanced non-small cell lung cancer (LA-NSCLC) experienced the shift from radiotherapy alone to combined chemotherapy and radiotherapy (CRT) over two decades ago. In 1980s, based on the result of RTOG7301, radical radiotherapy (RT) alone was the standard care of LA-NSCLC, with a 2-year survival of 25% and 5-year survival of only 5% (1,2). In 1995, a high-quality meta-analysis provided evidence that radical radiotherapy plus chemotherapy resulted in absolute benefits of 3% and 2% at 2- and 5-year survival compared with radiation alone (3). Thereafter, cisplatin-based chemotherapy plus radiotherapy became the new standard of care for LA-NSCLC. Among the two modes of combined CRT: concurrent CRT (CCRT) and sequential CRT (SCRT), several randomized trials and a meta-analysis based on the individual patients data showed that CCRT could increase the overall survival (OS) and progression-free survival (PFS) for patients with LA-NSCLC patients over SCRT, although at the expense of increased manageable acute esophageal toxicity (4). Moreover, several phase III randomized trials demonstrated induction (5) and consolidation (6,7) chemotherapy beyond CCRT had no further benefit. Thus, CCRT alone became the standard of care for LA-NSCLC before immunotherapy involved.
In the next two decades, many attempts have been made to improve the efficacy and/or the tolerance of CCRT in LA-NSCLC, all of which failed to introduce considerable advancement (7,8). Recently, multiple randomized trials demonstrated that inhibitors of the programmed cell death-1 (PD-1) pathway could provide significant clinical benefits for patients with metastatic NSCLC and several early phase clinical trials incorporating anti-programmed cell death-ligand 1(PD-L1) or anti-PD-1 agents showed encouraging antitumor activity for patients with LA-NSCLC (9,10). In the HRCN LUN14-179 study, consolidation with anti-PD-1 agent Pembrolizumab after CCRT was found to be well tolerated (11). In the DETERRED study, consolidation using anti-PD-L1 agent Atezolizumab in combination with two cycles of carboplatin plus paclitaxel after CRT showed preliminary efficacy (12). Finally, the landscape for the management of unresectable LA-NSCLC changed in 2017. Based on the result of the PACIFIC study (13), immunotherapy has opened up a new era in the treatment for LA-NSCLC. The PACIFIC study has corroborated the benefits brought by consolidation anti-PD-L1 agent durvalumab compared to placebo after CCRT (13,14). For PFS, the HR was 0.51 in favor of the durvalumab group, with 1-, 2-year, and median PFS results of 55.7%, 49.5%, and 17.2 months. For OS, the HR was 0.68, also favoring the immunotherapy arm. One-, two-year, and median OS results were 83.1%, 66.3%, and not reached, respectively (P=0.003). The standard of care for LA-NSCLC has then been updated to the PACIFIC treatment pattern by now.
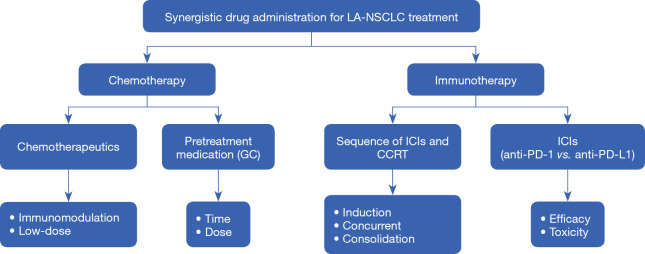
So currently, systematic therapy is vital in the management of LA-NSCLC, with now two kinds of therapeutic drugs involving in the standard treatment: chemotherapeutics and anti-PD-1/PD-L1 axis agents. The new treatment mode also raises important questions, such as the future directions of systematic therapy for LA-NSCLC in the era of immunotherapy. In this review, we will summarize the current status regarding the optimal choice of systematic drugs, with a particular focus on prospects and challenges in the era of cancer immunotherapy for LA-NSCLC (Figure 1). Information used to write this review was collected from PUBMED and EMBASE databases (date of the last search 10 January 2020). The key words used for searching are chemotherapy, radiotherapy for LA-NSCLC and their combination with immunotherapy. In addition, several reference lists of identified articles were searched manually.
Figure 1.
Consideration of the optimal choice of systematic drugs for unresectable locally advanced non-small cell lung cancer (LA-NSCLC). GC, glucocorticoid; ICIs, Immune checkpoint inhibitors; CCRT, concurrent chemo-radiotherapy; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand 1.
We present the following article in accordance with the Narrative Review Checklist (available at http://dx.doi.org/10.21037/tlcr-20-512).
Chemotherapy: the optimal regimen
There have been multiple prospective randomized controlled clinical trials (4) comparing the efficacy of different chemotherapy regimens used in CCRT, with most of them failing to show whichever was better, except for one study (15). This study is a multicenter randomized phase III study performed in China (15), a total of 200 LA-NSCLC patients were treated with 60–66 Gy of thoracic radiation therapy concurrent with either etoposide and cisplatin (EP arm), or paclitaxel and carboplatin weekly protocol (PC arm). They found EP arm was superior to the PC arm in terms of OS, with 3-year OS significantly higher in EP arm (P=0.024). However, this result was inconsistent with the analysis based on the big real-world data from Veteran Health Administration of the United States, which showed LA-NSCLC patients treated with EP versus PC had similar OS, but EP was associated with increased morbidity (16). Thus, the superiority of EP versus PC is still controversial. At present, several platinum-based chemotherapy protocols can be chosen in the clinical settings for LA-NSCLC during CCRT, according to the guidelines (17,18), except the agent associated with increased radiation toxicities, such as gemcitabine to pulmonary toxicity.
When the immunotherapy involved, several concerns should be taken into consideration because chemotherapeutics and even their pretreatment medications such as glucocorticoids (GCs) may have an impact on the immune system and thus affect the therapeutic response of immunotherapy.
Chemotherapeutics as immune modulators
Chemotherapy has long been recognized as an immunosuppressive intervention, due to the cytotoxic effects of chemotherapeutics to the immune cells as well as lymphoid tissues. The immunosuppressive side effects of chemotherapeutics mainly manifest as bone marrow suppression, including myelo- and lymphopenia (19), resulting from impaired T lymphocytes, B lymphocytes, and NK cells. For instance, in the preclinical researches, etoposide and camptothecin were shown to induce a rapid production of ceramide, which can initiate the apoptotic cascade signaling in peripheral T lymphocytes (20); paclitaxel could down-regulated the intracellular pathways involving JNK/p38 MAP kinases, thus producing a selective inhibition of LPS-induced B-cell proliferation in mouse model (21); and taxol treatment inhibited NK cell cytotoxicity by altering microtubule assembly (22). Chemotherapeutics induced immunosuppressive effects are also commonly seen in the clinical settings, for example, the populations of B cells, CD4+ and CD8+ T cells were shown to be significantly decreased by high-dose sequential chemotherapy and did not recover even long after the first cycle of therapy (23).
However, accumulative evidence emerged recently that chemotherapeutics also have the immunomodulation effects on tumor microenvironment, with some of them being positive to the antitumor immune response (24). For instance, among the commonly used chemotherapeutics in NSCLC, cisplatin has been found to have antitumor immune-stimulatory effect by upregulating MHC class I expression on cancer cells, promoting recruitment and proliferation of immune effector cells, and downregulating the immunosuppressive molecules (25); carboplatin could mediate the activation of murine macrophage and then enhance IL-1α and TNF-α activities (26); gemcitabine and docetaxel were found to reduce the inhibitory immune cells, such as MDSCs (27,28); and paclitaxel was able to increase immune stimulatory factors by promoting the tumoricidal activity of M1 macrophage as well as inducing apoptosis of immunosuppressive cells, such as Tregs (29-31). Tumor response to conventional chemotherapy has been indicated to be partly attributed to the increased immunogenicity of malignant cells and inhibited immunosuppressive circuitries, which are promoted by chemotherapeutic (32). On the other side, tumor microenvironment immunomodulation by chemotherapeutics also would be negative. A notable example is that some chemotherapeutic agents can induce PD-L1 expression on tumor cells (33), which is a process of immune escape of tumor and associated with poor outcomes in several cohorts of patients with cancer (32,34). A more comprehensive description of the immunomodulation effects of chemotherapeutics goes beyond the scope of our review and can be found elsewhere (24).
The immunomodulation effects of chemotherapeutics form the basis of combination treatment with chemotherapy and immunotherapy. The clear example comes from the success of combing anti-PD-1/PD-L1 with chemotherapeutic agents in NSCLC (35-38), to which tumor PD-L1 expression increased by chemotherapy should at least partly contribute. However, chemotherapy is a double-edged sword to immunotherapy, as described above. There exist clear variations in the immune stimulatory effects of different chemotherapeutics. So, the detailed knowledge of these differences may have a profound impact on the design of novel, effective treatment options, especially in chemotherapy agent choice for LA-NSCLC, when immunotherapy involved.
The other issue in chemotherapy lies in the administration schedule, among which the dosage of chemotherapeutics is of most importance. For more than half a century, to kill as many tumor cells as possible, chemotherapeutics are often administered in single doses, or short courses of therapy at the maximum tolerated dose (MTD) (39). Indeed, for some chemo-sensitive malignancies, such as acute lymphoblastic leukemia and Hodgkin’s lymphoma, MTD chemotherapy may lead to complete remission or even cure. However, significant advances of MTD seem to have reached a plateau for solitary tumors over the past two decades, due to drug resistance and highly toxic (40). In the meantime, the low-dose protocols such as metronomic chemotherapy attracted growing scientific and clinical interest (41), originating from the potential to overcome drug resistance by shifting the therapeutic target from tumor cells to tumor vasculature (42). Low-dose chemotherapy also holds merit in reducing specific toxicities (43). In unresectable LA-NSCLC, low-dose chemotherapy has also been tested in combination with radiotherapy as the radio-sensitizer, but with conflicting results (44).
However, in the era of immunotherapy, we need reconsideration of low-dose chemotherapy, because the effects of chemotherapeutics on immune system depend significantly on their dosage. For instance, in mouse models, cisplatin and paclitaxel administrated with MTD would induce the toxicity to all immune cells and thus cause significant myelosuppression, while the lower dose protocol could reduce immunosuppression of tumor microenvironment, and even elicit tumor-specific antitumor CD8+ T-cell responses (45); in the clinical setting, oral administration of high dosage cyclophosphamide (200 mg/day) was found to induce a profound decrease in the circulating lymphocyte number, NK cell-dependent cytotoxicity, and T cell proliferation capacity, while a lower dosage (100 mg/day) selectively depleted circulating immunosuppressive cell Tregs, and restored T cell and NK effector functions (46). The detailed mechanisms regarding the impact of low-dose chemotherapy on the immune system have been described elsewhere (47).
Low-dose chemotherapy has also been explored in combination with anti-PD-1/PD-L1 agent. A preclinical study showed combining low-dose gemcitabine, a CDK1 inhibitor, and PD-L1 antibody significantly increased anti-tumorigenic CD8+ cytotoxic T-cell, DC and M1 macrophage populations, and simultaneously decreased the populations of immunosuppressive M2 macrophage and MDSC in SCLC (48). An interim analysis of clinical study demonstrated that weekly low-dose carboplatin (AUC =1) and paclitaxel (25 mg/m2) combined with pembrolizumab was well tolerated in advanced NSCLC patients with poor performance status, and of noted,70% of the patients received combined therapy achieved PR, comparing to only 20% in the single pembrolizumab treatment group; the combination group experienced a significant decrease in absolute numbers of immunosuppressive MDSC subpopulation and an increase in activated CD4+ T cells in the blood (49).
There has been no report of low-dose chemotherapy in LA-NSCLC when immunotherapy involved. However, this field needs to be investigated in the era of immunotherapy due to the mechanisms described above, and additionally, the unique advantage of radio-sensitive effects of several chemotherapeutics was illustrated in their low-dose levels (50,51).
Pretreatment medication
GCs are commonly used to control the symptoms and alleviate the side effects of treatment modalities in cancer treatment. They are also administrated as preconditioning for some chemotherapy agents such as pemetrexed, paclitaxel, and docetaxel, which are often used in NSCLC. The reason for choosing GCs as the pretreatment medication is attributed to the well-known anti-inflammatory and immunosuppressive effects of GCs.
GCs modulate immune function through the glucocorticoid receptor (GR), which belongs to the nuclear receptor superfamily of ligand-activated transcription factors. Upon binding of GCs, GR translocated to the nucleus where it binds to DNA sequences and regulates the transcription of various kinds of immune-related genes, such as cytokines, chemokines, interferons, as well as other immune-modulating molecules, with the help of a large number of co-activators and co-repressors (52,53). At the cellular level, GCs suppress the immune response by modulating the differentiation, activation, and function of both lymphocytes and myeloid cells, especially T cells (54). GCs mediate their immunosuppression effect on T cells through both genomic actions by regulating the transcription of viral genes (such as GILZ, GITR) and non-genomic actions by the interaction between GR and the T-cell receptor complex (55,56). The immunosuppression effects of GCs are well recognized in their treatment for autoimmune diseases.
It is still an open question of whether GCs administration could significantly affect the efficacy of immune checkpoint inhibitors. In the Keynote-407 study, treatment efficacy was similar among the untreated metastatic squamous NSCLC patients who received paclitaxel, which needed preconditioning with GCs, and those who received GC-free nab-paclitaxel (36). Also, the response rate (RR) was similar in advanced melanoma patients who did receive system GCs or other suppressive immune-modulating agents and patients who did not (57). Furthermore, in a study of metastatic melanoma patients treated by CTLA-4 blockade, high-dose of system steroid administration in patients with high-grade immune-related adverse events (IRAE) had no significant effect on the duration of response (P=0.23) (58). However, Arbour et al. reported baseline GC use of ≥10 mg of prednisone equivalent to indicate poorer PFS (HR: 1.3) and OS (HR: 1.7) in NSCLC patients who treated with PD-1/PD-L1 inhibitors, and they recommended prudent use of GCs at the time of initiating immune checkpoint inhibitors (59).
Nevertheless, another study found that the differences in treatment efficacies between patients receiving a higher dose of baseline GCs and those who did not mainly depend on whether corticosteroids were administered for cancer-related palliative reasons or cancer-unrelated indications. Patients who received a higher dose of baseline GCs for palliative indications had significantly shorter survival, while no significant survival difference among patients receiving different levels of baseline GCs for cancer-unrelated indications (60). Until now, there is no consensus about the exact role of GCs on the efficacy of immunotherapy. Additionally, a recent study indicated that the administration of anti-TNF drugs for the treatment of steroid-refractory immune-related toxicity decreased patient’s survival among 1,250 melanoma patients receiving immune checkpoint inhibitors (61). Future researches examining the exact effect of GCs as well as other immune-modulating drugs on the efficacy of immunotherapy, with prospective design and larger sample size, are called for.
As for the treatment of LA-NSCLC in the era of immunotherapy, chemotherapy without preconditioning may be favored, and the administration of low dose GCs for a limited duration of time in the case of side effect intervention and symptom control may be safe. However, high dose GCs or extended duration of usage should be in caution.
Immunotherapy
Immune checkpoint inhibitors (ICIs) are emerging as a frontline treatment for many cancers by blocking the immune escape mechanisms. Currently, the US Food and Drug Administration (FDA) has approved two classes of ICIs for clinical use: anti-PD-1/PD-L1 agents and anti-cytotoxic T-cell lymphocyte-associated protein 4 (CTLA-4) antibody. PD-1/PD-L1 or CTLA-4 served as brakes on immune system by blocking anti-tumor responses, while ICIs could reverse this effect. Based on the PACIFIC study, durvalumab (anti-PD-L1) treatment after CCRT is recommended for LA-NSCLC (13). Along with radiotherapy and chemotherapy, immunotherapy becomes a pillar of LA-NSCLC care.
A functional immune system is essential for successful ICIs therapy. Based on the density and location of tumor-infiltrating lymphocytes, the phenotypes of tumor immune microenvironment are classified into three categories: immune-desert, immune-excluded and immune-inflamed. The first phenotype features the absence of T cells and is associated with poor response to ICIs therapy. The immune-excluded tumors feature the presence of immune cells located in the periphery, which can not enter the center of tumor. The immune-inflamed tumors are characterized by abundant T cells in the center of tumor, and usually accompanied by the presence of PD-L1 expression, and thus respond well to ICIs. To obtain better response to ICIs, it is worthy to explore how to turn a tumor into “immune inflamed” (62).
Except for chemotherapy we discussed above, radiotherapy also has been found to have impacts on immune system. On one hand, radiotherapy could stimulate antitumor immune response through releasing tumor antigens and immune-activating cytokine (e.g., high-mobility group protein B1, interleukin 1β, interferon γ,tumor necrosis factor α) (63). On the other hand, radiotherapy may also have immune toxicity by inducing radiation-induced lymphopenia or activating immunosuppressive cytokines (e.g., transforming growth factor β) and cells (e.g., Tregs, M2 macrophage) (63,64).
Since the effects of chemotherapy and radiotherapy on the immune system are two-sided, the impacts of CRT on the anti-tumor immune response are complicated. In patients with head and neck cancer, adjuvant CRT contributed to the cultivation of immunosuppressive microenvironment by increasing the number of Treg cells, elevating the secretion of TGF-β and decreasing the frequency of immune-active CD4+ T cells (65). However, preoperative CRT was found to promote the anti-tumor immune response through decreasing the number and inhibiting the function of Tregs in pancreatic cancer (66). In addition, CRT was reported to enhance anti-tumor immunity by increasing the expression of various polyfunctional cytokines of CD4+ T cells, leading to increased sensitivity to subsequent ICIs (67). As the immune status can be changed by CCRT, the response to anti-cancer therapy in LA-NSCLC may vary a lot according the sequence of immunotherapy and CCRT. The timing of immunotherapy related to CCRT in LA-NSCLC has three modes: induction, concurrent, and consolidation therapy.
Consolidation mode
At present, the standard of care supports the use of PD-L1 blockade durvalumab as consolidation treatment after CCRT for unresectable LA-NSCLC, which is derived from the success of PACIFIC trial (13,14). The timing of durvalumab involvement in the PACIFIC protocol design was initially based on the preclinical evidence suggesting that chemotherapy and radiotherapy may up-regulate PD-L1 expression on tumor cells (33,68,69), which is a predictive factor for a response to PD-1/PD-L1 antibodies. The phenomenon that CCRT could induce an elevator of cancer cell PD-L1 expression was also shown recently in the clinical setting in LA-NSCLC (70). The advantages of consolidation immunotherapy may also stem from that radiotherapy can alter the tumor microenvironment to promote more significant infiltration of immune effector cells (71-73), and the front CCRT can reduce the tumor burden, both of which will favor therapeutic response when immunotherapy participated. Also, the patients with progressive diseases, death, or with poor health after completion of CCRT will be picked out, so that those with good physical potential can be selected, and this group may be more suitable for immunotherapy. CCRT followed by durvalumab consolidation is the new standard care of LA-NSCLC, while CRT (either CCRT or SCRT) followed by other PD-1/PD-L1 inhibitors have also reported promising preliminary results (74-76).
Concurrent mode
Even with the PACIFIC trial, the best sequence of PD-1/PD-L1 blockades and CCRT in LA-NSCLC is still unclear. Of interest, the concurrent administration of a PD-1 inhibitor and radiation has been shown to increase immune activation over the sequential administration in mice models (69), suggesting there may be a room toward improving therapeutic effects through optimizing the timing of PD-1/PD-L1 blockade related to radiotherapy. Also, prior exploratory analysis from PACIFIC demonstrated that delivering durvalumab sooner after completion of CCRT (≤14 days) could potentially obtain more benefit (13). Thus, the field of combining concurrent PD-1/PD-L1 inhibitors and CCRT is of great clinical interest. However, there are several concerns about this mode in LA-NSCLC, with the first one about safety. Several preclinical types of research have shown that concurrent administration of anti-PD-1 antibody with thoracic irradiation will significantly increase pulmonary and cardio toxicities (77,78). And the toxicities enhanced by concomitant use of anti-PD-1/PD-L1 and chemotherapy compared to chemotherapy alone were also demonstrated in many large phase III trials in lung cancer (Keynote 189, Keynote 407, IMPOWER 130, 131, 132).
By now, two single-arm phases II trials (69,79) have been published to demonstrate the feasibility of combining concurrent anti-PD-1/PD-L1 antibody and definitive CCRT in LA-NSCLC according to the safeties. In the ETOP NICOLAS trial, LA-NSCLC patients received three cycles of platinum-based chemotherapy and concurrent radiotherapy (66 Gy/33 fractions), and the PD-1 antibody nivolumab started concurrently with radiotherapy. The interim safety analysis showed no unexpected AEs or increased toxicities were observed; among the 80 enrolled patients, 34% (19 grade 2 and 8 grade 3) experienced pneumonitis within 6 months (69). Although the pneumonitis was higher in NICOLAS than in the PACIFIC trial, it is comparable to the historical controls where patients treated with standard CCRT alone without immunotherapy have a 3–10% grade or higher rate of radiation pneumonitis (80). In the DETERRED study (79), the arm of concurrent treatment firstly applied CCRT with standard course radiotherapy (60–66 Gy/30–33 fractions) and weekly paclitaxel and carboplatin regimen and concurrent PD-L1 antibody atezolizumab to LA-NSCLC patients, the consolidation full dose paclitaxel and carboplatin with atezolizumab was then proceeded for 2 cycles for those with no evidence of disease progression, followed by maintenance atezolizumab for up to 1 year. The profile of general toxicity in the DETERRED trial was acceptable, with a 20% grade 3 or higher immune-related AE rate. Thirteen percent grade 2 and 3% grade 3 pneumonitis occurred among the 30 patients treated with concurrent CCRT-immunotherapy; the rates are comparable to the PACIFIC trial. The status of combining concurrent CCRT-immunotherapy in LA-NSCLC is presently inconclusive base on the currently published studies, due to the small sample sizes and none of the randomized comparative groups. However, the preliminary results of these trials warrant further investigation, and several large related trials are being developed or underway, including PACIFIC 2 (NCT 03519971) and EA 5181 (NCT 04092283).
Induction mode
The other way to involve immunotherapy in LA-NSCLC is induction therapy, in which the immunotherapy agents are administrated before CCRT. Although more attention is now paid to the immunomodulation of the conventional treatment modalities such as chemotherapy and radiotherapy, several pieces of evidence also showed the impact of the immune system on the treatment effects of conventional therapies. For example, both macrophage abundance and T-cell abundance in tumors represent prognostic indicators for recurrence-free and OS in breast cancer treated with chemotherapy, with a poor T-cell infiltrate linking to a poorer prognosis (81); and in animal models, the efficacy of radiation is partly dependent on functional T-cell responses (82,83), and radiotherapy can be made more effective by improving T-cell immune responses (82-84). Thus, the local immune environment within a tumor may potentially affect the success or failure of chemotherapy and radiotherapy. Several studies have indicated that salvage chemotherapy after PD-1/PD-L1 blockades produced potentially improved efficacy in metastatic NSCLC (85,86). Also, several preclinical types of research revealed that the tumor response to PD-1/PD-L1 blockades mainly results from recruiting the effective periphery T-cell to invade tumors (87); and the multispectral analyses of the tumor with primary pathological response after neoadjuvant PD-1 blockade illustrated the new infiltration with PD-1-positive CD8+ T cells in NSCLC (88), which suggest immunotherapy can remodel tumor local immune environment, with the potential of improving therapeutic effects of conventional treatments in some patients. All the above forms the theoretical basis of induction immunotherapy before CCRT. In the mice models, immunotherapy targeting another checkpoint, the anti-CTLA4 antibody was shown to be most effective when given before radiation, in part due to regulator T cell depletion (89). Other merits of induction immunotherapy lie in that the host systemic immune functions are not attacked by chemo and radiation therapy, which is suitable for the generation of antitumor immune responses, and that the possible tumor burden reduction by immunotherapy will favor the subsequence radiotherapy in respect of radiation toxicity.
There has been no report on the induction immunotherapy and CCRT in LA-NSCLC by now. The protocol of AFT-16 (NCT03102242) was announced in ASCO 2017, which is a single-arm phase II trial to explore the feasibility of induction atezolizumab followed by definitive CCRT in patients with LA-NSCLC, but the results have not yet been reported. The success or fail of induction mode depends on the effectiveness of immunotherapy because ineffective induction treatment will delay and thus be detrimental to the standard curative CCRT. However, the RR of the sole anti-PD-1/PD-L1 agents was relatively low in the non-selective population with NSCLC (90,91). PD-L1 expression on the tumor cell can predict the efficacy of anti-PD-1/PD-L1 alone in the first-line treatment for advanced NSCLC, with higher expression associated with better efficacy (92). So, there was a PD-L1 expression-driven trial designed in LA-NSCLC. In the ongoing SPRINT (NCT03523702) trial, the patients are stratified by the PD-L1 Tumor Proportion Score (TPS); the patients with TPS ≥50% will receive induction three cycles of pembrolizumab followed by radiation and sequential consolidation pembrolizumab, and otherwise will receive standard CCRT and consolidation therapy.
It should be noted that there are now several uncertainties in the PD-L1 test, including tumor heterogeneity, different potencies attributing to the different assays, variations in the criteria of pathologist judgment, etc. (93,94). Even when PD-L1 expression was higher than 50% (TPS ≥50%), the reported objective RRs were not high, such as 44.8% in Keynote 024 (95) and 39% in Keynote 042 (92), which indicate that potentially there are more than 50% patients will not benefit from induction PD-1 antibody even in this high-selected group. Combing immunotherapy and chemotherapy has been shown to improve treatment efficacy (35-38), but the impact of increased toxicities on the compliance of subsequent CCRT will be another issue. However, the idea of biomarker-driven immunotherapy is significant, which beckons for the more effective biomarkers. There should be a long way to the clinical practice of induction mode in LA-NSCLC, with other issues also need be concerned, including pseudoprogression after immunotherapy which will make the delineation of radiation targets more complex, and immunotherapy induced hyper-progression which may let some patients lost the opportunity of cure by radical CCRT.
PD-1 versus PD-L1 antibodies
There are two types of anti-PD-1/PD-L1 axis agents: PD-1 inhibitors and PD-L1 inhibitors. Mechanically, the significant difference between the two types lies in that PD-1 inhibitors simultaneously block the binding between PD-1 and its ligands PD-L1 and PD-L2, while PD-L1 inhibitors will not influence the interaction between PD-1 and PD-L2. Accumulating evidence suggests that PD-L2 is involved in the maintenance of immune tolerance and homeostasis of several non-hematopoietic tissues and vital organs, such as lung (96-99), liver (100), kidney (101) and pancreas (102). An example is that the absence of PD-L2 resulted in significantly enhanced severity of asthma, while only minimal inflammation and the airway hyper-responsiveness were reduced in deficiency of PD-L1 (97,98,103). And thus, it has long been suspected that PD-L1 inhibitors may have a favorable toxicity profile, especially in terms of immune-related adverse effects. On the other hand, PD-L2 is demonstrated to be another crucial inhibitory receptor, and the interaction between PD-1 and PD-L2 represses the activation of anti-tumor T cell response (99,104). Several studies have reported the predictive and prognostic value of PD-L2 expression in NSCLC, independently from PD-L1 expression (105-109), and blocking the interaction between PD-1 and PD-L2 could exhibit promising anti-tumor effect (104,110). Therefore, PD-1 inhibitors, with the potencies of blocking the interactions between PD-1 with both PD-L1 and PD-L2, may induce superior efficacy. Additionally, PD-1 inhibitors are usually designed as IgG4 antibodies, which have a low affinity for C1q and Fc receptors, and thus lead to reduce the chance of antibody-dependent cell-mediated cytotoxicity (ADCC) effect (111). Conversely, PD-L1 inhibitors are generally designed as IgG1 antibodies and could elicit potent ADCC against PD-L1 expressing tumor cells and immune regulatory cells (112-114). The differences of IgG isotypes and the potency of the ADCC effect contribute to the complexity of comparison between PD-1 and PD-L1 inhibitors.
At present, there have been several published meta-analyses of indirect comparisons among different anti-PD-1/PD-L1 agents, leading to conflicting results. In some studies, PD-1 inhibitors are found to induce superior efficacy to PD-L1 inhibitors in terms of objective RR (115), PFS (116) and OS (116,117) in metastatic NSCLC, while others reported no such significant difference of treatment efficacy and patient’s survival (80,118,119). Similarly, several studies indicated no significant difference in safety profiles between various PD-1 inhibitors and PD-L1 inhibitors, in terms of toxicity of any grade, immune-related AEs, AEs leading to treatment discontinuation and fatal toxic effects (116,120), but other studies found a significantly higher rate of immune-related toxicity among patients receiving PD-1 inhibitors than those receiving PD-L1 inhibitors (80,115,121,122), especially pneumonitis (121) and thyroid dysfunction (122). Additionally, even among the same type of antibodies, different drugs may have significantly different treatment efficacy and toxicity profiles, due to their distinct binding sites, various binding affinities and diverse interaction structures (111,123-125). For example, several clinical studies suggested that the PD-1 antibody pembrolizumab might have a higher rate of immune-related toxicity than another PD-1 antibody nivolumab when administered in advanced NSCLC (115,118). However, conclusions from these indirect comparisons need to be interpreted with caution as considerable biases exist. There were significant heterogeneousness and inconsistency in terms of cancer type, treatment line, biomarker expression, baseline characteristic, follow up and toxicity judgment, between different randomized clinical trials.
Up to now, durvalumab is recommended as the only PD-1/PD-L1 inhibitor among the stand care of LA-NSCLC, based on the result of the PACIFIC study. Other prospective trials are ongoing involving various kinds of other immune checkpoint inhibitors, and the best choice of PD-1/PD-L1 blockade under this indication is under debate. LUN 14-179 was a single-arm prospective phase II study that used consolidation pembrolizumab for LA-NSCLC similar to the duvalumab group in PACIFIC study, and the similar results were reported in terms of PFS and pneumonitis (126). Other anti-PD-1/PD-L1 axis agents are also being tested in the concurrent immunotherapy and CCRT mode, as described above (69,79). Although from the merely superficial numbers of these studies, PD-L1 inhibitors were safer than PD-1 inhibitors when combined with CCRT for LA-NSCLC, they are far from conclusive and needed to be further justified. In the routine clinical practice, whether the success of the PACIFIC trial can be generalized to other anti-PD-1/PD-L1 axis agents remain unclear, but the large randomized clinical trials directly comparing different kinds of PD-1/PD-L1 inhibitors are highly warranted.
Conclusion and future directions
As immunotherapy has opened a new era in the treatment for LA-NSCLC, understanding the impact of different systematic drugs on the immune system is becoming increasingly important. Indeed, chemotherapeutics is a double-edged sword to immunotherapy, with both suppressive and stimulatory effects on the immune system. Low-dose chemotherapy has been found to enhance host anti-tumor immune response reduced toxicities, which attracted growing interest in LA-NSCLC when anti-PD-1/PD-L1 agent is involved. In consideration of the immunosuppressive effects of GCs, chemotherapy without preconditioning may be favored. When necessary, low dose GCs for a limited duration of time may be safe, while a high dose or extended duration of usage should be careful. Although CCRT followed by durvalumab consolidation is the standard of care recommended by guidelines, it is possible to improve therapeutic effects by concurrent or induction immunotherapy, and it may be critical to distinguish particular patients for different combination strategies in the future. Furthermore, several pieces of evidence suggest that PD-L1 inhibitors may have lower toxicity, while PD-1 inhibitors may induce superior efficacy. In summary, more preclinical and clinical studies are called for to look for the optimal combination of CCRT and immunotherapy for LA-NSCLC, during which the above issues need to be concerned.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This project was supported by the National Science Foundation of China (No. 81572963 to Zhengfei Zhu)
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review Checklist. Available at http://dx.doi.org/10.21037/tlcr-20-512
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-512). Dr. ZZ reports receiving consulting fees and honoraria from AstraZeneca. The other authors have no conflicts of interest to declare.
References
- 1.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874-81. [DOI] [PubMed] [Google Scholar]
- 2.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 1980;45:2744-53. [DOI] [PubMed] [Google Scholar]
- 3.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. 10.1136/bmj.311.7010.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 5.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007;25:1698-704. 10.1200/JCO.2006.07.3569 [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008;26:5755-60. 10.1200/JCO.2008.17.7840 [DOI] [PubMed] [Google Scholar]
- 7.Ahn JS, Ahn YC, Kim JH, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol 2015;33:2660-6. 10.1200/JCO.2014.60.0130 [DOI] [PubMed] [Google Scholar]
- 8.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clini- cal activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Presented at the 41st European Society for Medical Oncology Annual Meeting, Co- penhagen, October 7-11, 2016. [Google Scholar]
- 11.Durm GA, Kio EA, Fisher WB, et al. Phase II trial of consolidation Pembrolizumab following concurrent chemoradiation in patients (pts) with unresectable or inoperable stage III non-small cell lung cancer (NSCLC): initial safety data from HCRN LUN 14-179. J Clin Oncol 2016;34:abstr e20025.
- 12.Lin SH, Lin Y, Price J, et al. DETERRED: PD-L1 blockade to evaluate the safety of lung cancer therapy using carboplatin, paclitaxel, and radiation combined with MPDL3280A (atezolizumab). J Clin Oncol 2017;35:abstr 3064.
- 13.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 14.Bang A, Schoenfeld JD, Sun AY. PACIFIC: shifting tides in the treatment of locally advanced non-small cell lung cancer. Transl Lung Cancer Res 2019;8:S139-46. 10.21037/tlcr.2019.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang J, Bi N, Wu S, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol 2017;28:777-83. 10.1093/annonc/mdx009 [DOI] [PubMed] [Google Scholar]
- 16.Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol 2015;33:567-74. 10.1200/JCO.2014.56.2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 18.NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer. Version 3.2020-Febraury 11,2020. https://www.nccn.org/professionals.
- 19.Bodey GP, Hersh EM, Valdivieso M, et al. Effects of cytotoxic and immunosuppressive agents on the immune system. Postgrad Med 1975;58:67-74. 10.1080/00325481.1975.11714222 [DOI] [PubMed] [Google Scholar]
- 20.Ferraro C, Quemeneur L, Fournel S, et al. The topoisomerase inhibitors camptothecin and etoposide induce a CD95-independent apoptosis of activated peripheral lymphocytes. Cell Death Differ 2000;7:197-206. 10.1038/sj.cdd.4400595 [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Yea SS, Jeon YJ. Paclitaxel causes mouse splenic lymphocytes to a state hyporesponsive to lipopolysaccharide stimulation. Int J Immunopharmacol 2000;22:615-21. 10.1016/S0192-0561(00)00024-2 [DOI] [PubMed] [Google Scholar]
- 22.Chuang LT, Lotzova E, Heath J, et al. Alteration of lymphocyte microtubule assembly, cytotoxicity, and activation by the anticancer drug taxol. Cancer Res 1994;54:1286-91. [PubMed] [Google Scholar]
- 23.Mackall CL, Fleisher TA, Brown MR, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994;84:2221-8. 10.1182/blood.V84.7.2221.2221 [DOI] [PubMed] [Google Scholar]
- 24.Galluzzi L, Buque A, Kepp O, et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28:690-714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 25.de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res 2014;20:5384-91. 10.1158/1078-0432.CCR-14-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palma JP, Aggarwal SK. Cisplatin and carboplatin-mediated activation of murine peritoneal macrophages in vitro: production of interleukin-1 alpha and tumor necrosis factor-alpha. Anticancer Drugs 1995;6:311-6. 10.1097/00001813-199504000-00016 [DOI] [PubMed] [Google Scholar]
- 27.Kodumudi KN, Woan K, Gilvary DL, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 2010;16:4583-94. 10.1158/1078-0432.CCR-10-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713-21. 10.1158/1078-0432.CCR-05-0883 [DOI] [PubMed] [Google Scholar]
- 29.Cullis J, Siolas D, Avanzi A, et al. Macropinocytosis of Nab-paclitaxel Drives Macrophage Activation in Pancreatic Cancer. Cancer Immunol Res 2017;5:182-90. 10.1158/2326-6066.CIR-16-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng B, Wang J, Cai W, et al. Changes in the tumor immune microenvironment in resected recurrent soft tissue sarcomas. Ann Transl Med 2019;7:387. 10.21037/atm.2019.07.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Dermawan K, Jin M, et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol 2008;129:219-29. 10.1016/j.clim.2008.07.013 [DOI] [PubMed] [Google Scholar]
- 32.Senovilla L, Vacchelli E, Galon J, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012;1:1323-43. 10.4161/onci.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Su DM, Liang M, et al. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol 2008;45:1470-6. 10.1016/j.molimm.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 34.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 35.Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:387-97. 10.1016/S1470-2045(19)30801-0 [DOI] [PubMed] [Google Scholar]
- 36.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 37.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 38.Jotte RMCF, Vynnychenko I, Stroyakovskiy D, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;2018;36:abstr LBA9000.
- 39.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 2004;4:423-36. 10.1038/nrc1369 [DOI] [PubMed] [Google Scholar]
- 40.Kerbel RS, Klement G, Pritchard KI, et al. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol 2002;13:12-5. 10.1093/annonc/mdf093 [DOI] [PubMed] [Google Scholar]
- 41.Biziota E, Mavroeidis L, Hatzimichael E, et al. Metronomic chemotherapy: A potent macerator of cancer by inducing angiogenesis suppression and antitumor immune activation. Cancer Lett 2017;400:243-51. 10.1016/j.canlet.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 42.Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000;60:1878-86. [PubMed] [Google Scholar]
- 43.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol 2001;2:733-40. 10.1016/S1470-2045(01)00587-3 [DOI] [PubMed] [Google Scholar]
- 44.Rowell NP, O'rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004;(4):CD002140. 10.1002/14651858.CD002140.pub2 [DOI] [PubMed] [Google Scholar]
- 45.Chang CL, Hsu YT, Wu CC, et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res 2013;73:119-27. 10.1158/0008-5472.CAN-12-2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007;56:641-8. 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YL, Chang MC, Cheng WF. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett 2017;400:282-92. 10.1016/j.canlet.2017.01.040 [DOI] [PubMed] [Google Scholar]
- 48.Sen T, Della Corte CM, Milutinovic S, et al. Combination Treatment of the Oral CHK1 Inhibitor, SRA737, and Low-Dose Gemcitabine Enhances the Effect of Programmed Death Ligand 1 Blockade by Modulating the Immune Microenvironment in SCLC. J Thorac Oncol 2019;14:2152-63. 10.1016/j.jtho.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonomi M, Ahmed T, Addo S, et al. Circulating immune biomarkers as predictors of the response to pembrolizumab and weekly low dose carboplatin and paclitaxel in NSCLC and poor PS: An interim analysis. Oncol Lett 2019;17:1349-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pauwels B, Vermorken JB, Wouters A, et al. The role of apoptotic cell death in the radiosensitising effect of gemcitabine. Br J Cancer 2009;101:628-36. 10.1038/sj.bjc.6605145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Revannasiddaiah S, Susheela SP. Chemically enhanced radiotherapy: visions for the future. Ann Transl Med 2016;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995;377:68-71. 10.1038/377068a0 [DOI] [PubMed] [Google Scholar]
- 53.Escoter-Torres L, Caratti G, Mechtidou A, et al. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front Immunol 2019;10:1859. 10.3389/fimmu.2019.01859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitajima T, Ariizumi K, Bergstresser PR, et al. A novel mechanism of glucocorticoid-induced immune suppression: the inhibiton of T cell-mediated terminal maturation of a murine dendritic cell line. J Clin Invest 1996;98:142-7. 10.1172/JCI118759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vacca A, Felli MP, Farina AR, et al. Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med 1992;175:637-46. 10.1084/jem.175.3.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowenberg M, Verhaar AP, van den Brink GR, et al. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol Med 2007;13:158-63. 10.1016/j.molmed.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 57.Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785-92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 58.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 2007;13:6681-8. 10.1158/1078-0432.CCR-07-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2872-8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 60.Ricciuti B, Dahlberg SE, Adeni A, et al. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol 2019;37:1927-34. 10.1200/JCO.19.00189 [DOI] [PubMed] [Google Scholar]
- 61.Verheijden RJ, May AM, Blank CU, et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res 2020;26:2268-74. 10.1158/1078-0432.CCR-19-3322 [DOI] [PubMed] [Google Scholar]
- 62.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133-50. 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol 2018;3:512-9. 10.1016/j.adro.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuler PJ, Harasymczuk M, Schilling B, et al. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin Cancer Res 2013;19:6585-96. 10.1158/1078-0432.CCR-13-0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuchikawa T, Hirano S, Tanaka E, et al. Novel aspects of preoperative chemoradiation therapy improving anti-tumor immunity in pancreatic cancer. Cancer Sci 2013;104:531-5. 10.1111/cas.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doescher J, Jeske S, Weissinger SE, et al. Polyfunctionality of CD4(+) T lymphocytes is increased after chemoradiotherapy of head and neck squamous cell carcinoma. Strahlenther Onkol 2018;194:392-402. 10.1007/s00066-018-1289-z [DOI] [PubMed] [Google Scholar]
- 68.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. 10.1158/0008-5472.CAN-14-1258 [DOI] [PubMed] [Google Scholar]
- 70.Yoneda K, Kuwata T, Kanayama M, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer 2019;121:490-6. 10.1038/s41416-019-0541-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatfield P, Merrick A, Harrington K, et al. Radiation-induced cell death and dendritic cells: potential for cancer immunotherapy? Clin Oncol (R Coll Radiol) 2005;17:1-11. 10.1016/j.clon.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 72.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol 2004;173:2462-9. 10.4049/jimmunol.173.4.2462 [DOI] [PubMed] [Google Scholar]
- 73.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005;174:7516-23. 10.4049/jimmunol.174.12.7516 [DOI] [PubMed] [Google Scholar]
- 74.Gerber DE, Urbanic JJ, Langer C, et al. Treatment Design and Rationale for a Randomized Trial of Cisplatin and Etoposide Plus Thoracic Radiotherapy Followed by Nivolumab or Placebo for Locally Advanced Non-Small-Cell Lung Cancer (RTOG 3505). Clin Lung Cancer 2017;18:333-9. 10.1016/j.cllc.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu YL, Chen M, Zhou Q, Li H, et al. Gemstone-301: A phase III clinical trial of CS1001 as consolidation therapy in subjects with locally advanced/unresectable (stage III) non-small cell lung cancer (NSCLC) who have not progressed after prior concurrent/sequential chemoradiotherapy (CRT). J Clin Oncol 2019;37:abstr TPS8572.
- 76.Luis G. Paz-Ares, Senan S, Planchard D, et al. Tislelizumab (BGB-A317) + concurrent chemoradiotherapy (cCRT) followed by tislelizumab monotherapy for newly diagnosed locally advanced, unresectable, stage III non-small cell lung cancer (NSCLC) in a phase III study (RATIONALE 001). J Clin Oncol 2019;37:abstr TPS8574.
- 77.Myers CJ, Lu B. Decreased Survival After Combining Thoracic Irradiation and an Anti-PD-1 Antibody Correlated With Increased T-cell Infiltration Into Cardiac and Lung Tissues. Int J Radiat Oncol Biol Phys 2017;99:1129-36. 10.1016/j.ijrobp.2017.06.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du S, Zhou L, Alexander GS, et al. PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes. J Thorac Oncol 2018;13:510-20. 10.1016/j.jtho.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin SH, Lin Y, Yao L, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol 2020;15:248-57. 10.1016/j.jtho.2019.10.024 [DOI] [PubMed] [Google Scholar]
- 80.Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271-7. 10.1002/cncr.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011;1:54-67. 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gough MJ, Crittenden MR, Sarff M, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother 2010;33:798-809. 10.1097/CJI.0b013e3181ee7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728-34. [PubMed] [Google Scholar]
- 85.Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017;112:90-5. 10.1016/j.lungcan.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 86.Kato R, Hayashi H, Chiba Y, et al. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L). J Immunother Cancer 2020;8:e000350. 10.1136/jitc-2019-000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yost KE, Satpathy AT, Wells DK, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 2019;25:1251-9. 10.1038/s41591-019-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young KH, Baird JR, Savage T, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One 2016;11:e0157164. 10.1371/journal.pone.0157164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 93.Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J Thorac Oncol 2020;15:499-519. 10.1016/j.jtho.2019.12.107 [DOI] [PubMed] [Google Scholar]
- 94.Evans M, O'Sullivan B, Smith M, et al. Predictive markers for anti-PD-1/PD-L1 therapy in non-small cell lung cancer-where are we? Transl Lung Cancer Res 2018;7:682-90. 10.21037/tlcr.2018.06.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 96.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 97.Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol 2010;3:81-91. 10.1038/mi.2009.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy 2011;66:155-62. 10.1111/j.1398-9995.2010.02458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao Y, Yu S, Zhu B, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med 2014;211:943-59. 10.1084/jem.20130790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mataki N, Kikuchi K, Kawai T, et al. Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am J Gastroenterol 2007;102:302-12. 10.1111/j.1572-0241.2006.00948.x [DOI] [PubMed] [Google Scholar]
- 101.Menke J, Lucas JA, Zeller GC, et al. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol 2007;179:7466-77. 10.4049/jimmunol.179.11.7466 [DOI] [PubMed] [Google Scholar]
- 102.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 2003;33:2706-16. 10.1002/eji.200324228 [DOI] [PubMed] [Google Scholar]
- 103.Matsumoto K, Inoue H, Nakano T, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol 2004;172:2530-41. 10.4049/jimmunol.172.4.2530 [DOI] [PubMed] [Google Scholar]
- 104.Ahmad SM, Martinenaite E, Holmstrom M, et al. The inhibitory checkpoint, PD-L2, is a target for effector T cells: Novel possibilities for immune therapy. Oncoimmunology 2018;7:e1390641. 10.1080/2162402X.2017.1390641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim MY, Koh J, Kim S, et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 2015;88:24-33. 10.1016/j.lungcan.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 106.Calles A, Liao X, Sholl LM, et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J Thorac Oncol 2015;10:1726-35. 10.1097/JTO.0000000000000687 [DOI] [PubMed] [Google Scholar]
- 107.Shibahara D, Tanaka K, Iwama E, et al. Intrinsic and Extrinsic Regulation of PD-L2 Expression in Oncogene-Driven Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:926-37. 10.1016/j.jtho.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 108.Takamori S, Takada K, Azuma K, et al. Prognostic Impact of Programmed Death-Ligand 2 Expression in Primary Lung Adenocarcinoma Patients. Ann Surg Oncol 2019;26:1916-24. 10.1245/s10434-019-07231-z [DOI] [PubMed] [Google Scholar]
- 109.Matsubara T, Takada K, Azuma K, et al. A Clinicopathological and Prognostic Analysis of PD-L2 Expression in Surgically Resected Primary Lung Squamous Cell Carcinoma. Ann Surg Oncol 2019;26:1925-33. 10.1245/s10434-019-07257-3 [DOI] [PubMed] [Google Scholar]
- 110.Tanegashima T, Togashi Y, Azuma K, et al. Immune Suppression by PD-L2 against Spontaneous and Treatment-Related Antitumor Immunity. Clin Cancer Res 2019;25:4808-19. 10.1158/1078-0432.CCR-18-3991 [DOI] [PubMed] [Google Scholar]
- 111.Picardo SL, Doi J, Hansen AR. Structure and Optimization of Checkpoint Inhibitors. Cancers (Basel) 2019;12:38. 10.3390/cancers12010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boyerinas B, Jochems C, Fantini M, et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res 2015;3:1148-57. 10.1158/2326-6066.CIR-15-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grenga I, Donahue RN, Lepone LM, et al. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunology 2016;5:e83. 10.1038/cti.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hicks KC, Fantini M, Donahue RN, et al. Epigenetic priming of both tumor and NK cells augments antibody-dependent cellular cytotoxicity elicited by the anti-PD-L1 antibody avelumab against multiple carcinoma cell types. Oncoimmunology 2018;7:e1466018. 10.1080/2162402X.2018.1466018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Passiglia F, Galvano A, Rizzo S, et al. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: An indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer 2018;142:1277-84. 10.1002/ijc.31136 [DOI] [PubMed] [Google Scholar]
- 116.Duan J, Cui L, Zhao X, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2020;6:375-84. 10.1001/jamaoncol.2019.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tartarone A, Roviello G, Lerose R, et al. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol 2019;15:2423-33. 10.2217/fon-2018-0868 [DOI] [PubMed] [Google Scholar]
- 118.Peng TR, Tsai FP, Wu TW. Indirect comparison between pembrolizumab and nivolumab for the treatment of non-small cell lung cancer: A meta-analysis of randomized clinical trials. Int Immunopharmacol 2017;49:85-94. 10.1016/j.intimp.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Zhou Y, Tang L, et al. Immune-Checkpoint Inhibitors as the First Line Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. J Cancer 2019;10:6261-8. 10.7150/jca.34677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271-81. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 122.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:173-82. 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tan S, Zhang H, Chai Y, et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun 2017;8:14369. 10.1038/ncomms14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scapin G, Yang X, Prosise WW, et al. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol 2015;22:953-8. 10.1038/nsmb.3129 [DOI] [PubMed] [Google Scholar]
- 125.Wang J, Fei K, Jing H, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs 2019;11:1443-51. 10.1080/19420862.2019.1654303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Durm GA, Althouse SK, Sadiq AA, et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. J Clin Oncol 2018 36:abstr 8500.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as