Abstract
Magnesium (Mg) and its alloys are promising biodegradable materials for orthopedic applications. However, one of the major problems is their rapid degradation rate with quick evolution of hydrogen gas. To overcome this problem, calcium phosphate (CaP) coatings have been used to improve the degradation resistance and the biocompatibility of Mg materials. This study focuses on the comparison and correlation of the in vitro and in vivo degradation and biocompatibility behaviors of these materials. A CaP coating consisting of dicalcium phosphate dihydrate (DCPD) was deposited on an AZ60 Mg alloy by the chemical conversion method. Then, the in vitro degradation testing including electrochemical and immersion tests, and in vivo implantation of the CaP coated Mg alloy were conducted to compare the degradation behaviors. Next, the in vitro cell behavior and in vivo bone tissue response were also compared on both uncoated and CaP-coated Mg samples. Data showed that the CaP coating provided the Mg alloy with significantly better biodegradation behavior and biocompatibility. The in vitro and in vivo biocompatibility tests exhibited good consistency while not the case for biodegradation. Results showed that the in vitro electrochemical test could be a quick screening tool for the biodegradation rate, while the in vitro immersion degradation rate was often 2–4 folds faster than the in vivo degradation rate.
Keywords: Magnesium implants, Calcium phosphate, Biodegradation, Cytocompatibility, Bone regeneration
Graphical abstract
Highlights
-
•
Calcium phosphate coating improves biocompatibility and inhibits degradation of Mg implants.
-
•
Good consistency between in vitro and in vivo biocompatibility tests.
-
•
Electrochemical test can offer rapid and reliable screening of biodegradation rates.
-
•
A factor of 2–4 between the faster in vitro and slower in vivo degradation rates.
1. Introduction
Biodegradable metals have been regarded as potential temporary implant materials due to their attractive biodegradation properties [[1], [2], [3], [4]]. The biodegradable implants could provide tissue support and regeneration in the initial stage and gradually disappear with the degradation process, thus, avoiding the secondary surgery and satisfying the clinical requirement as the temporary implants. As one of the promising biodegradable metals, magnesium (Mg) and its alloys have been studied in various biomedical applications because of the attractive mechanical properties and biocompatibility [[2], [3], [4], [5]]. However, Mg and its alloys have severe degradation reactions in the physiological environment, resulting in the quick evolution of hydrogen gas and loss of mechanical integrity before complete healing. These problems limit their clinical uses especially the load-bearing orthopedic applications.
Calcium phosphate (CaP) ceramics have intrinsic bioactivity and biocompatibility for orthopedic applications because they are the main components in bone tissue [6]. Different CaP compounds have been used as bone cement and drug delivery carrier in orthopedic applications [[7], [8], [9], [10]], including hydroxyapatite (HA), tricalcium phosphate (α-TCP), and their combination as biphasic calcium phosphate (BCP) [11,12]. The CaP bone cement has a unique function as bone substitutes and can accelerate the bone tissue healing processes [7,8]. The CaP ceramic particles can act as the carrier to load various proteins and drugs and are used in different pathological bone sites [9]. However, the low ductility of CaP ceramics limits their clinical applications as load-bearing implants.
The combination of biodegradable Mg alloys with biofunctional CaP ceramics can potentially make an ideal load-bearing implant material. There are two ways to realize the combination: metallic composites and CaP-based surface coating. Compared to the Mg–CaP composites, the surface modifications of Mg alloys with a biocompatible CaP coating might result in a better implant material because of better mechanical performance, an improved bone/implant interface, and an acceptable biodegradation rate in the human body [13,14]. There have been many studies on the feasibility of CaP coated Mg alloys as orthopedic biodegradable implants [15,16], for example, HA-coated Mg alloys showed excellent biocompatibility and osteoconductivity [[17], [18], [19]]. Different CaP-based composite coatings on metallic implants can also offer various degradation rates tailored for different applications [[20], [21], [22]].
These CaP-based coatings will serve as a biofunctional layer at the interface and provide improved biocompatibility and degradation resistance for Mg implants. In vitro and cell-based studies are important that they allow more rapid assessment of new biomaterials, and are relatively cheap and simple to procure, enabling a rapid screening and prediction in biomaterial studies [23]. A major drawback is their failure to capture the inherent complexity of organ systems [24]. This is also true when evaluating the degradation and biological performances of CaP coated Mg-based materials [[17], [18], [19]]. Therefore, the goal of this study is to examine the similarities and differences between the in vitro and in vivo performances of the CaP coated Mg alloy in a bid to establish some measurable correlations. Thus, we hope the in vitro data could provide better screening and prediction on the in vivo performances of these biomaterials.
2. Materials and methods
2.1. Coating preparation
An AZ60 Mg alloy (Jiangsu Maxi Metal, China) plate samples with dimensions of 10 mm × 10 mm × 5 mm were used for in vitro tests, including electrochemical, immersion, and cell behavior tests. Rod samples for in vivo experiments were 3 mm in diameter and 8 mm in length. After mechanically polish and alcohol cleaning, the samples were socked in the coating solution for 5 min at 40 °C to obtain the CaP coating. The coating solution includes: 0.7 M calcium nitrate and 1.5 M phosphate acid (85% V/V), pH of 2.8–3.0.
2.2. Surface characterization
The samples were characterized by X-ray diffraction (XRD, Rigaku Dymax, Japan) and scanning electron microscopy (SEM, ZEISS EV018, Germany). The XRD was tested with Cu Kα radiation and a monochromator at 40 kV and 200 mA with a scanning rate and a step of 4°/min and 0.02°, respectively.
2.3. In vitro degradation behaviors
In vitro degradation behaviors were evaluated by electrochemical polarization test and immersion test in simulated body fluid (SBF) at 37 °C [25,26]. The SBF composition is identical to previous studies [27,28]. A three-electrode cell was set up for the electrochemical polarization test with platinum plate and a saturated calomel electrode (SCE) as counter and reference electrode, respectively. A square area of 0.5 cm2 was exposed on the sample surface for the test. The test was carried out at a scanning rate of 5 mV/s. The corrosion rate (CRi, mm y−1) was obtained using the corrosion current density (icorr, μA cm−2), determined by the Tafel extrapolation method [29,30]:
| CRi = 22.85 × 10−3icorr | (1) |
Samples were socked completely in the SBF using fish line for the immersion test at 37 °C. The surface morphologies of the samples after immersion for 15 days were observed with SEM. The degradation rate (CRH, mm y−1) was evaluated from the average hydrogen evolution rate (VH, ml cm−2 d−1) [30,31]:
| CRH = 2.007VH | (2) |
where VH is measured at 37 °C and a pressure of 1 atm.
2.4. In vitro cell tests
Osteoblast MC3T3-E1 cells were cultured in complete cell medium of Dulbecco's modified Eagle's medium (DMEM, Gibco, US) [32,33]. The cell suspension (1 mL) was seeded onto the samples at a cell density of 1 × 105 cells/mL. After 3 days of culture in 24-well plates (Corning, NY, USA) at 37 °C in a humidified 5% CO2 incubator, the samples were washed three times with phosphate-buffered saline (PBS), fixed in 4% (w/v) paraformaldehyde solution for 24 h, gradually dehydrated in 30%, 50%, 70%, 90%, 100% alcohol solution, coated with Au, and then processed for SEM imaging.
Cell viability was evaluated by the Cell Counting Kit 8 assay (CCK-8, Dojindo, Kumamoto, Japan) according to ISO 10993–5:2009. The extract medium was prepared by incubating Mg samples in complete DMEM media at a ratio of 1.25 cm2/mL for 3 days. Osteoblast MC3T3-E1 cells were incubated in 96-well plates (Corning, NY, USA) with 2500 cells per 100 μL in each well and incubated for 24 h to allow attachment. Then, the medium was replaced with 100 μL of DMEM (control group) or different extract mediums. After incubation for 1, 3, 5, and 7 days, 10 μL of CCK-8 solution was added, and the plates were incubated for a further hour. Cell viability was determined from absorbance readings at a wavelength of 450 nm.
For the Alkaline phosphatase (ALP) tests, the cells were incubated in 24-well plates with 5 × 104 cells/mL in each well. The medium was replaced with DMEM (control group) or different extract medium after 24 h of culture following the above cell viability protocol. The differentiation behavior of the osteoblast MC3T3-E1 cells was estimated by measuring ALP activity after 14 days of culture [29,34]. The cells were lysed in 0.1% Triton X-100 solution in PBS and measured using p-nitrophenyl phosphate (pNpp, Sigma, MO, USA).
2.5. In vivo animal studies
All animal studies were approved by the IACUC at Stony Brook University following NIH guidelines. Twenty adult healthy New Zealand White rabbits of 2.5–3.0 kg in weight were anesthetized with 0.5% pentobarbital sodium solution for surgery. Coated or uncoated implants were inserted into a predrilled hole on one femora of each animal. All animals received subcutaneous injections of penicillin in the following three days after surgery. At 1 month and 3 months, five rabbits from each material groups were euthanized for micro-computed tomographic (micro-CT) and histological evaluation.
The femora containing implants were fixed in 75 vol% alcohol solution and scanned using a micro-CT system (Locus SP, GE Healthcare, USA) with a resolution of 10 μm. After the three-dimensional (3D) reconstruction, in vivo corrosion rate was calculated based on the metallic volume reduction after implantation [41]:
| CRv = (V0 – Vt) / At | (3) |
where Vt is the finial implant volume, V0 is initial implant volume, A is the initial implant surface area, and t is implantation time.
The femur samples with implants were explanted and fixed in 4% paraformaldehyde solution for 24 h, dehydrated in 30%, 50%, 70%, 90%, 100% alcohol solution, and then decalcified in ethylene diamine tetraacetate acid (EDTA). Subsequently, the samples were paraffin embedded and stained with hematoxylin and eosin (HE).
2.6. Statistical analysis
All data was displayed as mean ± standard deviation. Statistical analysis was conducted by analysis of variance (ANOVA) by the Turkey post hoc test. P < 0.05 was considered as statistically significant.
3. Results
3.1. Coating characteristics
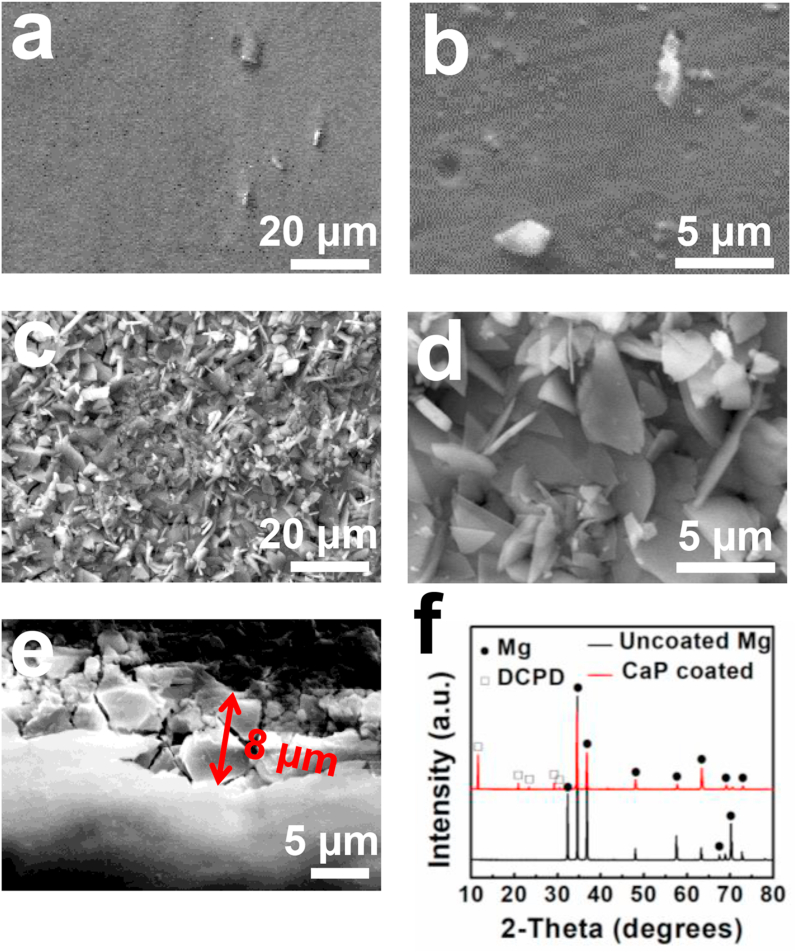
Surface morphology and XRD spectra of the uncoated and CaP-coated Mg alloy are shown in Fig. 1. The polished Mg surface showed a few white particles, corresponding to the intermetallic phases (Fig. 1a–b). The uniform coating is composed primarily of small leaf-like flakes with porous structure (Fig. 1c–d). The coating thickness is 8 ± 2 μm (Fig. 1e). Although some cracks appeared on the cross-section of the coating, there were no cracks between the coating and Mg substrate, indicating good coating adhesion. The XRD spectra show that the main phase of the CaP coating is DCPD (JCDPS No. 09–0077) (Fig. 1f).
Fig. 1.
Mg alloy and CaP coating characteristics: (a–b) Polished surface of Mg alloy, (c–d) surface coating morphology, (e) cross sectional coating morphology, (f) XRD spectra.
3.2. In vitro degradation behavior
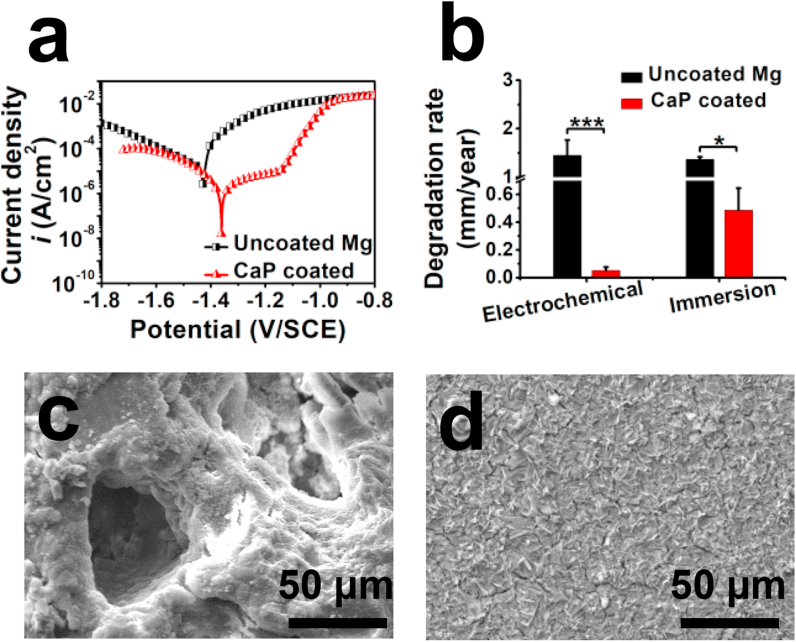
Determined from the electrochemical polarization curves in Fig. 2a, the uncoated Mg alloy had the corrosion potential of −1.43 ± 0.02 V/SCE and corrosion current density of 67 ± 14 μA cm−2. Compared with the uncoated alloy, the coating had more positive corrosion potential (−1.35 ± 0.03 V/SCE) and lower polarization current density (6 ± 2 μA cm−2). The immersion tests focused on the evolution of the CaP coating on the AZ60 alloy in SBF. The corrosion pits and cracks could be observed on the uncoated alloy (Fig. 2c). Some cracks appeared with pieces of coating plates on the CaP-coated Mg alloy surface (Fig. 2d), but the coating structure was complete to protect the Mg surface. After 2 weeks of immersion test, the pH value of the SBF after incubated with uncoated and CaP-coated Mg alloys were increased to 10.8 ± 0.3 and 9.3 ± 0.2, respectively (data not shown here). The degradation rates decreased significantly after the CaP coating treatment (Fig. 2b). Compared to the data from immersion test, the CaP coated sample showed much slower degradation rate in the electrochemical tests, while the uncoated Mg in electrochemical tests had a three times higher degradation rate.
Fig. 2.
In vitro degradation behaviors: (a) Electrochemical polarization curves, (b) degradation rates based on the electrochemical and immersion tests, (c–d) surface morphology of (c) uncoated Mg and (d) CaP coated samples after immersion tests for 15 days.
3.3. In vitro cell behavior
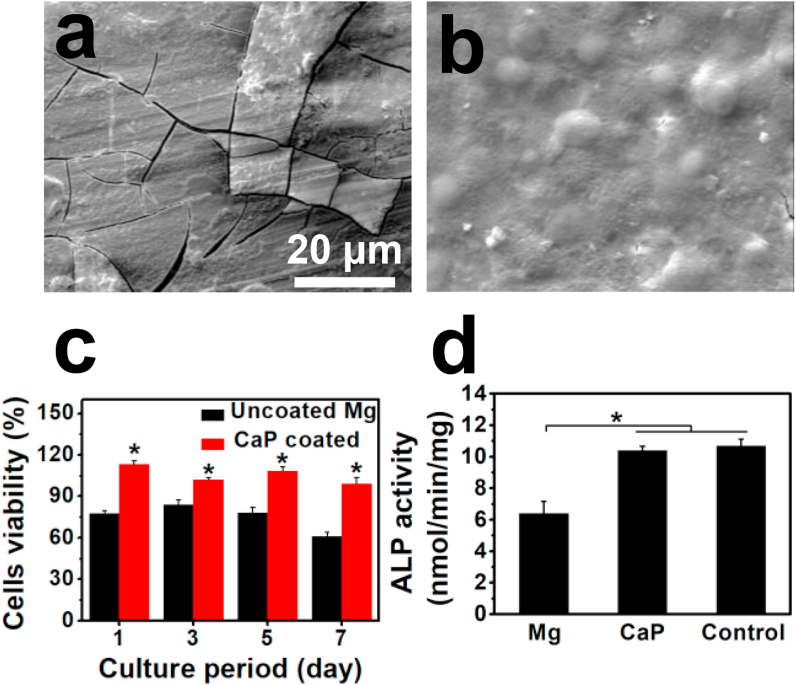
To determine the cytocompatibility and cell adhesion capability on the CaP coating, MC3T3-E1 cells were either cultured directly onto the samples or with material extracts. The results are shown in Fig. 3. There were distinct differences in the responses of the cells to different surfaces. From the observation of the uncoated Mg alloy (Fig. 3a), only a few cells are attached to the surface with round or spindle-like morphology. The surface was completely covered with severe cracks beneath the cells, due to the rapid degradation that took place in the cell culture fluid. On the other hand, numerous cells were observed on the CaP-coated Mg alloy (Fig. 3b), and a dense, uniform cell layer covered the whole surface.
Fig. 3.
In vitro biocompatibility: MC3T3-E1 cell morphologies after 3 days of culture on (a) uncoated and (b) CaP-coated samples, (c) cell viability and (d) ALP activity when cells were culture with sample extracts. *p < 0.05, compared to uncoated group.
Both cell viability and ALP activity were measured by indirect extract assays, as shown in Fig. 3c–d. The cell viability values of the uncoated sample remained at about 80% on the first 5 days until day 7 in which it decreased greatly to 61%. With CaP-coated alloy, a significant increase (P < 0.05) can be seen at all time intervals; the values were somewhat higher than 100% in the first 5 days (grade 0 cytotoxicity) and 99% on day 7 (grade 1 cytotoxicity), suggesting that the coating had significantly better surface bioactivity than the uncoated alloy and could promote cell growth and proliferation.
ALP activity is one of the most widely used markers for early osteoblastic differentiation. Compared with the uncoated sample, significantly increased ALP activity was observed for the coated sample (Fig. 3d). Besides, the difference between the coated sample and the control group was not significant (p > 0.05).
3.4. In vivo degradation behavior
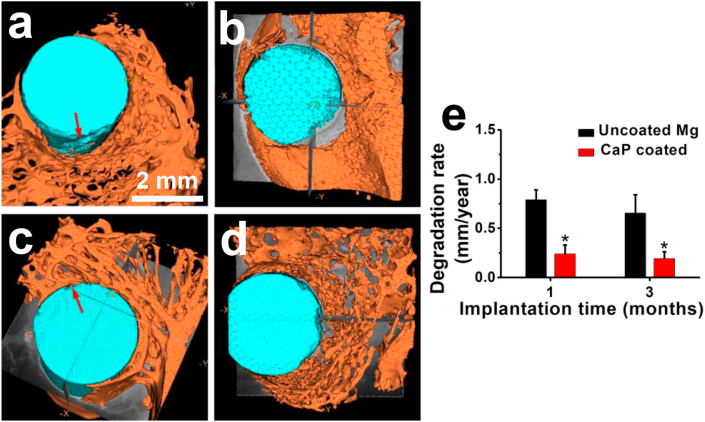
The in vivo degradation morphology of the implants in the femora was studied using micro-CT. 3D reconstruction images of the Mg implants at 1 month and 3 months postoperatively, as well as their in vivo degradation rates, are illustrated in Fig. 4. The uncoated alloy implant exhibited degradation pits (indicated by the red arrow in Fig. 4a) at 1 month, while there was only slight pitting degradation for the CaP-coated implant (indicated by the red arrow in Fig. 4c). Degradation of the uncoated alloy implant became worse over time, while the coated implant was also corroded in specific local area (Fig. 4b and d). The degradation rates of the uncoated implants are three times higher than those of the CaP-coated implants (Fig. 4e). The degradation rates for both implants decreased slightly with implantation time.
Fig. 4.
In vivo degradation: Representative micro-CT 3D reconstruction images of (a, b) uncoated and (c, d) CaP-coated magnesium implants after (a, c) 1 and (b, d) 3 months of implantation, and (e) their in vivo degradation rates.
3.5. Tissue response
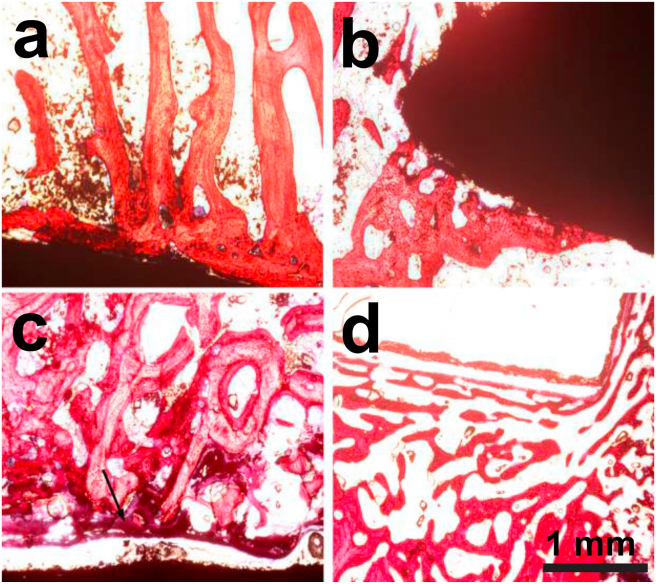
Fig. 5 shows the optical microstructures of the interfaces between the Mg implants and new bone stained by HE after 1 month and 3 months of implantation. For the uncoated Mg alloy, sparse newborn bone and bone trabeculae were in contact with portions of the implant surface, but no obvious inflammatory response was observed at 1 month post-implantation, as indicated in Fig. 5a. After 3 months of implantation, although severe degradation occurred on the uncoated implant (Fig. 4b), bone trabeculae were still connected around the implant. However, the amount of new bone seemed to be reduced, with gaps of uneven size were observed between the implant and the surrounding bone tissue, which were filled with fibrous connective tissue. This might be attributable to the high degradation rate, which caused the induced locally high pH value and cell cytotoxicity. For the CaP-coated implant, connective tissue and more newborn bone were seen at the interface at 1 month post-surgery, as indicated by the arrow in Fig. 5c, but the alignment of the connected bone trabeculae was irregular. After 3 months, osteoid tissue and newborn bone trabeculae almost covered the implant surface (Fig. 5d) with normal alignment. The increased number of bone trabeculae and their uniform alignment for the CaP-coated implant, as compared to the uncoated one, can be attributed to the greatest bioactivity of the CaP coating and the reduced in vivo degradation rate.
Fig. 5.
In vivo biocompatibility: HE stained photographs of bone tissues containing (a, b) uncoated and (c, d) CaP-coated implants after (a, c) 1 month; and (b, d) 3 months of implantation.
4. Discussion
In this study, a CaP coating composed of DCPD was prepared on Mg implant and the in vitro and in vivo biocompatibility and degradation behaviors were examined and compared systemically. When compared to the uncoated Mg implants, the CaP coated implants had a significantly decreased in vivo degradation rate of 0.22 ± 0.03 mm/year, which is in the range of a suggested degradation rate of 0.2–0.5 mm/year for bone implants to match bone healing process [35,36]. The CaP coating could significantly improve the cell adhesion, proliferation, and differentiation of osteoblasts from the in vitro tests. Similarly, the CaP coated implants promoted peri-implant new bone formation without inducing any significant adverse effects in vivo. Although the biological performances were consistent in vitro and in vivo, the CaP coated implants had a quite different biodegradation rate in vivo from electrochemical and immersion tests in vitro.
It is expected that there is a gap between the in vitro and in vivo behaviors when studying biomaterials including biodegradable metals. There are a few studies provided some perspectives on the correlation between the in vitro and in vivo degradation rates for Mg and its alloys [[37], [38], [39]]. Several influential factors include material compositions and surface status, the in vitro and in vivo protocols, the various in vitro corrosion medium, and the in vivo implantation sites. Generally, it is not easy to predict in vivo degradation rates of Mg alloys using the current ASTM standards for in vitro assays, and there is a possible correlation factor of 1–5 between the relative faster in vitro and the relative slower in vivo degradation rate when using the appropriate in vitro corrosion medium [37,39].
However, only a few studies touched on the discrepancies between in vitro and in vivo behavior of CaP coated Mg materials [15,40]. Here we summarized some key studies addressing both in vitro and in vivo biodegradation rates of CaP-coated Mg implants (Table 1) [17,[41], [42], [43], [44], [45], [46]]. It is shown that the degradation rates from electrochemical tests are 0.03–0.57 mm/year, which has a similar range to that from in vivo tests (0.03–0.81 mm/year). The degradation rates could be obtained in hours, making it suitable to rapidly screen different surface treatments and coating materials [28,47]. However, there are no corresponding time points in the electrochemical tests, so it cannot be used as a prediction tool for the in vivo behavior. The immersion degradation rates are usually 2–4 times higher than that in vivo and this correlation factor has a trend to increase with the implantation time. The addition of protein in the corrosion medium could potentially decrease the correlation factor [46]. The CaP ceramic coatings offer a physical barrier to protect the Mg substrates from severe localized corrosion, and the more uniform corrosion behavior is potentially more predictable.
Table 1.
Key studies on in vitro and in vivo biodegradation rates of CaP coatings on Mg and its alloys.
| Coating method | Coating/Substrate | Electrochemical corrosion rates (mm/year) | Immersion corrosion rates (mm/year) | In vivo corrosion rates (mm/year) | Ref. |
|---|---|---|---|---|---|
| Electron cyclotron resonance plasma sputtering | HA/Mg | 0.07 | 1.5 m: 0.08 3 m: 0.21 |
[17] | |
| Pulse electrodeposition | Ca-def HA/MgZnCa | 0.57 | 2 m: 0.15 3 m:0.56 6 m:0.81 |
[43,44] | |
| Chemical immersion | DCPD/JDBM | 0.03 | 10d: 0.39 | 1 m: 0.16 4 m: 0.1 7 m: 0.03 |
[41,42] |
| Micro-arc oxidation | Amorphous CaP/Mg–Sr | 0.37 | 2w: 3 | 2 m: 0.75 | [45] |
| Chemical immersion | DCPA/Mg | 3w: 0.3–0.4 | 3w: 0.15 | [46] | |
| Chemical immersion | DCPD/Mg | 3w: 0.4–0.7 | 3w: 0.2 | [46] | |
| Chemical conversion | DCPD/AZ60 | 0.05 | 2w: 0.48 |
1 m: 0.24 3 m: 0.19 |
This study |
On the other hand, biocompatibility is somehow easier to be predicted using in vitro cell-based testing. It is recommended to use a 6–10 times dilution of extracts for in vitro indirect cytotoxicity test for Mg and other biodegradable metals [[48], [49], [50], [51]]. There are also some sophisticated setups and methods suggested to be used in vitro to predict the blood compatibility of biodegradable metals [[52], [53], [54]]. The quick pH change and hydrogen release from the degradation of Mg and its alloys are the main factors to depress the interactions with mammalian cells, including blood cells. In addition to controlling the degradation rates of Mg-based substrates, CaP coating improved adhesion of osteoblasts cells. Adhesive interactions play critical roles in osteoblasts proliferation and differentiation, matrix mineralization, and bone formation [14,34,55]. The excellent biocompatibility of CaP coatings with the orthopedic tissues facilitates an improved in vitro and in vivo biological performances for Mg-based materials [16,56,57].
5. Conclusion
A CaP coating was obtained on a Mg alloy surface by using a simple and easily controlled chemical treatment. The CaP coating could significantly improve the cell adhesion, proliferation, and differentiation of osteoblasts in vitro. The in vivo tests also indicated that the CaP coated implants promoted peri-implant new bone formation without inducing any significant adverse effects. The in vitro and in vivo biocompatibility tests exhibited a good consistency. The electrochemical test in vitro could act as a fast and reliable screening method for the biodegradation of CaP coated Mg implants. Immersion tests showed a faster biodegradation rate than in vivo results, and a correlation factor of 2–4 is suggested between the in vitro immersion test and in vivo test.
CRediT authorship contribution statement
Julia Gao: Investigation, Data curation, Writing - original draft. Yingchao Su: Conceptualization, Investigation, Writing - review & editing, Supervision. Yi-Xian Qin: Resources, Writing - review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Liu Y., Zheng Y., Chen X.-H., Yang J.-A., Pan H., Chen D., Wang L., Zhang J., Zhu D., Wu S., Yeung K.W.K., Zeng R.-C., Han Y., Guan S. Fundamental theory of biodegradable metals-definition, criteria, and design. Adv. Funct. Mater. 2019;29(18) [Google Scholar]
- 2.Chen Q.Z., Thouas G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015;87:1–57. [Google Scholar]
- 3.Seitz J.M., Durisin M., Goldman J., Drelich J.W. Recent advances in biodegradable metals for medical sutures: a critical review. Adv Healthc Mater. 2015;4(13):1915–1936. doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. 0. [Google Scholar]
- 5.Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2017;112:287–302. doi: 10.1016/j.biomaterials.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Eliaz N., Metoki N. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. 2017;10(4):334. doi: 10.3390/ma10040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooms E., Wolke J., Van de Heuvel M., Jeschke B., Jansen J. Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials. 2003;24(6):989–1000. doi: 10.1016/s0142-9612(02)00438-6. [DOI] [PubMed] [Google Scholar]
- 8.Ambard A.J., Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J. Prosthodont. 2006;15(5):321–328. doi: 10.1111/j.1532-849X.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Verron E., Khairoun I., Guicheux J., Bouler J.-M. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov. Today. 2010;15(13–14):547–552. doi: 10.1016/j.drudis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Bose S., Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8(4):1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouler J.-M., Pilet P., Gauthier O., Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: a review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 12.Eliaz N., Metoki N. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. 2017;10(4):334. doi: 10.3390/ma10040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan P., Tan L., Yang K. Surface modification on biodegradable magnesium alloys as orthopedic implant materials to improve the bio-adaptability: a review. J. Mater. Sci. Technol. 2016;32(9):827–834. [Google Scholar]
- 14.Su Y., Luo C., Zhang Z., Hermawan H., Zhu D., Huang J., Liang Y., Li G., Ren L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018;77:90–105. doi: 10.1016/j.jmbbm.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Shadanbaz S., Dias G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: a review. Acta Biomater. 2012;8(1):20–30. doi: 10.1016/j.actbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Su Y., Cockerill I., Zheng Y., Tang L., Qin Y.X., Zhu D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact Mater. 2019;4:196–206. doi: 10.1016/j.bioactmat.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S.M., Jo J.H., Lee S.M., Kang M.H., Kim H.E., Estrin Y., Lee J.H., Lee J.W., Koh Y.H. Hydroxyapatite‐coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2014;102(2):429–441. doi: 10.1002/jbm.a.34718. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Li X., Chen M., Zhao Y., You C., Li Y., Chen G. In vitro and in vivo degradation behavior and biocompatibility evaluation of microarc oxidation-fluoridated hydroxyapatite-coated Mg–Zn–Zr–Sr alloy for bone application. ACS Biomater. Sci. Eng. 2019;5(6):2858–2876. doi: 10.1021/acsbiomaterials.9b00564. [DOI] [PubMed] [Google Scholar]
- 19.Hiromoto S., Inoue M., Taguchi T., Yamane M., Ohtsu N. In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 2015;11:520–530. doi: 10.1016/j.actbio.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y., Su Y., Gu R., Zhang Z., Li G., Lian J., Ren L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coating. Technol. 2020;401 [Google Scholar]
- 21.Su Y., Lu C., Hu X., Guo Y., Xun X., Zhang Z., Li G., Lian J., Ren L. Improving the degradation resistance and surface biomineralization ability of calcium phosphate coatings on a biodegradable magnesium alloy via a sol-gel spin coating method. J. Electrochem. Soc. 2018;165(3):C155–C161. [Google Scholar]
- 22.Guo Y., Jia S., Qiao L., Su Y., Gu R., Li G., Lian J. Enhanced corrosion resistance and biocompatibility of polydopamine/dicalcium phosphate dihydrate/collagen composite coating on magnesium alloy for orthopedic applications. J. Alloys Compd. 2020;817:152782. [Google Scholar]
- 23.Kohli N., Ho S., Brown S.J., Sawadkar P., Sharma V., Snow M., García-Gareta E. Bone remodelling in vitro: where are we headed?:-A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone. 2018;110:38–46. doi: 10.1016/j.bone.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Pearce A., Richards R., Milz S., Schneider E., Pearce S. Animal models for implant biomaterial research in bone: a review. Eur. Cell. Mater. 2007;13(1):1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 25.Su Y., Li D., Su Y., Lu C., Niu L., Lian J., Li G. Improvement of the biodegradation property and biomineralization ability of magnesium–hydroxyapatite composites with dicalcium phosphate dihydrate and hydroxyapatite coatings. ACS Biomater. Sci. Eng. 2016;2(5):818–828. doi: 10.1021/acsbiomaterials.6b00013. [DOI] [PubMed] [Google Scholar]
- 26.Su Y., Lu Y., Su Y., Hu J., Lian J., Li G. Enhancing the corrosion resistance and surface bioactivity of a calcium-phosphate coating on a biodegradable AZ60 magnesium alloy via a simple fluorine post-treatment method. RSC Adv. 2015;5(69):56001–56010. [Google Scholar]
- 27.Song G. Control of biodegradation of biocompatible magnesium alloys. Corrosion Sci. 2007;49(4):1696–1701. [Google Scholar]
- 28.Su Y., Li G., Lian J. A chemical conversion hydroxyapatite coating on AZ60 magnesium alloy and its electrochemical corrosion behaviour. Int. J. Electrochem. Sci. 2012;7(11):11497–11511. [Google Scholar]
- 29.Su Y., Wang K., Gao J., Yang Y., Qin Y.X., Zheng Y., Zhu D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019;98:174–185. doi: 10.1016/j.actbio.2019.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z., Atrens A. An innovative specimen configuration for the study of Mg corrosion. Corrosion Sci. 2011;53(1):226–246. [Google Scholar]
- 31.Su Y., Guo Y., Huang Z., Zhang Z., Li G., Lian J., Ren L. Preparation and corrosion behaviors of calcium phosphate conversion coating on magnesium alloy. Surf. Coating. Technol. 2016;307:99–108. [Google Scholar]
- 32.Guo Y., Jia S., Qiao L., Su Y., Rui G., Li G., Lian J. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surf., B. 2020;194:111186. doi: 10.1016/j.colsurfb.2020.111186. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y., Su Y., Jia S., Sun G., Gu R., Zhu D., Li G., Lian J. Hydroxyapatite/titania composite coatings on biodegradable magnesium alloy for enhanced corrosion resistance, cytocompatibility and antibacterial properties. J. Electrochem. Soc. 2018;165(14):C962–C972. [Google Scholar]
- 34.Cockerill I., Su Y., Lee J.H., Berman D., Young M.L., Zheng Y., Zhu D. Micro-/Nanotopography on bioresorbable zinc dictates cytocompatibility, bone cell differentiation, and macrophage polarization. Nano Lett. 2020;20(6):4594–4602. doi: 10.1021/acs.nanolett.0c01448. [DOI] [PubMed] [Google Scholar]
- 35.Cho S.Y., Chae S.W., Choi K.W., Seok H.K., Kim Y.C., Jung J.Y., Yang S.J., Kwon G.J., Kim J.T., Assad M. Biocompatibility and strength retention of biodegradable Mg‐Ca‐Zn alloy bone implants. J. Biomed. Mater. Res. B Appl. Biomater. 2013;101(2):201–212. doi: 10.1002/jbm.b.32813. [DOI] [PubMed] [Google Scholar]
- 36.Shuai C., Li S., Peng S., Feng P., Lai Y., Gao C. Biodegradable metallic bone implants. Mat Chem Front. 2019;3(4):544–562. [Google Scholar]
- 37.Sanchez A.H.M., Luthringer B.J., Feyerabend F., Willumeit R. Mg and Mg alloys: how comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015;13:16–31. doi: 10.1016/j.actbio.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Walker J., Shadanbaz S., Kirkland N.T., Stace E., Woodfield T., Staiger M.P., Dias G.J. Magnesium alloys: predicting in vivo corrosion with in vitro immersion testing. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100(4):1134–1141. doi: 10.1002/jbm.b.32680. [DOI] [PubMed] [Google Scholar]
- 39.Witte F., Fischer J., Nellesen J., Crostack H.-A., Kaese V., Pisch A., Beckmann F., Windhagen H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27(7):1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 40.Su Y., Zheng Y., Tang L., Qin Y.-X., Zhu D. Springer; 2017. Calcium Phosphate Coatings for Metallic Orthopedic Biomaterials, Orthopedic Biomaterials; pp. 167–183. [Google Scholar]
- 41.Guan X., Xiong M., Zeng F., Xu B., Yang L., Guo H., Niu J., Zhang J., Chen C., Pei J. Enhancement of osteogenesis and biodegradation control by brushite coating on Mg–Nd–Zn–Zr alloy for mandibular bone repair. ACS Appl. Mater. Interfaces. 2014;6(23):21525–21533. doi: 10.1021/am506543a. [DOI] [PubMed] [Google Scholar]
- 42.Niu J., Yuan G., Liao Y., Mao L., Zhang J., Wang Y., Huang F., Jiang Y., He Y., Ding W. Enhanced biocorrosion resistance and biocompatibility of degradable Mg–Nd–Zn–Zr alloy by brushite coating. Mater. Sci. Eng. C. 2013;33(8):4833–4841. doi: 10.1016/j.msec.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Guan S., Wang X., Ren C., Wang L. In vitro degradation and mechanical integrity of Mg–Zn–Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta Biomater. 2010;6(5):1743–1748. doi: 10.1016/j.actbio.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Wang H., Guan S., Wang Y., Liu H., Wang H., Wang L., Ren C., Zhu S., Chen K. In vivo degradation behavior of Ca-deficient hydroxyapatite coated Mg–Zn–Ca alloy for bone implant application. Colloids Surf., B. 2011;88(1):254–259. doi: 10.1016/j.colsurfb.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 45.Han J., Wan P., Sun Y., Liu Z., Fan X., Tan L., Yang K. Fabrication and evaluation of a bioactive Sr–Ca–P contained micro-arc oxidation coating on magnesium strontium alloy for bone repair application. J. Mater. Sci. Technol. 2016;32(3):233–244. [Google Scholar]
- 46.Shadanbaz S., Walker J., Woodfield T.B., Staiger M.P., Dias G.J. Monetite and brushite coated magnesium: in vivo and in vitro models for degradation analysis. J. Mater. Sci. Mater. Med. 2014;25(1):173–183. doi: 10.1007/s10856-013-5059-2. [DOI] [PubMed] [Google Scholar]
- 47.Muster T., Hughes A., Furman S., Harvey T., Sherman N., Hardin S., Corrigan P., Lau D., Scholes F., White P. A rapid screening multi-electrode method for the evaluation of corrosion inhibitors. Electrochim. Acta. 2009;54(12):3402–3411. [Google Scholar]
- 48.Fischer J., Pröfrock D., Hort N., Willumeit R., Feyerabend F. Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng., B. 2011;176(11):830–834. [Google Scholar]
- 49.Wang J., Witte F., Xi T., Zheng Y., Yang K., Yang Y., Zhao D., Meng J., Li Y., Li W., Chan K., Qin L. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015;21:237–249. doi: 10.1016/j.actbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Su Y., Cockerill I., Wang Y., Qin Y.X., Chang L., Zheng Y., Zhu D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019;37(4):428–441. doi: 10.1016/j.tibtech.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Y., Yang H., Gao J., Qin Y.X., Zheng Y., Zhu D. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. 2019;6(14):1900112. doi: 10.1002/advs.201900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feyerabend F., Wendel H.-P., Mihailova B., Heidrich S., Agha N.A., Bismayer U., Willumeit-Römer R. Blood compatibility of magnesium and its alloys. Acta Biomater. 2015;25:384–394. doi: 10.1016/j.actbio.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Fu J., Su Y., Qin Y.-X., Zheng Y., Wang Y., Zhu D. Evolution of metallic cardiovascular stent materials: a comparative study among stainless steel, magnesium and zinc. Biomaterials. 2020;230:119641. doi: 10.1016/j.biomaterials.2019.119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowen P.K., Drelich J., Goldman J. A new in vitro–in vivo correlation for bioabsorbable magnesium stents from mechanical behavior. Mater. Sci. Eng. C. 2013;33(8):5064–5070. doi: 10.1016/j.msec.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 55.Kasemo B. Biological surface science. Surf. Sci. 2002;500(1–3):656–677. [Google Scholar]
- 56.Surmenev R.A., Surmeneva M.A. A critical review of decades of research on calcium phosphate–based coatings: how far are we from their widespread clinical application? Current Opinion in Biomedical Engineering. 2019;10:35–44. [Google Scholar]
- 57.Xu L., Pan F., Yu G., Yang L., Zhang E., Yang K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009;30(8):1512–1523. doi: 10.1016/j.biomaterials.2008.12.001. [DOI] [PubMed] [Google Scholar]