Abstract
N6‐methyladenosine (m6A) RNA modification, first discovered in 1974, is the most prevalent, abundant and penetrating messenger RNA (mRNA) modification in eukaryotes. This governs the fate of modified transcripts, regulates RNA metabolism and biological processes, and participates in pathogenesis of numerous human diseases, especially in cancer through the reciprocal regulation of m6A methyltransferases (“writers”) and demethylases (“erasers”) and the binding proteins decoding m6A methylation (“readers”). Accumulating evidence indicates a complicated regulation network of m6A modification involving multiple m6A‐associated regulatory proteins whose biological functions have been further analysed. This review aimed to summarize the current knowledge on the potential significance and molecular mechanisms of m6A RNA modification in the initiation and progression of cancer.
N6‐methyladenosine (m6A) RNA modification in human cancer. m6A modification is a dynamic and reversible process. m6A methylation is catalysed by methyltransferase complexes (writers), reversed by demethylases (erasers) and functionally facilitated by m6A‐binding proteins (readers). m6A methylation participates in carcinogenesis and tumor progression.
![]()
1. INTRODUCTION
More than 170 kinds of chemical modifications have been found in various types of RNAs. 1 The common examples of RNA modification include N6‐methyladenosine (m6A), 5‐hydroxymethylcytosine (m5C), 1‐methyladenine, N7‐methylguanosine, N6, 2‐O‐dimethyladenosine (m6Am), inosine, 8‐oxo‐7,8‐dihydroguanosine, pseudouridine (Ψ), 2′‐O‐methylation and so forth, 2 , 3 which confer instinct or complicated manifestations to pathophysiologic changes by embedding additional transcript information into their base sequences. Among these, m6A methylation is the primary form of post‐transcriptional modification of RNAs, accounting for 0.1%‐0.4% of adenylation in mammalian RNAs and 50% of all methylated ribonucleotides. 4 , 5 m6A methylation is installed and exerted effects through methyltransferases (writers), demethylases (erasers) and m6A‐binding proteins (readers) to regulate the post‐transcription of genes without altering base sequences. 6 The profile and characteristic of m6A modification remained unidentified due to technical bottleneck and less knowledge about regulators involved, until the first demethylases fat‐mass and obesity‐associated protein (FTO) was reported in 2011, triggering the upsurge of RNA epigenetic transcriptome researches.
m6A methylation was discovered in purified poly(A) RNA fraction in 1974. 4 It is the most pervasive and abundant internal modification in eukaryotic cells, which has been confirmed by other researchers in various eukaryotes, from yeast, Arabidopsis and Drosophila to mammals, and even in viruses. Researchers detected an enormous number of highly conserved m6A sites and also determined more than 12 000 m6A signal peaks on 7676 mammalian genes by m6A sequencing. 8 m6A messenger RNA (mRNA) methylation primarily appears in the consensus motif of RRm6ACH ([G/A/U][G>A]m6AC[U>A>C]) and is enriched in the transcription initiation region, coding sequence (CDS) and 3′‐untranslated regions (3′‐UTR), especially around stop codons of the CDS and the first quarter of 3′‐UTR. 9 m6A methylation is also found near the start codon in Arabidopsis thaliana. 10 In mammals, m6A modification exists widely in many tissues, with the highest level of m6A modification in the liver, kidney and brain, 9 showing a character of tissue universality and preference.
m6A modification was initially thought to exist only in mRNAs primarily due to the limitations of detection techniques. Subsequent studies found that m6A methylation occurred in various types of RNAs, such as small ribosome RNA, transfer RNA, small nuclear RNA (snRNA), small nucleolar RNA, microRNA (miRNA), long non‐coding RNA (lncRNA) and circular RNA. 11 , 12 The functions and roles of m6A methylation in different biological processes have gained renewed attention. m6A modification can regulate RNA metabolism (Figure 1), affect cell fate and functionally participate in a variety of pathophysiological processes, such as stem cell differentiation, cell division, immune homeostasis, mitosis, gametogenesis, sex determination and biological rhythm, and occurrence of numerous human diseases. 13 , 14 In this review, starting from m6A modification, the related regulators and research progresses on tumours were elaborated, which were expected to become novel markers for molecular diagnosis and potential therapeutic strategies for tumours.
Figure 1.
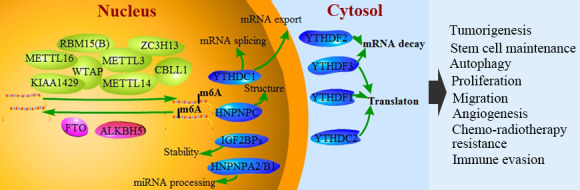
N6‐methyladenosine (m6A) RNA modification in human cancer. m6A modification is a dynamic and reversible process. m6A methylation is catalysed by methyltransferase complex (writers), reversed by demethylases (erasers) and functionally facilitated by m6A‐binding proteins (readers). m6A methylation participates in carcinogenesis and tumour progression
1.1. m6A‐Writers
The deposition of m6A modification is catalysed by a multicomponent methyltransferase complex composed mainly of methyltransferase‐like protein 3 (METTL3), methyltransferase‐like protein 14 (METTL14) and Wilms’ tumour 1‐associated protein (WTAP), which are all the earliest known writer proteins. 15 , 16 Subsequently, more new writers, such as methyltransferase‐like protein 16 (METTL16), zinc finger CCCH domain–containing protein 13 (ZC3H13), KIAA1429 (VIRMA), RNA‐binding motif protein 15 (RBM15) and CBLL1 (an E3 ubiquitin ligase) were reported. 1 , 17 METTL3 is an S‐adenosylmethionine (SAM)–binding protein and a core component of methyltransferase complex 15 ; it can identify potential m6A sites and transfer the methyl groups attached on SAM to these sites. METTL14 is another component of the m6A methyltransferase complex. Structural studies indicated that METTL3 interacted with METTL14 to form a stable heterodimer structure in the ratio of 1:1 to contribute to recognizing target RNAs. 18 The physical relationship between METTL3 and METTL14 has a synergistic effect; METTL14 can increase the activity of METTL3 methyltransferase. 18 Knocking down METTL3 or METTL14 in mouse embryonic stem cells can eliminate up to 99% of total m6A level at thousands of sites. 19 WTAP without catalytic methylation domain and methylase activity is also a component of methyltransferase complex and required for the localization of METTL3‐METTL14 heterodimers to nuclear speckles and the activation of methyltransferase complexes, thereby promoting m6A deposition. 18 , 20 It is responsible for the recruitment of other m6A writers to target RNAs. 18 ZC3H13 can potentiate the immobilization of WTAP on the nucleus and subsequently strengthen the methyltransferase complexes, 21 assembling enough complexes for the m6A modification. METTL16, recently proposed as a methyltransferase, can bind to U6 snRNA, non‐coding RNA (ncRNA) and precursor messenger RNA (pre‐mRNA), and participate in regulating intracellular homeostasis and mRNA splicing. 22 , 23 KIAA1429 is found to catalyse the m6A modification. The homologous proteins of KIAA1429 and WTAP in Drosophila melanogaster regulates sex determination by the selective splicing of pre‐mRNAs, affecting Sex‐lethal gene expression. 24 , 25 The knockdown of KIAA1429 results in a decrease of in the m6A content, which is greater than that achieved by METTL3 and METTL14 knockdown in A549 cells. 26 RBM15 and its paralog RBM15B can bind to the U‐rich region and catalyse m6A modification in some mRNAs and lncRNA XIST. 27 In 2018, ZC3H13 was identified as a new m6A writer in mice and D melanogaster.
1.2. m6A‐Erasers
Until today, only two m6A demethylases, FTO and ALKB homolog 5 (ALKBH5), have been identified, both of which belong to the ALKB dioxygenase family and rely on the cofactors Fe2+ and α‐ketoglutarate to execute catalytic functions. FTO was initially identified as an obesity‐related gene by genome‐wide association studies. 28 FTO was the first m6A demethylase discovered in 2011, which confirmed that m6A modification was a dynamic and reversible process. 7 FTO affects mRNA stability and translation efficiency by regulating m6A modification. 29 In 2019, FTO was verified to demethylate the m6Am modification of snRNA and modulate alternative splicing of mRNAs. 30 FTO‐mediated m6A demethylation involves two steps concomitant with two intermediates, N6‐hydroxymethyladenosine and N6‐formyladenosine. 31 ALKBH5 belongs to the ALKB family, but unlike other AIkBs, only ALKBH5 can demethylate m6A modification. 32 ALKBH5 can directly catalyse the methylation of m6A‐modified adenosines without producing intermediates. 33 The expression patterns of FTO and ALKBH5 are different. FTO exists widely in adults and embryos, especially in the brain, while ALKBH5 is expressed in testis. Besides FTO and ALKBH5, more m6A demethylases need to be further discovered.
1.3. m6A‐Readers
m6A‐binding proteins mainly include YT521‐B homology (YTH) domain proteins including two subtypes of YTH domain–containing family protein (YTHDFs; YTHDF1/2/3) and YTH domain–containing proteins (YTHDCs; YTHDC1/2); all have a conserved m6A‐binding domain and preferentially bind to the consensus RRm6ACH sequence. 27 , 34 Besides the YTH domain family, other readers, certain members of the heterogeneous nuclear ribonucleoprotein (HNRNP) family (HNRNPA2B1, HNRNPC and HNRNPG), insulin‐like growth factor 2 mRNA‐binding proteins (IGF2BPs, including IGF2BP1/2/3) and eukaryotic translation initiation factor 3 (eIF3) have been found. 35 , 36 Reader proteins displaying a 10‐ to 50‐fold enhancement of m6A‐modified mRNA‐binding affinity over unmodified mRNA exercise various downstream effects by interacting with modified RNAs and encode m6A modification information. 37 YTHDF1 promotes the ribosome loading of m6A‐modified RNA and improves targeted RNA translation by interacting with translation initiation factors. 34 YTHDF2 anchors mRNA in decay site processing bodies, inducing mRNA degradation. 38 Interestingly, YTHDF3 combined with YTHDF1 can enhance mRNA translation, while YTHDF3 combined with YTHDF2 can promote its degradation. 39 , 40 However, other studies found that YTHDF2 binding to the m6A site prevented FTO from removing m6A methylation in the 5′‐UTR region, thereby promoting the cap‐independent translation of mRNAs. 41 Besides, HNRPNPA2/B1 is involved in the transcription of miRNA precursors (pre‐miRNA). 35 However, HNRPNPC can affect the secondary structures of mRNAs and lncRNAs. 6 m6A modification can destroy base pairing and improve the accessibility of single‐stranded RNA motifs, thus being recognized by HNRPNPC and HNRPNPG. 6 , 42 Although HNRNPC and HNRNPG cannot directly bind to the m6A site, they can mediate the selective splicing of m6A‐modified transcripts by recognizing and combining m6A‐dependent structural switches. 6 , 43 Prrc2a has been recently confirmed as an m6A reader, and recombinant Prrc2a can stabilize m6A‐modified transcripts required for myelin formation. 44 The newly discovered IGF2BPs are considered to belong to the m6A reader family. IGF2BP2 selectively binds to m6A‐modified mRNA via K homolog and flanking domains, promoting the translation and stability of mRNA, which is quite different from that of readers with the YTH domain. 45 , 46
2. M6A AND CANCERS
The list of pathophysiology processes regulated by m6A modification continues to expand with increasing research and technological breakthroughs. It includes mRNA metabolism, immune modulation, biological rhythm, neural development, autophagy, R‐loop regulation, embryonic and reproductive development and various diseases. 11 , 47 , 48 The m6A modification can be traced from systemic lupus erythematosus, 49 single nucleotide polymorphism (genetic variant), 50 type 2 diabetes, 51 inflammatory response and so on. 52
Recently, emerging evidence indicates the crucial parts of m6A methylation in carcinogenesis and tumour progression by regulating the expression of oncogenes and tumour suppressor genes. 1 , 14 , 53 Analogous to other epigenetic modifications, m6A methylation participates in cell proliferation, apoptosis, migration, angiogenesis, autophagy, chemoradiotherapy resistance, energy metabolism, immune escape, self‐renewal and differentiation during the initiation and progression of cancers. 54 , 55 Inhibitors of m6A regulators have been found and identified as potential inhibitors of cancer progression, suggesting that m6A might be a potential target for cancer treatment. The article will review the relationships between m6A modification and various types of tumours were discussed further (Table 1, Figure 2).
Table 1.
Roles of m6A regulators in different cancers
| Cancer | m6A regulator | Role in cancer | Mechanism | References |
|---|---|---|---|---|
| AML | METTL3 | Oncogene | Promotes AML progression by promoting the translation of c‐MYC, BCL‐2 and PTEN mRNAs | 57 |
| METTL3 | Oncogene | Be recruited by CEBPZ to promoters of a specific set of active gene (SP1) and increased translation, resulting in the maintenance of a leukaemic state | 58 | |
| METTL14 | Oncogene | Enhances MYB and MYC expression, promotes myeloid differentiation of HSPCs and AML cells and leukaemogenesis | 56 | |
| WTAP | Oncogene | Induces abnormal proliferation and arrested differentiation of leukaemia cells | 24 | |
| FTO | Oncogene | Promotes leukaemic oncogene‐mediated cell transformation and leukemogenesis and weakens the therapeutic effect of ATRA | 62 | |
| FTO | Oncogene | Be inhibited by R‐2HG, resulting in anti‐tumour effect via targeting FTO/m6A/MYC/CEBPA signalling | 63 | |
| Glioblastoma | METTL3, METTL14 | Tumour suppressor | Inhibits GSC self‐renewal and tumorigenesis by down‐regulating oncogenes (ADAM19, EPHA3 and KLF4) and up‐regulating tumour suppressor genes (CDKN2A, BRCA2 and TP53I11) | 68 |
| WTAP | Oncogene | Be correlated with glioma grade and poor postoperative survival in glioma patients | 71 | |
| ALKBH5 | Oncogene | Maintains tumorigenicity of GSC by sustaining FOXM1 expression and cell proliferation programme | 72 | |
| BC | METTL3 | Oncogene | Promotes cell proliferation via HBXIP/let‐7g/METTL3/HBXIP positive feedback loop | 74 |
| KIAA1429 | Oncogene | Promotes BC progression by regulating CDK1 by an m6A‐independent manner | 75 | |
| ALKBH5 | Oncogene | Promotes NANOG mRNA and protein expression and mediates enrichment of BCSCs in the hypoxic tumour microenvironment | 76 | |
| FTO | Oncogene | Promoted cell proliferation, colony formation and metastasis by abrogating the m6A modification of BNIP3 mRNA and epigenetically down‐regulating BNIP3 | 78 | |
| HCC | METTL3 | Oncogene | Promoted cell proliferation, colony formation and metastasis by abrogating the m6A modification of BNIP3 mRNA and epigenetically down‐regulating BNIP3 | 79 |
| METTL14 | Tumour suppressor | Be inversely correlated with OS and RFS of HCC patients and inhibits tumour metastasis in vitro and in vivo by modulating the pri‐miRNA 126 process in an m6A‐dependent manner via interacting with DGCR8 | 81 | |
| FTO | Oncogene | Be correlated with poor prognosis of HCC individuals and promotes tumorigenesis by mediating demethylation of PKM2 mRNA | 83 | |
| Hepatoblastoma | METTL3 | Oncogene | Serves as a diagnostic and prognostic biomarker and promotes tumour growth in vivo by regulating CTNNB1 expression via m6A modification and activating Wnt/β‐catenin signalling | 80 |
| GC | METTL3 | Oncogene | P300‐mediated H3K27 acetylation promotes METTL3 expression, which mediates m6A modification on HDGF mRNA and enhances its stability by an IGF2BP3‐dependent manner, resulting in tumour angiogenesis, GC growth and liver metastasis | 89 |
| FTO | Oncogene | Be an increased expression in 375 GC from TCGA data set | 92 | |
| CRC | METTL3 | Tumour suppressor | Acts as an independent prognostic factor and suppresses cell proliferation and migration through p38/ERK pathways | 94 |
| METTL3 | Oncogene | Mediates m6A modification of CBX8 mRNA and promotes its expression, which maintains the stemness and inhibits the chemosensitivity of CRC | 95 | |
| METTL3 | Oncogene | Promotes lncRNA RP11 expression by an m6A‐dependent manner, which targets Siah1/Fbxo45/Zeb1 axis to drive cell dissemination and development of CRC | 96 | |
| FTO | Oncogene | Low expression of microRNA‐1266 promotes CRC progression via targeting FTO | 97 | |
| YTHDC2 | Oncogene | Be correlated with CRC progression and promotes the cell metastatic ability via promoting translation of HIF‐1α | 99 | |
| Lung cancer | METTL3 | Oncogene | miR‐33a or miR‐600 suppresses cell proliferation, migration and invasion by targeting METTL3 | 101, 102 |
| ALKBH5 | Oncogene | Promotes cell proliferation and invasion by decreasing m6A level on FOXM1 mRNA and increasing its translation under intermittent hypoxia | 106 | |
| FTO | Oncogene | Promotes cell proliferation by increasing the stability of USP7 mRNA | 104 | |
| FTO | Oncogene | Promotes progression of lung squamous cell carcinoma by reversing m6A modification of MZF1 mRNA and increasing its stability | 105 | |
| Bladder cancer | METTL3 | Oncogene | Be correlated with poor prognosis of bladder cancer patients and promotes cell proliferation in vitro and in vivo and tumorigenesis by modulating the pri‐miR221/222 process in an m6A‐dependent manner via interacting DGCR8 | 108 |
| METTL3 | Oncogene | Promotes bladder cancer progression via AFF4/NF‐kappaB/MYC signalling network | 109 | |
| RCC | METTL14 | Tumour suppressor | m6A (METTL14) suppressed P2RX6 activation promotes cell migration and invasion through ATP‐induced Ca2 + influx modulating ERK1/2 phosphorylation and MMP9 signalling pathway | 111 |
| WTAP | Oncogene | Be related to poor prognosis of RCC patients and stabilizes CDK2 transcript to enhance CDK2 expression to promote cell proliferation and tumorigenesis in vivo | 112 | |
| Pancreatic cancer | METTL3 | Oncogene | Be correlated with poor prognosis of patients with pancreatic cancer and promotes cell proliferation, invasion, migration and chemo‐ and radioresistance | 114, 115 |
| WTAP | Oncogene | Be associated with poor overall survival for pancreatic ductal adenocarcinoma | 116 | |
| ALKBH5 | Tumour suppressor | Inhibits pancreatic cancer motility by decreasing lncRNA KCNK15‐AS1 methylation | 118 | |
| FTO | Oncogene | Promotes cell proliferation by enhancing stability of c‐Myc mRNA | 117 | |
| YTHDF2 | Unknown | Promotes cell proliferation possibly via Akt/GSK3β/Cyclin D1 pathway and inhibits cell migration and invasion probably via up‐regulation of total YAP | 119 | |
| Cervical cancer | FTO | Oncogene | Contributes to the chemoradiotherapy resistance of cervical squamous cell carcinoma through up‐regulation β‐catenin via mRNA demethylation and the subsequent activation of ERCC1 | 122 |
| FTO | Oncogene | Be correlated with cervical cancer progression and promotes cell proliferation and migration via controlling m6A modification of E2F1 and Myc transcripts | 123 | |
| Endometrial cancer | METTL3, METTL14 | Tumour suppressor | Inhibits cell proliferation, anchorage‐independent growth, and migration and in vivo tumour growth through activation of the AKT pathway | 120 |
| Ovarian epithelial cancer | ALKBH5 | Oncogene | Be related to poor OS and progression‐free survival, promotes cell proliferation and invasion, and inhibits autophagy through the interaction between Bcl‐2 and Beclin‐1 and the EGFR‐PI3K‐AKT‐mTOR pathway | 124 |
| Cutaneous cancer | METTL3 | Oncogene | Promotes colony formation and invasion of melanoma cells by regulating MMP2 | 125 |
| METTL3 | Oncogene | Promotes cSCC cell colony‐forming ability in vitro and tumorigenicity in vivo through modulating ΔNp63 in an m6A‐dependent manner | 126 | |
| Nasopharyngeal carcinoma | METTL3 | Oncogene | Mediates m6A modification of ZNF750 mRNA and blocks its expression, which promotes cell growth through regulating FGF14 | 127 |
| Osteosarcoma | METTL3 | Oncogene | Promotes cell progression by regulating the m6A level of LEF1 and activating Wnt/β‐catenin signalling pathway | 128 |
| Cholangiocarcinoma | WTAP | Oncogene | Increases cell motility and tumorigenicity in vivo | 129 |
Figure 2.
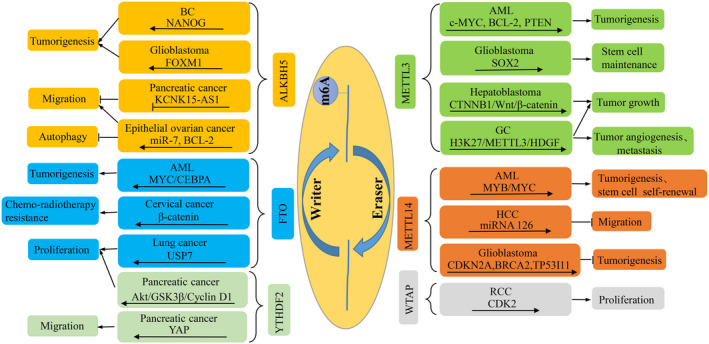
Multiple mechanisms of m6A regulators in cancer. Abnormal expression of m6A writers, erasers and readers has multiple roles in various types of cancers
2.1. m6A in leukemia
METTL3 and METTL14 are highly expressed in acute myeloid leukaemia (AML) cells compared with normal hematopoietic progenitor cells. 56 , 57 METTL3 inhibits cell differentiation and apoptosis and promotes cell proliferation in AML. 57 , 58 Two possible pathways are responsible for the involvement of METTL3 in the initiation and progression of AML. Firstly, METTL3 increases the expression of the target genes, c‐MYC, BCL‐2 and PTEN, by m6A methylation‐mediated translation of mRNAs. 57 Second, METTL3 combines with the CCAAT‐box‐binding factor of m6A‐modified mRNAs in a CEBPZ‐dependent manner, promoting AML deterioration. 58 METTL14 is essential in the self‐renewal of leukaemic stem/progenitor cells. 59 In SPI1‐METTL14‐MYB/MYC axis, METTL14 is down‐regulated by SPI1 and enhances MYB and MYC expression through m6A modification. It is carcinogenic in enhancing the self‐renewal of haematopoietic stem and progenitor cells (HSPCs) and inhibiting bone marrow differentiation. 56 RBM15 can maintain the differentiation of HSPCs and megakaryocytic leukaemia cells by controlling mRNA splicing of the crucial differentiation‐related genes including GATA1, RUNX1, TAL1 and C‐MPL. 60 , 61 WTAP is also described as a new carcinogen of AML. Increased expression of WTAP promotes cell proliferation and inhibits cell differentiation in AML. 24 m6A writers, METTL3, METTL14 and WTAP, elicit potent oncogenic effects in AML, which provide new thought for AML therapy from a multitude of perspectives.
Most noteworthy, erasers and writers provide complementary mechanisms in AML progression. FTO also acts as an oncogene in AML. 62 It is highly expressed in some subtypes of AML, such as t (11q23)/MLL‐rearrangement, t (15; 17)/PML‐RARA, FLT3‐ITD and/or NPM1 mutation. It promotes cell proliferation and inhibits differentiation and apoptosis in AML. 62 It can inhibit all‐trans‐retinoic acid (ATRA)–mediated leukaemia cell differentiation and weaken the therapeutic effect of ATRA. 62 R‐2‐hydroxyglutarate (R‐2HG), the metabolite of isocitrate dehydrogenase 1 and 2 (IDH1/2) mutant, suppresses cell proliferation, induces apoptosis and displays anti‐leukaemic activity by targeting the FTO/m6A/MYC/CEBPA axis. 63 , 64 Mechanically, R‐2HG binds to FTO and competitively suppresses its demethylase activity, thus resulting in the up‐regulation of global m6A modification, which in turn impairs the stability of MYC/CEBPA mRNAs and MYC/CEBPA involving signalling pathways in leukaemia cells sensitive to R‐2HG. 63 , 64 FTO depletion reduces the sensitivity of leukaemic cells to R‐2HG. IDH mutations can weaken FTO activity in about 20% of patients with AML; they improve m6A modification without affecting FTO expression, 65 which provides a novel target for the clinical treatment of leukaemia. YTHDF2 is not necessary to maintain the function of hematopoietic stem cells (HSCs); on the contrary, the absence of YTHDF2 helps in enhancing the activity of HSCs. 66
Currently, conclusions on the m6A regulation of tumour progression are inconsistent. METTL3 can bind to the promoter region of target genes and promote translation in an m6A‐dependent manner, causing AML deterioration. 58 However, increased FTO can decrease the tumour suppressor genes ASB2 and RARA and promote the proliferation of AML cells. 62 Given the inconsistent role of METTL3 and FTO in tumour progression, it is presumed that the discrepancy may be due to METTL3 and FTO acting on different downstream targets. m6A modification can regulate the occurrence and development of AML by regulating the mRNAs of critical genes in AML. 67 Compared with other epigenetic modifications, the m6A methylation in AML can be easily targeted chemical medications, thus providing a theoretical basis for the investigation of new drugs against AML. 67 Inhibiting METTL3/14 may be a novel strategy for the treatment of malignant myeloid tumours.
2.2. m6A in glioblastoma
At present, the role of METTL3 in the progression of glioblastoma is still controversial. The blockade of METTL3 or METTL14 enhances the proliferation, self‐renewal and tumorigenesis of glioblastoma stem cells (GSCs) concomitant with increased expressions of the ADAM19, EPHA3 and KLF4, and decreased expression of the tumour suppressor genes TP53I11, CDKN2A and BRCA2. 68 Wang et al reported that m6A modification was weaker after knocking out METTL14 in GSCs than knocking out METTL3. 69 Enforced expression of METTL3 or treatment with FTO inhibitor (MA2) impairs GSC‐initiated tumorigenesis and prolongs the lifespan of GSC‐grafted mice, 68 implying that METTL3 may serve as an anti‐oncogene in the development of GSCs. Paradoxically, other studies proved that METTL3 was amplified in GSCs and was required for GSC maintenance. METTL3 promotes tumour growth and predicts poor survival in patients with glioblastoma. 70 Besides, Xi et al 71 found that increased WTAP is associated with the prognosis in glioblastoma patients. The role of METTL3 and its exact molecular mechanism in GSCs still needs further exploration, which encourages researchers to reveal treatment prospects.
As other m6A writers, ALKBH5 participates in the stemness of GSCs. Increased ALKBH5 indicates poor prognosis of patients with glioblastoma while ALKBH5 depletion interferes with self‐renewal ability and inhibits the proliferation and tumorigenesis of GSCs by sustaining FOXM1 expression via ALKBH5‐mediated m6A demethylation of FOXM1 nascent transcripts. 72 In 2019, Chai et al showed that methylation‐related enzymes were closely associated with malignant progression, mesenchymal subtypes and temozolomide sensitivity in glioma through the analysis of RNA sequencing data from 904 glioma samples, providing paramount evidence for the role of m6A methylation in glioma progression, prognostic stratification and treatment development. 73
2.3. m6A in breast cancer (BC)
Increasing numbers of studies have reported that abnormal m6A is involved in BC initiation and progression. HBXIP promotes METTL3 expression through inhibiting tumour suppressor, miRNA let‐7g that targets the 3′‐UTR of METTL3 mRNA. In turn, METTL3 increases the HBXIP level via stimulating its m6A modification, which creates a positive feedback loop of HBXIP/let‐7g/METTL3/HBXIP, thus accelerating the malignant proliferation of BC cells. 74 KIAA1429 regulates CDK1 expression in an m6A‐independent manner to promote BC progression.
Zhang C et al found that HIF‐1α and HIF‐2α determined ALKBH5 expression in BC stem cells (BCSCs) exposed to hypoxia. ALKBH5 enhances NANOG expression by abating m6A modification and accelerates the amplification of BCSCs in hypoxic microenvironment, 76 illustrating the cancer‐promoting function of ALKBH5 in BC. ALKBH5 deletion reverses hypoxia‐induced BCSCs enrichment and tumorigenesis. 72 Further research showed a complementary role of ALKBH5 and ZNF217 in the negative regulation of m6A modification. Although ALKBH5 can induce the m6A demethylation of NANOG mRNA, hypoxia‐induced ZNF217 inhibits the m6A methylation of NANOG mRNA, maintaining the stability of NONG and KLF4 mRNAs, which ultimately accelerates the malignant progression of BCSCs. 77 Both of them can elevate the levels of NANOG mRNA and protein, besides enriching BCSCs. 77 These findings confirmed the role of ALKBH5 in promoting the carcinogenesis of BC. FTO can potentiate the growth and metastasis of BC cells by abrogating the m6A modification of BNIP3 mRNA. 78 It suggests that both writers and erasers of m6A seem to show cancer‐promoting effects on BC.
2.4. m6A in hepatocellular carcinoma (HCC)
Most of the m6A‐related enzymes are reported to be up‐regulated in HCC, but METTL14 and YTHDF2 are still debatable. METTL3 is significantly elevated in HCC and associated with poor prognosis in patients with HCC. 79 METTL3 reduction inhibits cell migration, colony formation and proliferation in vitro and lung metastasis and tumorigenicity of HCC in vivo. 79 Mechanistically, METTL3 potentiates the degradation of SOCS2 mRNA (tumour suppressor gene) via the m6A‐YTHDF2–dependent pathway. 79 Equally, METTL3‐mediated m6A modification activates Wnt/β‐catenin signalling by promoting CTNNB1 expression to drive the growth of hepatoblastoma. 80 Ma et al demonstrated that an anti‐cancer role of METTL14 in HCC. METTL14 has significantly lower expression in HCC tissues compared with adjacent tissues. Decreased METTL14 expression is associated with poor prognosis and short recurrence time in 130 patients with patients. 81 Moreover, METTL14 expression has a negative correlation with the proliferation ability of HCC cells. 81 METTL14 can regulate primary transcripts of miRNA (pri‐miRNA) 126 maturation by interacting with DGCR8. Targeted inhibition of miRNA 126 can reverse the inhibitory effect of METTL14 on HCC progression. 81 On the contrary, Chen et al found that the expression of METTL14 slightly increased in HCC, and knockdown of METTL14 markedly inhibited the proliferation and metastasis of HCC cells. 79 KIAA1429 inhibits ID2 expression and promotes the invasion and metastasis of HCC cells. 82 FTO is linked to an unfavourable prognosis in patients with HCC. It enhances the translation of PKM2 mRNA by relieving m6A modification and accelerates HCC aggressiveness. 83 YTHDF1 is strongly expressed in HCC and closely related to a poor prognosis. 84 , 85 Downstream molecules regulated by YTHDF1 are implicated in cell cycle, degradation of amino acids and metabolism of lipids. 84 m6A regulators are more prone to regulate the stability, translation and splicing of mRNA in HCC, and these molecules can be regarded as potential biomarkers and prospective therapeutic targets of HCC.
2.5. m6A in gastric cancer (GC)
METTL3 can serve as an oncogene‐promoting GC tumorigenesis and progression. 86 , 87 , 88 , 89 P300‐mediated H3K27 acetylation activation in the METTL3 promoter accounts for the ectopic expression of METTL3 in GC, leading to the enrichment m6A modification on HDGF mRNA. IGF2BP3 subsequently recognizes and binds to the methylated HDGF mRNA and augments the stability of HDGF mRNA. 89 Secreted HDGF drives tumour angiogenesis, while nuclear HDGF elevates ENO2 and GLUT4 levels, followed by a potentiation of the glycolysis ability, resulting in GC growth and liver metastasis. 89 miRNA‐4429 inhibits GC progression by targeting METTL3 to abrogate the m6A‐mediated stabilization of SEC62 mRNA. 86 Liu et al reported that the expression of mRNAs of METTL3, rather than METTL14, FTO, and ALKBH5, was significantly elevated in GC tissues compared with paired normal tissues; decreased expression of METTL3 suppressed the migration and proliferation of GC cells. 87 Paradoxically, Xu et al 90 found that the mRNA and protein levels of FTO were significantly higher in GC tissues than in adjacent tissues. Increased FTO is closely related to poor differentiation, lymph node metastasis, TNM stage and an unfavourable prognosis, and the down‐regulation of FTO inhibits the proliferation, invasion and metastasis of GC cells. 90 , 91 The expression of most the 13 widely reported m6A regulators, especially FTO, increased in 375 GC cases from the TCGA data set. 91 Jeopardizing m6A modification was associated with oncogene signalling and phenotypes by scrutinizing bioinformatics, 92 and overriding m6A with METTL14 silencing accelerated the proliferation and invasion of GC cells by activating Wnt/PI3K/Akt signalling, which was reversed by FTO depletion. 92 In GC, low YTHDF2 expression retarded cell proliferation and promote cell apoptosis. 93 Conclusively, histone modification and ncRNAs affects the expression and regulation of METTL3, contributing to better understand the physiological functions and regulatory mechanisms of METTL3 in cancers. The recent researches mainly concentrate on the role of m6A regulators in GC and whether the expression of m6A‐related proteins is up‐regulated in the early stage of GC needs further study.
2.6. m6A in colorectal cancer (CRC)
Increased METTL3 expression acts as an independent and favourable prognostic marker in CRC and inhibits cell proliferation, migration and invasion by activating p38/ERK signalling. 94 METTL3 potentiates cell stemness properties in vitro and accelerates tumorigenesis and metastasis of CRC in vivo, which can be deciphered by IGF2BP2 that recognizes methylated SOX2 and maintains the stability of SOX2 mRNA. 94 Similarly, CBX8 potentiates stemness properties and alleviates chemosensitivity to irinotecan (CPT‐11, a camptothecin derivative) and oxaliplatin (L‐OHP, a platinum‐based chemotherapeutic) in colon cancer cells by recruiting Pol II and KMT2b to the LGR5 promoter to enforce its expression. 95 METTL3‐mediated m6A methylation of CBX8 mRNA and IGF2BP1‐induced mRNA stability coordinately sustain CBX expression in colon cancer. 95 The lncRNA RP11‐targeted Siah1/Fbxo45/Zeb1 axis drives cell dissemination and development of CRC. The up‐regulation of lncRNA RP11 is implemented partly by m6A modification. 96 Shen et al 97 demonstrated that FTO was highly expressed in CRC tissues and negatively regulated by miRNA 1266. Collectively, it can be certain that METTL3 plays important role in CRC progression, but whether it serves an oncogene or a tumour suppressor gene is controversial.
YTHDF1 is associated with malignant phenotypes in CRC. The blockade of YTHDF1 suppresses cell proliferation and enhances the chemosensitivity of cancer cells to 5‐fluorouracil (5‐FU) and L‐OHP. Oncogene MYC promotes the transcriptional activation of YTHDF1 rather than other YTH domain family members. 98 Immunohistochemical staining showed that YTHDF2 positively correlated with the stage of CRC. 99 YTHDF2 promotes the metastasis of CRC by promoting the HIF‐1α translation, and knocking out YTHDF2 can weaken HIF‐1α expression and inhibit the metastasis of CRC cells in vitro and in vivo. 99 Liu et al recently discovered that most of the m6A regulators, except YTHDC2, showed significant differences in comparing of cancer tissues and adjacent mucosal tissues. Higher HNRNPC and lower YTHDF1 expression may act as the prognostic predictors of poor overall survival (OS) in colon cancer through excavating the TCGA data set including 331 colorectal adenocarcinoma samples. 100
2.7. m6A in lung cancer
miRNA‐33a suppresses the proliferation of non–small cell lung cancer (NSCLC) cells via targeting 3′‐UTR of METTL3. 101 METTL3 silencing expedites cell apoptosis via AKT signalling pathway and inhibits the proliferation, migration and invasion of lung cancer cells. miRNA‐600 reverses METTL3‐induced tumorigenesis and progression of lung cancer. 102 Lin et al 103 also confirmed that METTL3 promoted cell growth, proliferation and invasion in lung adenocarcinoma. Besides being an m6A “writer”, METTL3 may serve as an m6A “reader” in the cytoplasm by identifying and interacting with translation initiation factor, enhancing the translation of EGFR and TAZ in lung cancer. 103 It indicates that METTL3 conveys oncogenic signals to potentiate aggressiveness in lung cancer.
FTO acts as a proto‐oncogene in NSCLC. 104 The knockdown of FTO inhibits the proliferation of lung cancer cells by modulating the mRNA stability of USP7 via FTO‐mediated m6A demethylase. 104 Furthermore, FTO rather than METTL3, METTL14 and ALKBH5 is considered as the main factor causing the dysfunction of m6A modification in lung squamous cell carcinoma. 105 FTO can enhance the stability of MZF1 mRNA by reversing m6A modification, inducing MZF1 expression and ultimately promoting the progression of lung squamous cell carcinoma. 105 All these indicate that FTO may be a potential therapeutic target in lung cancer. Intermittent hypoxia‐induced ALKBH5 amplification in lung adenocarcinoma promotes cell proliferation and invasion by decreasing the m6A level of FOXM1 mRNA and increasing the translation of FOXM1 mRNA. 106
2.8. m6A in bladder cancer and renal cell carcinoma (RCC)
Enrichment of m6A modification is associated with bladder cancer. Bioinformatics analysis revealed that m6A regulators were associated with the malignant progression of bladder cancer. 107 METTL3 may serve as an oncogene in bladder cancer, contributes to cell proliferation and drives tumorigenesis, which is mediated by interacting with DGCR8 and subsequently accelerating the pri‐miR221/222 process in an m6A‐dependent manner concomitant with PTEN down‐regulation, and METTL3 correlates with poor prognosis and may be proposed as a potential therapeutic target for bladder cancer. 108 The ectopic expression of METTL3 potentiates bladder cancer progression through the AFF4/NF‐κB/MYC network. 109
m6A abnormity plays a crucial role in ccRCC. PI3K/mTOR and p53 pathways may function as downstream targets of m6A modification in RCC. 110 ATP/P2RX6 modulates Ca2+ influx–mediated MAPK ERK1/2 phosphorylation and MMP9 signalling to potentiate the migration and invasion of RCC cells. 111 Noteworthy, METTL14 is down‐regulated and abrogates P2RX6 expression through modulating m6A modification, which affects the malignant progression of RCC. 111 WTAP activates the proliferation of RCC cells by regulating the stability of CDK2 mRNA, leading to the occurrence and development of RCC; patients with RCC and overexpression of WTAP have a dismal prognosis. 112 According to the latest research, transcriptome‐wide m6A patterns in clear cell RCC (ccRCC) tissues are significantly different from that of neighbouring non‐cancerous tissues. A total of 6919 new m6A peaks appeared concurrent with the disappearance of 5020 peaks in ccRCC samples were compared with non‐cancerous tissues; the unique distribution profile of m6A regulated gene expression and pathways in ccRCC. 113
2.9. m6A in pancreatic cancer
The expression of METTL3 increases at both protein and mRNA levels in pancreatic cancer; METTL3 knockdown inhibits cell proliferation, invasion and migration. 114 Pancreatic cancer cells with METTL3 silencing are more susceptible to irradiation, gemcitabine, 5‐FU and L‐OHP, but cell morphology and proliferation are not affected. 115 METTL3 and METTL14 are proposed as novel and promising targets for enhancing chemosensitivity and radiosensitivity of pancreatic cancer cells. 115 In pancreatic cancer, METTL3 correlates with carcinogenesis and may act as a potential therapeutic target. 114 , 115 In pancreatic ductal adenocarcinoma, increased WTAP expression is significantly related to the sex and tumour stage and appears to be an independent and valid prognostic factor. 116 FTO markedly promotes cell proliferation, suppresses cell apoptosis and enhances mRNA stability of c‐MYC in pancreatic cancer. 117 Low expression of ALKBH5 in pancreatic cancer cells reverses the m6A modification of lncRNA KCNK15‐AS1, resulting in a decrease in the ability of cancer cells to invade and metastasize. 118
The expression of YTHDF2 is up‐regulated in pancreatic cancer and significantly associated with tumour stage. 119 YTHDF2 has a reverse regulatory effect on cell proliferation and invasion in pancreatic cancer. The down‐regulation of YTHDF2 reduce the cloning density and proliferation curve by regulating Akt/GSK3β/cyclin D1 pathway. 119 However, the down‐regulation of the expression of YTHDF2 promotes cell migration, invasion and EMT through the YAP signalling pathway. 119 The relationship between YTHDF2 and YAP in pancreatic cancer cells needs further exploration, especially whether the reverse regulation of YTHDF2 exists in other tumours.
2.10. m6A in gynecological cancer
m6A modification is frequently found at low levels in gynaecological cancers, such as endometrial cancer, 120 cervical cancer 121 , 122 , 123 and epithelial ovarian cancer. 124 Low m6A level, as an independent prognostic indicator, was found to be associated with cancer progression and poor outcome through screening 286 cervical cancer samples, and augmenting m6A modification with the FTO inhibitor MA2 suppressed the tumour development in mouse models. 121 , 122 FTO is frequently overexpressed in cervical cancer and associated with cervical cancer progression. 121 , 123 Patients with cervical cancer and co‐expression of FTO and β‐catenin have a worse prognosis. 122 FTO contributes to the proliferation and migration of cervical cancer cells by directly interacting with E2F1 and MYC transcripts and impairing their translation efficiency in a demethylase‐dependent manner. 123 Overall, it offers the possibility of FTO for a clinical diagnostic and therapeutic target in cervical cancer.
In 2018, Liu et al observed that 70% of endometrial cancer exhibited decreased m6A methylation probably through either METTL14 (R298P) mutation or METTL3 down‐regulation. 120 Reduced m6A methylation is accompanied by increased expression of mTORC2 and decreased expression of PHLPP2 and potentiates cell proliferation and invasion in endometrial cancer partly by activating the AKT pathway. 120 Through abolishing m6A methylation, ALKBH5 enhances the stability of Bcl‐2 mRNA, potentiates the interaction between Bcl‐2 and Beclin‐1, and activates the EGFR/PIK3CA/AKT/mTOR pathway to promote the proliferation, invasion and autophagy of ovarian epithelial cancer cells. 124
2.11. m6A in cutaneous cancer
METTL3 functions as an oncogene in cutaneous cancer. 125 , 126 Its expression increases in cutaneous squamous cell carcinoma (cSCC). Administration of cSCC cells with the methylation inhibitor cycloleucine or silencing METTL3 expression affects the colony‐forming efficiency in vitro and carcinogenesis in vivo partly through modulating ΔNp63 in an m6A‐dependent manner. 126 METTL3 promotes cell invasion and migration and increases MMP2 and N‐cadherin levels in melanoma cells, which are completely reversed in cells transfected with inactivated METTL3 through site mutant. 125 Inhibiting m6A methylation may provide a good prospect in the treatment of melanoma.
2.12. m6A in other tumors
Zhang et al uncovered that the ZNF750/FGF14 axis accelerated cell apoptosis and inhibited the growth of nasopharyngeal carcinoma in vitro and in vivo; METTL3‐dependent m6A modification was enriched in the CDS of ZNF750 mRNAs and impaired ZNF750 expression, 127 thus proving the oncogene role of METTL3 in nasopharyngeal carcinoma. The m6A level in total RNA increased in osteosarcoma tissues and cell lines, and METTL3 promoted the cell proliferation, migration and invasion of osteosarcoma by modulating the m6A level of LEF1 and activating Wnt/β‐catenin. 128 WTAP is highly expressed in cholangiocarcinoma, especially in cholangiocarcinoma cells with metastasis to lymph nodes or vessels. The overexpression of WTAP can significantly increase the metastasis and invasion of cholangiocarcinoma cells. 129
3. M6A AND RELATED INHIBITORS
Considering that epigenetics is characterized by regulating transcription and post‐transcriptional products, previous studies showed that m6A methylation has crucial role in malignant biological behaviours. Therefore, developing specific inhibitors of m6A‐related proteins is of great scientific significance and clinical value, thus being vital in tumour therapy. 130 , 131 , 132 , 133 Gemcitabine has emerged as an inducer of apoptosis in pancreatic cancer cells with low METTL3 expression. 115 CRC cells with YTHDF1 silencing are more sensitive to L‐OHP and 5‐FU. 98 METTL3 drives the ectopic expression of CBX8 in an m6A‐dependent manner, which obviates the sensitivity of colon cancer cells to chemotherapy of CPT‐11 and L‐OHP. 95 Single medication is prone to produce drug resistance in the clinical application. The aforementioned findings on m6A methylation and chemotherapeutic drugs provide a promising strategy for tumour therapy. 1 , 134
At present, the development of inhibitors based on m6A‐related enzymes is focused mainly on the first discovered RNA demethylase FTO. Yang et al proposed that Rhein might serve as a competitive inhibitor of FTO through the inhibition of the catalytic domain of FTO. 131 Subsequently, fluorescein derivatives, 135 MA (a non‐steroidal anti‐inflammatory drug), 132 IOX3 136 and radicicol 133 were also identified to decrease FTO expression. MA, by competently binding to FTO at the m6A site, can significantly increase the m6A level without affecting the demethylase activity of ALKBH5. 132 At the same time, MA2, an ethyl esterification isomer of MA, possesses better cell penetration ability and contributes to m6A modification, thus providing a chemical basis and guidance for the development of specific FTO inhibitors. Moreover, N‐CDPCB, CHTB and entacapone can also exert potent suppressive activities against FTO. 130 , 133 , 137 , 138 As the first obesity‐related gene identified by genome‐wide association analysis analysis, 28 not only FTO is closely associated with obesity and tumour, but also its common variant rs9939609 may be associated with central nervous system diseases, such as brain volume loss and alcohol dependence. 139 Therefore, FTO inhibitors may also be developed as drugs for neurological diseases, besides their anti‐cancer and weight‐reducing effects in addition to anti‐tumour and weight loss, may also be developed as drugs for neurological diseases. The progress on methyltransferase inhibitors is relatively slow. So far, only 3‐deazaadenosine has been proven to inhibit METTL3, but it has a broad‐spectrum efficacy and inhibits the activity of all m6A “readers”. 140 Furthermore, chemical oxidation eliminates the m6A modification of mRNA. 141 , 142 Although several m6A demethylase agents have been reported in the literature, their specificity or efficacy cannot achieve the goal of precise and suboptimal treatment. Precise and effective m6A targeted drugs still need further research and development.
4. CONCLUSIONS AND PERSPECTIVES
Various biological processes and diseases have been revealed in detail with increasing investigations on the composition of m6A regulatory network and its significance in mRNA processing and metabolism. 143 As the most prevalent modifications of mRNAs, m6A modification, characterized by widespread existence, unique distribution and dynamic reversibility, is involved in almost the whole course of mRNA biology from production to degradation. It participates in the regulation of biological functions of miRNAs and lncRNAs, 12 , 144 which acts as an important regulator of stress response, biological rhythm, cell differentiation, immune response, virus replication and infection, adipogenesis, embryonic development, sex determination, carcinogenesis, tumour progression, and so on. 14 , 54
Many issues related to the function and action mechanism of m6A methylation remain unresolved. Novel m6A readers and other components of m6A methyltransferase complex have emerged successively. No eraser has been uncovered except the known ALKBH5 and FTO. Considering the reciprocal effects on mRNA stability and degradation, the m6A reader‐mediating biological functions warrant further exploration. The rapid development of the detection technology of m6A methylation (sites) can make it easier to clarify the m6A regulatory network. Although several inhibitors of m6A‐related factors have been discovered, clinical applications have a far way to go. Effectuating the dynamic monitoring and tracking of m6A methylation is a fundamental issue confronting researchers.
From the epitranscriptome perspective, the tissue‐specific and inhomogeneous distribution of m6A modification provides a new direction for comprehending the pathogenesis in numerous diseases, especially in tumours. m6A modification, as a “double‐edged sword”, can promote or inhibit the initiation and progression of tumours mainly by regulating mRNA levels of oncogenes or tumour suppressor genes. It regulates cell proliferation, migration, invasion, differentiation and sensitivity to radiochemotherapy. However, whether m6A modification is implicated in a tumour microenvironment has not been reported. Further studying the molecular mechanisms of m6A methylation and its regulators can shed light on the clinical diagnosis and targeted therapy of tumours.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
FCH and ZMZ wrote and discussed the manuscript. DSP designed and drafted the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 81872080 and 81572349), Jiangsu Provincial Medical Talent (ZDRCA 2016055), the Science and Technology Department of Jiangsu Province (BK20181148) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the 333 high‐level talents of Jiangsu Province (BRA2019083).
Huo F‐C, Zhu Z‐M, Pei D‐S. N6‐methyladenosine (m6A) RNA modification in human cancer. Cell Prolif 2020;53:e12921 10.1111/cpr.12921
Fu‐Chun Huo and Zhi‐Man Zhu equal contributors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pinello N, Sun S, Wong JJ. Aberrant expression of enzymes regulating m(6)A mRNA methylation: implication in cancer. Cancer Biol Med. 2018;15(4):323‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arribas‐Hernandez L, Brodersen P. Occurrence and functions of m(6)A and other covalent modifications in plant mRNA. Plant Physiol. 2020;182(1):79‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livneh I, Moshitch‐Moshkovitz S, Amariglio N, Rechavi G, Dominissini D. The m(6)A epitranscriptome: transcriptome plasticity in brain development and function. Nat Rev Neurosci. 2020;21(1):36‐51. [DOI] [PubMed] [Google Scholar]
- 4. Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71(10):3971‐3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975;4(4):379‐386. [DOI] [PubMed] [Google Scholar]
- 6. Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)‐methyladenosine‐dependent RNA structural switches regulate RNA‐protein interactions. Nature. 2015;518(7540):560‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jia G, Fu Y, Zhao X, et al. N6‐methyladenosine in nuclear RNA is a major substrate of the obesity‐associated FTO. Nat Chem Biol. 2011;7(12):885‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dominissini D, Moshitch‐Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature. 2012;485(7397):201‐206. [DOI] [PubMed] [Google Scholar]
- 9. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao BS, Roundtree IA, He C. Post‐transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang H, Weng H, Chen J. The biogenesis and precise control of RNA m(6)A methylation. Trends Genet. 2020;36(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma S, Chen C, Ji X, et al. The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. 2019;12(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28(6):616‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao W, Qi X, Liu L, Liu Z, Ma S, Wu J. Epigenetic regulation of m(6)A modifications in human cancer. Mol Ther Nucleic Acids. 2019;19:405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet‐binding subunit of the human mRNA (N6‐adenosine)‐methyltransferase. RNA. 1997;3(11):1233‐1247. [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6‐methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16(2):191‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horiuchi K, Kawamura T, Iwanari H, et al. Identification of Wilms' tumor 1‐associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288(46):33292‐33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Yue Y, Han D, et al. A METTL3‐METTL14 complex mediates mammalian nuclear RNA N6‐adenosine methylation. Nat Chem Biol. 2014;10(2):93‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dezi V, Ivanov C, Haussmann IU, Soller M. Nucleotide modifications in messenger RNA and their role in development and disease. Biochem Soc Trans. 2016;44(5):1385‐1393. [DOI] [PubMed] [Google Scholar]
- 20. Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6‐methyladenosine methyltransferase. Cell Res. 2014;24(2):177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wen J, Lv R, Ma H, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self‐renewal. Mol Cell. 2018;69(6):1028‐1038 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pendleton KE, Chen B, Liu K, et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169(5):824‐835.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Warda AS, Kretschmer J, Hackert P, et al. Human METTL16 is a N(6)‐methyladenosine (m(6)A) methyltransferase that targets pre‐mRNAs and various non‐coding RNAs. EMBO Rep. 2017;18(11):2004‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bansal H, Yihua Q, Iyer SP, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28(5):1171‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ortega A, Niksic M, Bachi A, et al. Biochemical function of female‐lethal (2)D/Wilms' tumor suppressor‐1‐associated proteins in alternative pre‐mRNA splicing. J Biol Chem. 2003;278(5):3040‐3047. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz S, Mumbach MR, Jovanovic M, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep. 2014;8(1):284‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST‐mediated transcriptional repression. Nature. 2016;537(7620):369‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mauer J, Luo X, Blanjoie A, et al. Reversible methylation of m(6)Am in the 5' cap controls mRNA stability. Nature. 2017;541(7637):371‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mauer J, Sindelar M, Despic V, et al. FTO controls reversible m(6)Am RNA methylation during snRNA biogenesis. Nat Chem Biol. 2019;15(4):340‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu Y, Jia G, Pang X, et al. FTO‐mediated formation of N6‐hydroxymethyladenosine and N6‐formyladenosine in mammalian RNA. Nat Commun. 2013;4:1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tong J, Flavell RA, Li HB. RNA m(6)A modification and its function in diseases. Front Med. 2018;12(4):481‐489. [DOI] [PubMed] [Google Scholar]
- 33. Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kasowitz SD, Ma J, Anderson SJ, et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14(5):e1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A‐dependent nuclear RNA processing events. Cell. 2015;162(6):1299‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao YL, Liu YH, Wu RF, et al. Understanding m(6)A function through uncovering the diversity roles of YTH domain‐containing proteins. Mol Biotechnol. 2019;61(5):355‐364. [DOI] [PubMed] [Google Scholar]
- 37. Liao S, Sun H, Xu C. YTH domain: a family of N(6)‐methyladenosine (m(6)A) readers. Genomics Proteomics Bioinformatics. 2018;16(2):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Lu Z, Gomez A, et al. N6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N(6)‐methyladenosine‐modified RNA. Cell Res. 2017;27(3):315‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li A, Chen YS, Ping XL, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526(7574):591‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6‐methyladenosine alters RNA structure to regulate binding of a low‐complexity protein. Nucleic Acids Res. 2017;45(10):6051‐6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin BY, Shih CJ, Hsieh HY, Chen HC, Tu SL. Phytochrome coordinates with a hnRNP to regulate alternative splicing via an exonic splicing silencer. Plant Physiol. 2020;182(1):243‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu R, Li A, Sun B, et al. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29(1):23‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waly AA, El‐Ekiaby N, Assal RA, et al. Methylation in MIRLET7A3 gene induces the expression of IGF‐II and its mRNA binding proteins IGF2BP‐2 and 3 in hepatocellular carcinoma. Front Physiol. 2018;9:1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang H, Weng H, Sun W, et al. Recognition of RNA N(6)‐methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang J, Yin P. Structural insights into N(6)‐methyladenosine (m(6)A) modification in the transcriptome. Genomics Proteomics Bioinformatics. 2018;16(2):85‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dorn LE, Lasman L, Chen J, et al. The N(6)‐methyladenosine mRNA methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139(4):533‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li LJ, Fan YG, Leng RX, Pan HF, Ye DQ. Potential link between m(6)A modification and systemic lupus erythematosus. Mol Immunol. 2018;93:55‐63. [DOI] [PubMed] [Google Scholar]
- 50. Meng Y, Li S, Gu D, et al. Genetic variants in m6A modification genes are associated with colorectal cancer risk. Carcinogenesis. 2020;41(1):8‐17. [DOI] [PubMed] [Google Scholar]
- 51. Yang Y, Shen F, Huang W, et al. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(3):665‐673. [DOI] [PubMed] [Google Scholar]
- 52. Yu R, Li Q, Feng Z, Cai L, Xu Q. m6A Reader YTHDF2 Regulates LPS‐Induced Inflammatory Response. Int J Mol Sci. 2019;20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tuncel G, Kalkan R. Importance of m N(6)‐methyladenosine (m(6)A) RNA modification in cancer. Med Oncol. 2019;36(4):36. [DOI] [PubMed] [Google Scholar]
- 54. Chen XY, Zhang J, Zhu JS. The role of m(6)A RNA methylation in human cancer. Mol Cancer. 2019;18(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lan Q, Liu PY, Haase J, Bell JL, Huttelmaier S, Liu T. The critical role of RNA m(6)A methylation in cancer. Cancer Res. 2019;79(7):1285‐1292. [DOI] [PubMed] [Google Scholar]
- 56. Weng H, Huang H, Wu H, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22(2):191‐205.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vu LP, Pickering BF, Cheng Y, et al. The N(6)‐methyladenosine (m(6)A)‐forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23(11):1369‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barbieri I, Tzelepis K, Pandolfini L, et al. Promoter‐bound METTL3 maintains myeloid leukaemia by m(6)A‐dependent translation control. Nature. 2017;552(7683):126‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ianniello Z, Paiardini A, Fatica A. N(6)‐methyladenosine (m(6)A): a promising new molecular target in acute myeloid leukemia. Front Oncol. 2019;9:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mercher T, Coniat MB, Monni R, et al. Involvement of a human gene related to the Drosophila spen gene in the recurrent t(1;22) translocation of acute megakaryocytic leukemia. Proc Natl Acad Sci U S A. 2001;98(10):5776‐5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang L, Tran NT, Su H, et al. Cross‐talk between PRMT1‐mediated methylation and ubiquitylation on RBM15 controls RNA splicing. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Z, Weng H, Su R, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)‐methyladenosine RNA demethylase. Cancer Cell. 2017;31(1):127‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Su R, Dong L, Li C, et al. R‐2HG exhibits anti‐tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172(1–2):90‐105.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. R‐2HG targets FTO to increase m(6)A levels and suppress tumor growth. Cancer Discov. 2018;8(2):137. [DOI] [PubMed] [Google Scholar]
- 65. Elkashef SM, Lin AP, Myers J, et al. IDH Mutation, Competitive Inhibition of FTO, and RNA Methylation. Cancer Cell. 2017;31(5):619‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paris J, Morgan M, Campos J, et al. Targeting the RNA m(6)A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell. 2019;25(1):137‐148.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen QN, Chen Y, Ji TT, Yu L. Research progress of m6A‐methylation in acute leukemia – review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(6):2014‐2018. [DOI] [PubMed] [Google Scholar]
- 68. Cui Q, Shi H, Ye P, et al. m(6)A RNA Methylation Regulates the Self‐Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017;18(11):2622‐2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang S, Sun C, Li J, et al. Roles of RNA methylation by means of N(6)‐methyladenosine (m(6)A) in human cancers. Cancer Lett. 2017;408:112‐120. [DOI] [PubMed] [Google Scholar]
- 70. Visvanathan A, Patil V, Arora A, et al. Essential role of METTL3‐mediated m(6)A modification in glioma stem‐like cells maintenance and radioresistance. Oncogene. 2018;37(4):522‐533. [DOI] [PubMed] [Google Scholar]
- 71. Xi Z, Xue Y, Zheng J, Liu X, Ma J, Liu Y. WTAP expression predicts poor prognosis in malignant glioma patients. J Mol Neurosci. 2016;60(2):131‐136. [DOI] [PubMed] [Google Scholar]
- 72. Zhang S, Zhao BS, Zhou A, et al. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem‐like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31(4):591‐606.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chai RC, Wu F, Wang QX, et al. m(6)A RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gliomas. Aging (Albany NY). 2019;11(4):1204‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cai X, Wang X, Cao C, et al. HBXIP‐elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let‐7g. Cancer Lett. 2018;415:11‐19. [DOI] [PubMed] [Google Scholar]
- 75. Qian JY, Gao J, Sun X, et al. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6‐methyladenosine‐independent manner. Oncogene. 2019;38(33):6123‐6141. [DOI] [PubMed] [Google Scholar]
- 76. Zhang C, Samanta D, Lu H, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF‐dependent and ALKBH5‐mediated m(6)A‐demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113(14):E2047‐E2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang C, Zhi WI, Lu H, et al. Hypoxia‐inducible factors regulate pluripotency factor expression by ZNF217‐ and ALKBH5‐mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7(40):64527‐64542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niu Y, Lin Z, Wan A, et al. RNA N6‐methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen M, Wei L, Law CT, et al. RNA N6‐methyladenosine methyltransferase‐like 3 promotes liver cancer progression through YTHDF2‐dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67(6):2254‐2270. [DOI] [PubMed] [Google Scholar]
- 80. Liu L, Wang J, Sun G, et al. m(6)A mRNA methylation regulates CTNNB1 to promote the proliferation of hepatoblastoma. Mol Cancer. 2019;18(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ma JZ, Yang F, Zhou CC, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) ‐methyladenosine‐dependent primary MicroRNA processing. Hepatology. 2017;65(2):529‐543. [DOI] [PubMed] [Google Scholar]
- 82. Li J, Meng S, Xu M, et al. Downregulation of N(6)‐methyladenosine binding YTHDF2 protein mediated by miR‐493‐3p suppresses prostate cancer by elevating N(6)‐methyladenosine levels. Oncotarget. 2018;9(3):3752‐3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li J, Zhu L, Shi Y, Liu J, Lin L, Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am J Transl Res. 2019;11(9):6084‐6092. [PMC free article] [PubMed] [Google Scholar]
- 84. Zhao X, Chen Y, Mao Q, et al. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21(4):859‐868. [DOI] [PubMed] [Google Scholar]
- 85. Lin X, Chai G, Wu Y, et al. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun. 2019;10(1):2065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86. He H, Wu W, Sun Z, Chai L. MiR‐4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A‐caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517(4):581‐587. [DOI] [PubMed] [Google Scholar]
- 87. Liu T, Yang S, Sui J, et al. Dysregulated N6‐methyladenosine methylation writer METTL3 contributes to the proliferation and migration of gastric cancer. J Cell Physiol. 2020;235(1):548‐562. [DOI] [PubMed] [Google Scholar]
- 88. Lin S, Liu J, Jiang W, et al. METTL3 promotes the proliferation and mobility of gastric cancer cells. Open Med (Wars). 2019;14:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang Q, Chen C, Ding Q, et al. METTL3‐mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2019. [DOI] [PubMed] [Google Scholar]
- 90. Xu D, Shao W, Jiang Y, Wang X, Liu Y, Liu X. FTO expression is associated with the occurrence of gastric cancer and prognosis. Oncol Rep. 2017;38(4):2285‐2292. [DOI] [PubMed] [Google Scholar]
- 91. Su Y, Huang J, Hu J. m(6)A RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gastric cancer. Front Oncol. 2019;9:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang C, Zhang M, Ge S, et al. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K‐Akt signaling in gastric cancer. Cancer Med. 2019;8(10):4766‐4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang J, Pi J, Liu Y, Yu J, Feng T. Knockdown of YTH N(6)‐methyladenosine RNA binding protein 2 (YTHDF2) inhibits proliferation and promotes apoptosis in MGC‐803 gastric cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33(12):1628‐1634. [PubMed] [Google Scholar]
- 94. Deng R, Cheng Y, Ye S, et al. m(6)A methyltransferase METTL3 suppresses colorectal cancer proliferation and migration through p38/ERK pathways. Onco Targets Ther. 2019;12:4391‐4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang Y, Kang M, Zhang B, et al. m(6)A modification‐mediated CBX8 induction regulates stemness and chemosensitivity of colon cancer via upregulation of LGR5. Mol Cancer. 2019;18(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Wu Y, Yang X, Chen Z, et al. m(6)A‐induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shen XP, Ling X, Lu H, Zhou CX, Zhang JK, Yu Q. Low expression of microRNA‐1266 promotes colorectal cancer progression via targeting FTO. Eur Rev Med Pharmacol Sci. 2018;22(23):8220‐8226. [DOI] [PubMed] [Google Scholar]
- 98. Nishizawa Y, Konno M, Asai A, et al. Oncogene c‐Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9(7):7476‐7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tanabe A, Tanikawa K, Tsunetomi M, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF‐1alpha mRNA is translated. Cancer Lett. 2016;376(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 100. Liu T, Li C, Jin L, Li C, Wang L. The prognostic value of m6A RNA methylation regulators in colon adenocarcinoma. Med Sci Monit. 2019;25:9435‐9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Du M, Zhang Y, Mao Y, et al. MiR‐33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem Biophys Res Commun. 2017;482(4):582‐589. [DOI] [PubMed] [Google Scholar]
- 102. Wei W, Huo B, Shi X. miR‐600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell. 2016;62(3):335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li J, Han Y, Zhang H, et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512(3):479‐485. [DOI] [PubMed] [Google Scholar]
- 105. Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y. m(6)A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun. 2018;502(4):456‐464. [DOI] [PubMed] [Google Scholar]
- 106. Chao Y, Shang J, Ji W. ALKBH5‐m(6)A‐FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem Biophys Res Commun. 2020;521(2):499‐506. [DOI] [PubMed] [Google Scholar]
- 107. Chen M, Nie ZY, Wen XH, Gao YH, Cao H, Zhang SF. m6A RNA methylation regulators can contribute to malignant progression and impact the prognosis of bladder cancer. Biosci Rep. 2019;39(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Han J, Wang JZ, Yang X, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri‐miR221/222 maturation in m6A‐dependent manner. Mol Cancer. 2019;18(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cheng M, Sheng L, Gao Q, et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF‐kappaB/MYC signaling network. Oncogene. 2019;38(19):3667‐3680. [DOI] [PubMed] [Google Scholar]
- 110. Zhou J, Wang J, Hong B, et al. Gene signatures and prognostic values of m6A regulators in clear cell renal cell carcinoma – a retrospective study using TCGA database. Aging (Albany NY). 2019;11(6):1633‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gong D, Zhang J, Chen Y, et al. The m(6)A‐suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP‐induced Ca(2+) influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tang J, Wang F, Cheng G, et al. Wilms' tumor 1‐associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J Exp Clin Cancer Res. 2018;37(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen Y, Zhou C, Sun Y, He X, Xue D. m(6)A RNA modification modulates gene expression and cancer‐related pathways in clear cell renal cell carcinoma. Epigenomics. 2019. [DOI] [PubMed] [Google Scholar]
- 114. Xia T, Wu X, Cao M, et al. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract. 2019;215(11):152666. [DOI] [PubMed] [Google Scholar]
- 115. Taketo K, Konno M, Asai A, et al. The epitranscriptome m6A writer METTL3 promotes chemo‐ and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52(2):621‐629. [DOI] [PubMed] [Google Scholar]
- 116. Li BQ, Huang S, Shao QQ, et al. WT1‐associated protein is a novel prognostic factor in pancreatic ductal adenocarcinoma. Oncol Lett. 2017;13(4):2531‐2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tang X, Liu S, Chen D, Zhao Z, Zhou J. The role of the fat mass and obesity‐associated protein in the proliferation of pancreatic cancer cells. Oncol Lett. 2019;17(2):2473‐2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. He Y, Hu H, Wang Y, et al. ALKBH5 inhibits pancreatic cancer motility by decreasing long non‐coding RNA KCNK15‐AS1 methylation. Cell Physiol Biochem. 2018;48(2):838‐846. [DOI] [PubMed] [Google Scholar]
- 119. Chen J, Sun Y, Xu X, et al. YTH domain family 2 orchestrates epithelial‐mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16(23):2259‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu J, Eckert MA, Harada BT, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20(9):1074‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wang X, Li Z, Kong B, et al. Reduced m(6)A mRNA methylation is correlated with the progression of human cervical cancer. Oncotarget. 2017;8(58):98918‐98930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhou S, Bai ZL, Xia D, et al. FTO regulates the chemo‐radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta‐catenin through mRNA demethylation. Mol Carcinog. 2018;57(5):590‐597. [DOI] [PubMed] [Google Scholar]
- 123. Zou D, Dong L, Li C, Yin Z, Rao S, Zhou Q. The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhu H, Gan X, Jiang X, Diao S, Wu H, Hu J. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR‐7 and BCL‐2. J Exp Clin Cancer Res. 2019;38(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dahal U, Le K, Gupta M. RNA m6A methyltransferase METTL3 regulates invasiveness of melanoma cells by matrix metallopeptidase 2. Melanoma Res. 2019;29(4):382‐389. [DOI] [PubMed] [Google Scholar]
- 126. Zhou R, Gao Y, Lv D, Wang C, Wang D, Li Q. METTL3 mediated m(6)A modification plays an oncogenic role in cutaneous squamous cell carcinoma by regulating DeltaNp63. Biochem Biophys Res Commun. 2019;515(2):310‐317. [DOI] [PubMed] [Google Scholar]
- 127. Zhang P, He Q, Lei Y, et al. m(6)A‐mediated ZNF750 repression facilitates nasopharyngeal carcinoma progression. Cell Death Dis. 2018;9(12):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Miao W, Chen J, Jia L, Ma J, Song D. The m6A methyltransferase METTL3 promotes osteosarcoma progression by regulating the m6A level of LEF1. Biochem Biophys Res Commun. 2019;516(3):719‐725. [DOI] [PubMed] [Google Scholar]
- 129. Jo HJ, Shim HE, Han ME, et al. WTAP regulates migration and invasion of cholangiocarcinoma cells. J Gastroenterol. 2013;48(11):1271‐1282. [DOI] [PubMed] [Google Scholar]
- 130. Peng S, Xiao W, Ju D, et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci Transl Med. 2019;11(488):eaau7116. [DOI] [PubMed] [Google Scholar]
- 131. Chen B, Ye F, Yu L, et al. Development of cell‐active N6‐methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134(43):17963‐17971. [DOI] [PubMed] [Google Scholar]
- 132. Huang Y, Yan J, Li Q, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43(1):373‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang R, Han Z, Liu B, et al. Identification of natural compound radicicol as a potent FTO inhibitor. Mol Pharm. 2018;15(9):4092‐4098. [DOI] [PubMed] [Google Scholar]
- 134. Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613. [DOI] [PubMed] [Google Scholar]
- 135. Wang T, Hong T, Huang Y, et al. Fluorescein derivatives as bifunctional molecules for the simultaneous inhibiting and labeling of FTO protein. J Am Chem Soc. 2015;137(43):13736‐13739. [DOI] [PubMed] [Google Scholar]
- 136. McMurray F, Demetriades M, Aik W, et al. Pharmacological inhibition of FTO. PLoS One. 2015;10(4):e0121829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Han X, Wang N, Li J, Wang Y, Wang R, Chang J. Identification of nafamostat mesilate as an inhibitor of the fat mass and obesity‐associated protein (FTO) demethylase activity. Chem Biol Interact. 2019;297:80‐84. [DOI] [PubMed] [Google Scholar]
- 138. Qiao Y, Zhou B, Zhang M, et al. A novel inhibitor of the obesity‐related protein FTO. Biochemistry. 2016;55(10):1516‐1522. [DOI] [PubMed] [Google Scholar]
- 139. Milaneschi Y, Lamers F, Mbarek H, Hottenga JJ, Boomsma DI, Penninx BW. The effect of FTO rs9939609 on major depression differs across MDD subtypes. Mol Psychiatry. 2014;19(9):960‐962. [DOI] [PubMed] [Google Scholar]
- 140. Fustin JM, Doi M, Yamaguchi Y, et al. RNA‐methylation‐dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155(4):793‐806. [DOI] [PubMed] [Google Scholar]
- 141. Wu J, Xiao H, Wang T, et al. N(6)‐Hydroperoxymethyladenosine: a new intermediate of chemical oxidation of N(6)‐methyladenosine mediated by bicarbonate‐activated hydrogen peroxide. Chem Sci. 2015;6(5):3013‐3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xie LJ, Yang XT, Wang RL, et al. Identification of flavin mononucleotide as a cell‐active artificial N(6) ‐methyladenosine RNA demethylase. Angew Chem Int Ed Engl. 2019;58(15):5028‐5032. [DOI] [PubMed] [Google Scholar]
- 143. Berlivet S, Scutenaire J, Deragon JM, Bousquet‐Antonelli C. Readers of the m(6)A epitranscriptomic code. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):329‐342. [DOI] [PubMed] [Google Scholar]
- 144. Dai D, Wang H, Zhu L, Jin H, Wang X. N6‐methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018;9(2):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


