Abstract
Background:
Human epididymis protein 4 (HE4) is a novel cancer biomarker. This study evaluates the prognostic role of HE4 in determining the survival of endometrial cancer patients.
Methods:
Literature search was conducted in electronic databases (Embase, Ovid, PubMed, Scopus, and Web of Science). Studies were selected if they reported the relationship between HE4 and the survival of endometrial cancer patients. Random-effects meta-analyses were performed to achieve estimates of baseline serum HE4 levels, the 5-year survival with high and low serum HE4 levels/expression, and the hazard ratios (HRs) of the survival between patients with high and low serum HE4 levels.
Results:
9 studies (1404 patients; age 63.1 years [95% confidence interval (CI): 61.2, 64.9]; follow-up 35.9 months [95% CI: 32.2, 39.6]) were included. In these patients, serum HE4 levels were 83.36 picomole/liter (pM) [95% CI: 70.15, 96.56] overall but these were higher in patients with recurrence (108.13 pM [95% CI: 63.09, 153.18] and lower in patients with no recurrence (67.88 pM [95% CI: 65.09, 70.67]). The 5-year overall survival rate was higher in patients with low HE4 levels/expression (86% [95% CI: 79, 92] but lower in patients with high HE4 levels/expression (63% [95% CI: 58, 68]. A pooled HR of survival between patients with high and low serum HE4 levels of 2.25 [95% CI: 1.56, 2.94] indicated shorter survival in patients with high serum HE4 levels.
Conclusion:
High HE4 concentrations in patients with endometrial cancer are found to be associated with shorter survival.
Keywords: human epididymis protein 4, cancer biomarker, survival, endometrial cancer
Introduction
Endometrial cancer is the most common cancer of female reproductive tract.1 Excessive unopposed estrogen exposure to endometrium, earlier menarche, later menopause, tamoxifen exposure, nulliparity / infertility, and polycystic ovarian syndrome are the major risk factors for endometrial cancer incidence. Older age, higher body mass index, high blood pressure, hyperglycemia, and Lynch syndrome also pose significant risk of endometrial cancer incidence.2,3
It is estimated that 65,620 new cases will be diagnosed and approximately 12,590 women will die from endometrial cancer in the United States in 2020.4 In Europe and North America, endometrial cancer accounts for approximately 6% of new cancer cases and 3% of cancer deaths each year.5 Globally, the incidence of endometrial cancer is increasing.6 It is foreseen that there can be 2-fold increase in the number of endometrial cancer patients by the year 2030 in the United States.7 Some other countries such as South Korea have also reported increasing trends in the incidence of endometrial cancer.8 Aging population and increased prevalence of obesity are important factors behind this increased incidence.9
Major histological subtype of endometrial cancer is the endometrioid adenocarcinoma which comprises approximately 75% of all cases.10 Earlier diagnosis can result in better prognosis (5-year overall survival rate of 80–85%).11 Up to 70% of the cases are diagnosed at an early stage. However, up to 30% of women remain asymptomatic and may be diagnosed at an advanced stage which is associated with worse prognosis.12 The identification of biomarkers predicting the course of disease therefore remains an important part of uterine cancer research aimed at improving individualized treatment.
Human epididymis protein 4 (HE4), is a promising novel cancer biomarker which is approved for monitoring disease progression in ovarian cancer patients.13 Many tumors overexpress HE4 which is also associated with cancer progression.14 HE4 is a “whey acidic protein” that acts as “whey acidic four-disulfide core” trypsin inhibitor.15 Serum HE4 levels are found to be significantly elevated in both pre- and post-menopausal endometrial cancer patients.16 It has also been reported that HE4 is more sensitive and specific than the cancer antigen 125 (CA125) for early detection of endometrial cancer regardless of the age and hormonal status of patients.17
Although, the role of HE4 in the diagnosis of endometrial cancer is well-recognized, its long-term prognostic value for endometrial cancer patients needs to be elucidated. Many studies have reported the prognostic value of HE4 in determining the survival of endometrial cancer patients. However, the outcome measures and outcomes are variable in these studies which necessitates a systematic review of this area. The aim of the present study was to undertake a comprehensive literature search for the identification of relevant studies and to perform meta-analyses estimating the strength of relationship between HE4 and the survival of endometrial cancer patients.
Materials and Methods
Eligibility Criteria
Inclusion criterion was: A study investigated the prognostic role of HE4 in endometrial cancer patients using a longitudinal design and reported the relationship between the recurrence or survival and baseline HE4 serum levels or HE4 histologic expression. Studies were excluded if they investigated the prognostic role of HE4 in combination with other biomarker/s or reported the prognostic outcomes other than the survival or recurrence. In vitro studies, diagnostic studies, and research articles without quantitative information were also excluded.
Literature Search
Relevant research articles were searched in Embase, Ovid, PubMed, Scopus, and Web of Science databases using relevant keywords. These included “endometrial cancer,” “adenocarcinoma,” “carcinoma,” “carcinosarcoma,” “human epididymis protein 4,” “HE4,” “prognosis,” “prognostic factor,” “hazard,” “survival,” “mortality,” “recurrence”, “progression,” and “metastasis.” References lists of relevant research and review articles and database software indicated relevant articles were also screened.
Data Analyses
Demographic, clinicopathological, and oncological data of patients, methodological information, outcome measures and outcome data were extracted from respective research articles of the included studies. The survival data were preferably taken from text, but if were not found in the text, these were extracted from the survival curves. In studies which used immunohistochemistry to measure HE4, low or high HE4 expression was based on chromatic intensity where less than 25% chromatic intensity was considered low HE4 expression and 25% to 100% chromatic intensity was considered as high HE4 expression.
Meta-analyses were performed using the Stata software (Stata Corporation, Texas, USA). Random-effects model was used for the meta-analyses based on a methodological review of the included studies. Serum HE4 levels reported by the individual studies were pooled to achieve inverse variance weighted overall and subgroup (low / high) estimates. The hazard ratios (HRs) of survival between high and low serum HE4 levels reported by the individual studies were also pooled to achieve inverse variance weighted overall and subgroup estimates.
For the estimation of 5-year progression/disease free survival and overall survival in low and high serum HE4 levels / histologic expression groups, a meta-analysis of proportions was performed. The 5-years survival probability was converted to proportions and then meta-analyzed to achieve overall estimates where within-study variability was estimated from the binomial distribution and 95% confidence intervals of the estimates were calculated by using the score statistics and the exact binomial method.18 Meta-analysis of proportions incorporated the Freeman-Tukey double arcsine transformation for variance stabilization.18
Results
Nine studies19-27 fulfilled the eligibility criteria (Figure 1). Of these, 2 were prospective and 7 were retrospective in design. Overall, these studies reported the outcomes of 1404 women with endometrial cancer. Average age of these patients was 63.1 years [95% confidence interval (CI): 61.2, 64.9]. Follow-up duration of these studies was 35.9 months [95% CI: 32.2, 39.6]. Important characteristics of the included studies are presented in Table 1.
Figure 1.
A flowchart of the study screening and selection process.
Table 1.
Important Characteristics of the Included Studies.
| Study | n | Design | Follow-up (months) | HE4 detection | Age (years) | % Endo-metroid | FIGO (%) | Grade (%) | Lymph node (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | +ive | -ive | |||||||
| Abbink 201819 | 157 | RET | 60 | CLEIA | 63 ± 4.3 | 78 | 63 | 7 | 17 | 13 | 17 | 45 | 38 | 25 | 75 |
| Bignotti 201120 | 138 | RET | 33 ± 21 | ELISA | 79 | 77 | 23 | 18 | 82 | 18 | 82 | ||||
| Brennan 201421 | 373 | PROSP | 37 ± 18 | CLMEIA | 61.2 ± 10 | 85 | 75 | 8 | 6 | 1 | 49 | 31 | 20 | 10 | 90 |
| Brennan 201522 | 98 | RET | 60 | CLMEIA | 65 ± 22 | 70 | 59 | 7 | 26 | 8 | 17 | 39 | 42 | 23 | 77 |
| Deng 201523 | 72 | PROSP | IHC | 58.9 ± 12 | 47 | 63 | 8 | 25 | 4 | 28 | 72 | ||||
| Li 201524 | 102 | RET | 9-116 | IHC | 58.1 ± 14 | 48 | 60 | 7 | 27 | 6 | 59.8 | 6.86 | 22 | 28 | 52 |
| Mutz-dehbalaie 201225 | 183 | RET | 36 ± 35 | CLMEIA | 68 ± 15 | 72 | 56 | 9 | 27 | 7 | 20 | 38 | 42 | 25 | 75 |
| Stiekema 201726 | 88 | RET | 48 ± 40 | ECLIA | 65 ± 13 | 64 | 68 | 32 | 0 | 48 | 52 | 37 | 63 | ||
| Zanotti 201227 | 193 | RET | 31 ± 25 | CLMEIA | 66 ± 19 | 79 | 55 | 18 | 16 | 5 | 18 | 46 | 36 | 16 | 84 |
Abbreviations: CLEIA, chemiluminescent enzyme immunoassay; CLMEIA, chemiluminescent microparticle immunoassays; ECLIA, electrochemiluminescensce immunoassay; ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemistry; PROSP, prospective; RET, retrospective.
Histologically, the type of tumor was endometroid in 70% [95% CI: 62, 78] and non-endometroid in 30% [95% CI: 22, 38] of the patients. The serous papillary / clear cell types were in 21% [95% CI: 9, 37] whereas carcinosarcoma was in 8% [95% CI: 5, 11] of the patients. The percentages of patients with the International Federation of Gynecology and Obstetrics (FIGO) grades 1, 2, 3 and 4 were 60% [95% CI: 52, 68], 14% [95% CI: 8, 21], 17% [95% CI: 10, 25], and 4% [95% CI: 1, 7] respectively. The percentages of patients with World Health Organization (WHO) grades were 23% [95% CI: 13, 34] for grade I, 44% [95% CI: 31, 57] for grade II and 29% [95% CI: 15, 46] for grade III. Lymph node positive patients were 22% [95% CI: 17, 29] and lymph node negative patients were 76% [95% CI: 67, 83].
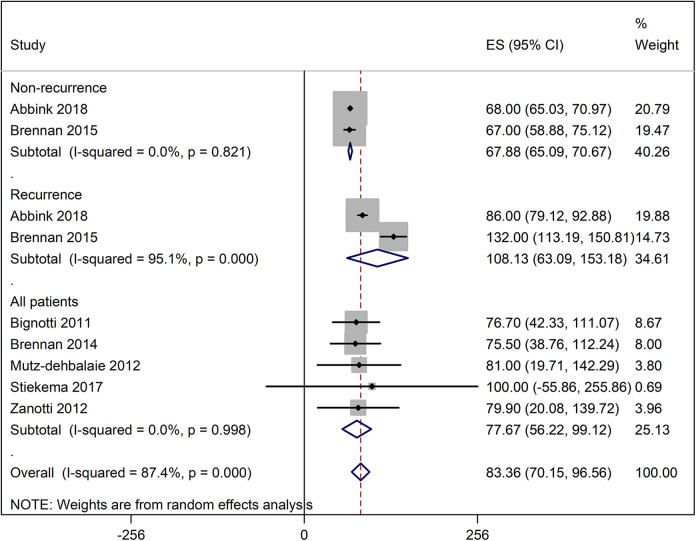
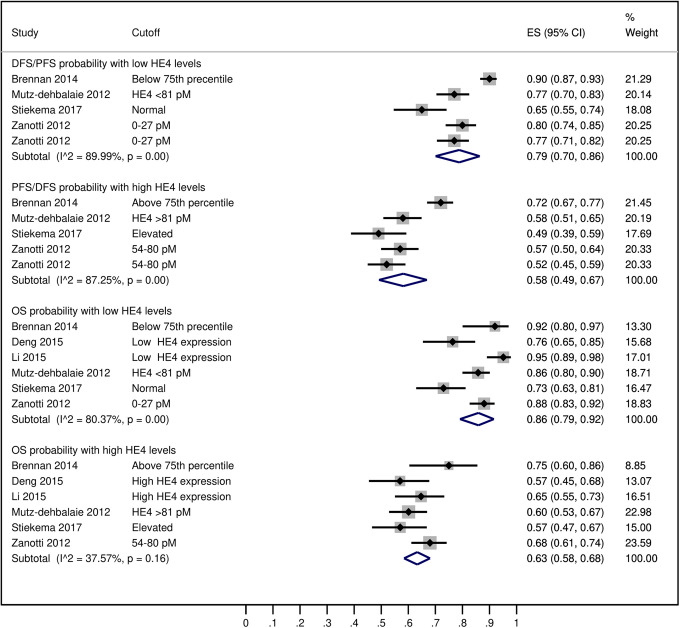
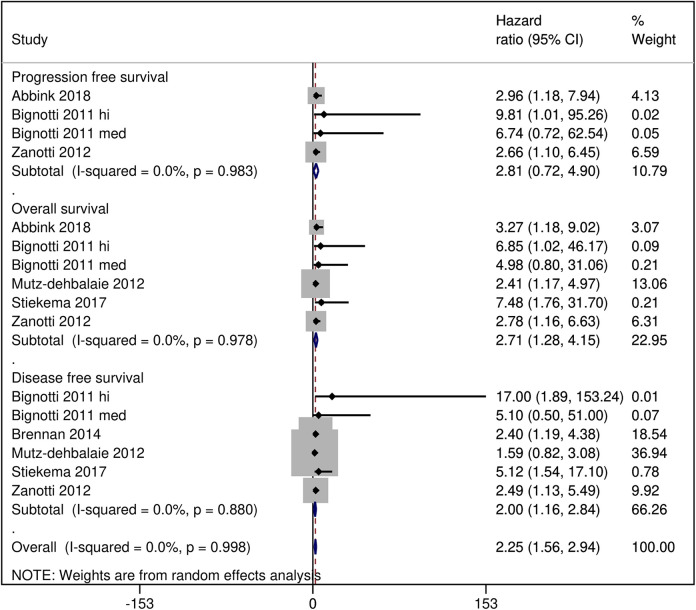
In these patients, the average serum HE4 levels were 83.36 picomole/liter (pM) [95% CI: 70.15, 96.56] overall but these were higher in patients with recurrence (108.13 pM [95% CI: 63.09, 153.18] and lower in patients with no recurrence (67.88 pM [95% CI: 65.09, 70.67]) (Figure 2). The probability of 5-year disease/progression free survival was 79% [95% CI: 70, 86] in patients with low HE4 levels/expression and 58% [95% CI 49, 67] in patients with high HE4 levels/expression. The probability of 5-year overall survival in patients with low HE4 levels/expression was 86% [95% CI: 79, 92] and in patients with high HE4 levels/expression it was 63% [95% CI: 58, 68] (Figure 3). A pooled analysis of the HRs of survival between high and low serum HE4 levels reported by the included studies revealed an overall HR of 2.25 [95% CI: 1.56, 2.94] which indicated shorter survival in patients with high HE4 levels (Figure 4).
Figure 2.
A forest graph showing the outcomes of the pooled analysis of the serum HE4 levels.
Figure 3.
A forest graph showing the outcomes of the pooled analysis of the 5-year disease/progression free survival (DFS/PFS) and overall survival (OS) probability in endometrial cancer patients with high and low HE4 levels/expression.
Figure 4.
A forest graph showing the outcomes of the pooled analysis of the hazard ratios of survival between patients with high vs low serum HE4 levels.
Discussion
In this meta-analysis we have found HE4 to be a valuable prognostic factor for predicting the survival of endometrial cancer patients as HE4 levels were higher in patients with recurrence and lower in patients with no recurrence; the 5-year survival probability was higher in patients with low HE4 concentrations but lower in patients with high HE4 concentrations; and a pooled analysis of HRs also showed that high HE4 levels were associated with shorter survival in patients with endometrial cancer.
A meta-analysis of studies that evaluated the prognostic role of HE4 in cancer patients also found that higher HE4 levels predicted worse survival in cancer patients including endometrial cancer patients.14 Serum HE4 levels are also found to be higher in endometrial cancer patients with ≥2 centimeters tumor diameter, deep myometrial invasion, later tumor stage, advanced stage of cancer, or lymph node metastasis.28 Significantly lower serum HE4 levels are found in low-risk patients (myometrial invasion of less than 50% and lesion diameter of less than 2 centimeter) than in high-risk patients with stage I endometrial carcinoma.29 Capriglione et al. found significantly lower HE4 levels in endometrial cancer patients with endometrioid than with non-endometroid histology (78.3 ± 20.1 vs 121.1 ± 24.6 pM; p = 0.04) and HE4 levels increased from 48.7 ± 9.1 pM in grade I to 83.9 ± 13.6 pM in grade II and 111.7 ± 34.1 pM in Grade III consistenly.30
Dobrzycka et al. also found similar increasing trends of HE4 levels where the stage I of endometrioid adenocarcinomas had significantly lower HE4 levels in comparison with IB and II stages. In this study, HE4 levels correlated well with the tumor grade and were also significantly lower in the low risk group. Authors suggested that HE4 could serve as a preoperative biomarker to appraise the need for pelvic and para-aortic lymphadenectomy.31 Preoperative serum HE4 levels and their regression rate before the interval debulking surgery predict favorable surgical outcomes of the ovarian cancer patients which suggests that HE4 is a valuable marker for the identification of patients with inoperable disease.32 Moreover, serum HE4 levels measured before first-line chemotherapy were found useful in predicting response to chemotherapy in the epithelial ovarian cancer.33
Mechanistically, it is postulated that HE4 causes cancer cells to skip G1 phase and to enter S phase, helps maintain cellular viability, promote proliferation, inhibit apoptosis, and contribute to drug resistance mechanisms.34 Recombinant HE4 protein promotes the proliferation of endometrial cancer cells.35 Moreover, overexpression of HE4 in endometrial cancerous cell lines significantly enhances the cell proliferation both in vivo and in vitro.36 It has also been reported that HE4 can mediate the expression of matrix metalloproteinase 2 via the annexin A2 to promote cell migration.37 Several variants of HE4 are identified and there is some evidence to suggest that HE4 may affect survival in a variant-specific manner.38
HE4 is found superior to the CA125 for the detection of endometrial malignancies,39 and it has been reported that HE4 has higher sensitivity and negative predictive value for the lymph node metastasis than the CA125.26 The combinational use of preoperative HE4 and CA125 predict the metastatic endometrial carcinoma better than either HE4 or CA125.40 Presl et al. have also opined that a parallel estimation of HE4 and CA125 may be used to improve pre-biopsy validation of the endometrial ultrasonographic findings and may help in preoperative staging of the endometrial cancer.41 Another biomarker, the annexin A2, which co-localizes with HE4 in the endometrial carcinoma, also shows interactive behavior with HE4 in determining the prognosis of endometrial cancer.23 A positive association has also been found between HE4 and thyroid transcription factor-1 (TTF-1) in circulating tumor cells to predict the survival rates in endometrial cancer pateints.42
An important limitation of the present study is that a smaller number of studies could be included in the meta-analysis. However, statistical heterogeneity was low which indicated good between-studies consistency in the outcomes. Among other factors, the use of different techniques to measure HE4 may have some impact on overall outcomes. However, because the meta-analysis of the HRs endorsed the findings of other meta-analyses, therefore such an impact could be minimal on overall outcomes. Finally, the retrospective designs of most included studies could have influenced the outcomes of the present study.
Abbreviations
- CA125
cancer antigen 125
- CI
confidence interval
- FIGO
The International Federation of Gynecology and Obstetrics
- HR
hazard ratio
- HE4
Human epididymis protein 4
- pM
picomole/liter
- WHO
World Health Organization
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yan-Hua Kang  https://orcid.org/0000-0001-8742-791X
https://orcid.org/0000-0001-8742-791X
References
- 1. American College of Obstetricians and Gynecologists. Endometrial cancer. Updated February 4, 2019. Accessed July 28, 2019 https://www.acog.org/patient-resources/faqs/gynecologic-problems/endometrial-cancer#
- 2. Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15(3):341–354. [DOI] [PubMed] [Google Scholar]
- 3. Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93(6):468–474. [PubMed] [Google Scholar]
- 4. American Cancer Society. Key Statistics for endometrial cancers. Updated July 14, 2019. Accessed August 12, 2019 https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html
- 5. Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268–278. [DOI] [PubMed] [Google Scholar]
- 6. Constantine GD, Kessler G, Graham S, Goldstein SR. Increased incidence of endometrial cancer following the women’s health initiative: an assessment of risk factors. J Womens Health (Larchmt). 2019;28(2):37–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. (corrigendum in Cancer Res. 2014;74(14):4006) [DOI] [PubMed] [Google Scholar]
- 8. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46(2):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother’s cancer. Cancer. 2016;122(18):2787–2798. [DOI] [PubMed] [Google Scholar]
- 10. Minar L, Klabenesova I, Jandakova E, Zlamal F, Bienertova-Vasku J. Prognostic value of human epididymis protein 4 in endometrial cancer and its utility for surgical staging. J Obstet Gynaecol Res. 2015;41(10):1644–1652. [DOI] [PubMed] [Google Scholar]
- 11. Gottwald L, Pluta P, Piekarski J, et al. Long-term survival of endometrioid endometrial cancer patients. Arch Med Sci. 2010;6(6):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angioli R, Miranda A, Aloisi A, et al. A critical review on HE4 performance in endometrial cancer: where are we now? Tumour Biol. 2014;35(2):881–887. [DOI] [PubMed] [Google Scholar]
- 13. Steffensen KD, Waldstrøm M, Brandslund I, et al. Identification of high-risk patients by human epididymis protein 4 levels during follow-up of ovarian cancer. Oncol Lett. 2016;11(6):3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai C, Zheng Y, Li Y, et al. Prognostic values of HE4 expression in patients with cancer: a meta-analysis. Cancer Manag Res. 2018;10(6):4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol. 2006;7(2):167–174. [DOI] [PubMed] [Google Scholar]
- 16. Cymbaluk-Ploska A, Chudecka-Glaz A, Pius-Sadowska E, et al. Clinical importance of serum HE4 and MMP2 levels in endometrial cancer patients. Onco Targets Ther. 2017;10(6):3169–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Zhao F, Hu L, Sun Y. Value of detection of serum human epididymis secretory protein 4 and carbohydrate antigen 125 in diagnosis of early endometrial cancer of different pathological subtypes. Onco Targets Ther. 2015;8(5):1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39 doi:10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abbink K, Zusterzeel PL, Geurts-Moespot AJ, et al. HE4 is superior to CA125 in the detection of recurrent disease in high-risk endometrial cancer patients. Tumour Biol. 2018;40(2):1010428318757103. [DOI] [PubMed] [Google Scholar]
- 20. Bignotti E, Ragnoli M, Zanotti L, et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer. 2011;104(9):1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brennan DJ, Hackethal A, Metcalf AM, et al. Serum HE4 as a prognostic marker in endometrial cancer—a population based study. Gynecol Oncol. 2014;132(1):159–165. [DOI] [PubMed] [Google Scholar]
- 22. Brennan DJ, Hackethal A, Mann KP, et al. Serum HE4 detects recurrent endometrial cancer in patients undergoing routine clinical surveillance. BMC Cancer. 2015;15:33 doi:10.1186/s12885-015-1028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deng L, Gao Y, Li X, et al. Expression and clinical significance of annexin A2 and human epididymis protein 4 in endometrial carcinoma. J Exp Clin Cancer Res. 2015;34(1):96 doi:10.1186/s13046-015-0208-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Gao Y, Tan M, et al. Expression of HE4 in endometrial cancer and its clinical significance. Biomed Res Int. 2015. 10.1155/2015/437468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mutz-Dehbalaie I, Egle D, Fessler S, et al. HE4 is an independent prognostic marker in endometrial cancer patients. Gynecol Oncol. 2012;126(2):186–191. [DOI] [PubMed] [Google Scholar]
- 26. Stiekema A, Lok C, Korse CM, et al. Serum HE4 is correlated to prognostic factors and survival in patients with endometrial cancer. Virchows Arch. 2017;470(6):655–664. [DOI] [PubMed] [Google Scholar]
- 27. Zanotti L, Bignotti E, Calza S, et al. Human epididymis protein 4 as a serum marker for diagnosis of endometrial carcinoma and prediction of clinical outcome. Clin Chem Lab Med. 2012;50(12):2189–2198. [DOI] [PubMed] [Google Scholar]
- 28. Wang Y, Han C, Teng F, Bai Z, Tian W, Xue F. Predictive value of serum HE4 and CA125 concentrations for lymphatic metastasis of endometrial cancer. Int J Gynaecol Obstet. 2017;136(1):58–63. [DOI] [PubMed] [Google Scholar]
- 29. Kalogera E, Scholler N, Powless C, et al. Correlation of serum HE4 with tumor size and myometrial invasion in endometrial cancer. Gynecol Oncol. 2012;124(2):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Capriglione S, Plotti F, Miranda A, et al. Utility of tumor marker HE4 as prognostic factor in endometrial cancer: a single-center controlled study. Tumour Biol. 2015;36(6):4151–4156. [DOI] [PubMed] [Google Scholar]
- 31. Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM, Kinalski M, Terlikowski SJ. Utility of HE4 to identify patients with endometrioid endometrial cancer who may require lymphadenectomy. Adv Med Sci. 2016;61(1):23–27. [DOI] [PubMed] [Google Scholar]
- 32. Shen Y, Li L. Serum HE4 superior to CA125 in predicting poorer surgical outcome of epithelial ovarian cancer. Tumour Biol. 2016;37(11):14765–14772. [DOI] [PubMed] [Google Scholar]
- 33. Angioli R, Capriglione S, Aloisi A, et al. Can HE4 predict platinum response during first-line chemotherapy in ovarian cancer? Tumour Biol. 2014;35(7):7009–7015. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Chen H, Curcuru JR, et al. Serum HE4 level as a biomarker to predict the recurrence of gynecologic cancers. Curr Drug Targets. 2017;18(10):1158–1164. [DOI] [PubMed] [Google Scholar]
- 35. Lu Q, Chen H, Senkowski C, et al. Recombinant HE4 protein promotes proliferation of pancreatic and endometrial cancer cell lines. Oncol Rep. 2016;35(1):163–170. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Chen H, Mariani A, et al. HE4 (WFDC2) promotes tumor growth in endometrial cancer cell lines. Int J Mol Sci. 2013;14(3):6026–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Deng L, Zhuang H, et al. Interaction of HE4 and ANXA2 exists in various malignant cells-HE4-ANXA2-MMP2 protein complex promotes cell migration. Cancer Cell Int. 2019;19:161 doi:10.1186/s12935-019-0864-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang SW, Chen H, Dowdy S, et al. HE4 transcription- and splice variants-specific expression in endometrial cancer and correlation with patient survival. Int J Mol Sci. 2013;14(11):22655–22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdalla N, Pazura M, Slomka A, Piorkowski R, Sawicki W, Cendrowski K. The role of HE4 and CA125 in differentiation between malignant and non-malignant endometrial pathologies. Ginekol Pol. 2016;87(12):781–786. [DOI] [PubMed] [Google Scholar]
- 40. Saarelainen SK, Peltonen N, Lehtimaki T, Perheentupa A, Vuento MH, Maenpaa JU. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma. Am J Obstet Gynecol. 2013;209(2):142.e141–146. [DOI] [PubMed] [Google Scholar]
- 41. Presl J, Novotny Z, Topolcan O, et al. CA125 and HE4 levels in a Czech female population diagnosed with endometrial cancer in preoperative management. Anticancer Res. 2014;34(1):327–331. [PubMed] [Google Scholar]
- 42. Zhang Y, Qu X, Qu PP. Value of circulating tumor cells positive for thyroid transcription factor-1 (TTF-1) to predict recurrence and survival rates for endometrial carcinoma. J BUON. 2016;21(6):1491–1495. [PubMed] [Google Scholar]