Abstract
We investigated changes in peripheral blood metabolites, oxidative stress markers (malondialdehyde, potential antioxidant capacity, and glutathione peroxidase [GPX]), and hepatic gene expression related to oxidative stress in Holstein cows with and without subacute ruminal acidosis (SARA) during the periparturient period. Eighteen multiparous Holstein cows were categorized into SARA (n=9) or non-SARA (n=9) groups depending on whether they developed SARA; reticulo-ruminal pH was <5.6 for more than 3 hr per day, during the 2 weeks after parturition. Blood and liver tissue samples were collected 3 weeks prepartum and 2 and 6 weeks postpartum, with an additional blood sample collected 0 and 4 weeks postpartum. Blood aspartate transaminase (AST) and nonesterified fatty acid (NEFA) increased significantly (P<0.05) after parturition in both groups. GPX activity decreased gradually after parturition in the SARA group. In the SARA group, gene expression of GPX 1 and microsomal glutathione S-transferase 3 (MGST3) decreased significantly (P<0.05), and expression of metallothionein 2A increased significantly (P<0.05) after parturition in the SARA group. Superoxide dismutase 1 and MGST3 decreased significantly (P<0.05) 2 weeks postpartum in the non-SARA group. Gene expression related to oxidative stress was negatively correlated with AST, NEFA and total ketone body levels. Therefore, the hepatic gene expression related to oxidative stress might change associated with a negative energy balance, and might relate the high oxidative stress in the SARA group during periparturient period.
Keywords: hepatic gene expression, Holstein cow, oxidative stress, periparturient period, subacute ruminal acidosis
Major physiological, nutritional, metabolic, and immunological changes occur during the transition period defined as 3 weeks before and after parturition to adapt to parturition and the onset of lactation [29]. A high-grain postpartum diet is necessary after parturition to meet the energy requirements for lactation while increasing the risk of subacute ruminal acidosis (SARA) [3, 16]. SARA reduces productivity by decreasing dry matter intake and milk production, and is involved in various disease occurrences by translocation of ruminal lipopolysaccharides (LPS) into the bloodstream [16, 22].
Oxidative stress, which reflects an imbalance between oxidant and antioxidant status, causes oxidative damage to macromolecules, such as lipids, proteins, and DNA [21]. Oxidative stress develops during early lactation in cows and increases the risk of various diseases by causing dysfunction in the inflammatory response [3, 29]. Furthermore, oxidative stress can be measured using biological markers, including malondialdehyde (MDA), glutathione peroxidase (GPX), and potential antioxidant capacity (PAO). Among these, MDA is the end product of lipid peroxidation caused by the generation of reactive oxygen species (ROS) [32]. GPX is an antioxidant enzyme involved in lipid peroxidation that produces MDA and converts hydrogen peroxide to water in the presence of glutathione [32]. PAO is a measure of the total antioxidant capacity using the reduction reaction of copper ions [23]. In addition, Gessner et al. [11] reported that upregulation of the nuclear factor E2-related factor 2 (NEF2L2) gene controls the transcription of genes encoding various antioxidant enzymes and antioxidant material in the liver after parturition in Holstein cows.
Serum L-lactate concentration, a marker of ruminal activity, correlates with serum oxidative stress markers in early postpartum cows [2]. In addition, it has reported that there is a relationship between increased oxidative stress and occurrence of SARA in Holstein cows. SARA-induced cows fed a high-grain diet show increased oxidative stress [1, 13], and exhibit changes in oxidative stress markers in the liver and plasma and hepatic NEF2L2 target gene expression with increasing LPS translocated into the bloodstream [1]. However, the relationships among the severity of SARA, peripheral blood metabolites, and peripheral blood oxidative stress markers, and hepatic gene expression relative to oxidative stress are unclear in periparturient Holstein cows. Therefore, the objective of this study was to investigate changes in peripheral blood metabolites, peripheral blood oxidative stress markers, and expression of hepatic genes involved in oxidative stress and metabolism in Holstein cows with and without postpartum SARA. We re-analyzed our previously published data regarding blood measurements [30] to evaluate their relationships with oxidative stress markers and liver gene expression related to oxidative stress and metabolism.
MATERIALS AND METHODS
Animals and group assignment
This experimental protocol was approved by the Iwate University Laboratory Animal Care and Use Committee (A201452-2; Morioka, Japan). Eighteen multiparous Holstein cows were obtained from Farm A and B, and were fed dry and lactation period diets ad libitum before and after parturition. Feed composition and amounts were based on the requirements of the Japanese Feeding Standard for Dairy Cattle. Nutrient compositions (% of dry matter) of the diets fed lactation period in Farm A and B, total digestible nutrient was 66.0% and 74.7%, crude protein was 16.1% and 15.0%, neutral detergent fiber was 41.1% and 37.4%, acid detergent fiber was 25.1% and 18.4%, starch was 16.7% and 23.3%, respectively. SARA was diagnosed when reticulo-ruminal pH was <5.6 for more than 3 hr per day [12] during the 2 weeks following parturition. Based on these criteria, cows were assigned to the SARA (n=9) or non-SARA (n=9) group. The 7 day mean reticulo-ruminal pH decreased after parturition in both groups and decreased significantly 1 week after parturition in the SARA group compared with the non-SARA group. Cows were milked twice daily at 0830 and 1600 hr, and the quantity of milk was determined and recorded at each milking using a milk meter installed in the milking parlor. Dry matter intake (DMI) were measured daily throughout the experiment period.
Blood sampling, biochemical components, and oxidative stress parameter analyses
Blood samples were collected 3 weeks before and 0, 2, 4, and 6 weeks after parturition. Samples were collected from the jugular vein into 10 ml evacuated tubes containing heparin (BD Vacutainer, Franklin Lakes, NJ, USA). Samples were centrifuged (1,500 ×g, 15 min, 4°C) immediately to separate the plasma and stored at −80°C until analysis. For blood biochemical analysis, triglyceride (TG), total cholesterol (T-CHO), total protein (TP), albumin (ALB), blood urea nitrogen (BUN), aspartate transaminase (AST), γ-glutamyl transpeptidase (GGT), total calcium (Ca), phosphate (IP), glucose (GLU), non-esterified fatty acid (NEFA), and total ketone body (T-KB) were measured using an automated biochemistry analyzer (Accute, Toshiba Ltd., Tokyo, Japan). For blood oxidative stress parameter analysis, plasma MDA was measured using a commercially available kit (Malondialdehyde Assay; Northwest Life Science Specialties LCC, Vancouver, WA, USA) as described elsewhere [6]. Plasma PAO was measured using a commercially available kit (PAO antioxidant capacity measurement kit; Nikken Zile Japan Aging Control Laboratory, Shizuoka, Japan). GPX was measured using a commercially available kit (Glutathione Peroxidase Assay; Northwest Life Science Specialties LCC).
Liver biopsy and RNA extraction
The liver tissue was biopsied from intercostal space 9–11 under ultrasound guidance by a skilled veterinarian as 3 weeks prepartum and 2 and 6 weeks postpartum. A sterile, percutaneous needle biopsy (Acecut; TSK Laboratory, Tochigi, Japan) was used under local anesthesia to biopsy the tissue. Samples were collected three times from each cow during each sample collection in the 2 hr after the morning feeding (1100 hr). Then, the liver tissue samples were temporarily stocked in a liquid nitrogen tank and stored at −80°C until use. Total RNA was extracted from liver tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) as described previously [15]. The purity of the extracted RNA was improved using an RNeasy RNA Clean-up Kit (Qiagen, Valencia, CA, USA). Total RNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA samples were treated with TURBO DNase (Applied Biosystems, Foster City, CA, USA) to remove contaminating DNA. Total RNA (600 ng) was converted to first-strand cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). qPCR was performed using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and the StepOne™ Plus Real-Time PCR system (Applied Biosystems) as described previously [15]. Primers designed for the genes involved in oxidative stress, metabolism, and negative energy balance (NEB) are shown in Table 1. The results were recorded as relative changes in gene expression normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein L27 (RPL27), and β-actin (ACTB) by the 2–∆∆Ct method [18]. We examined GAPDH, RPL27, and ACTB to evaluate their use as reference genes for qPCR, and there were no significant differences among them.
Table 1. Gene symbols and sequences of the primers used for quantitative real-time PCR.
| Gene symbol a) | Primer Sequences (5′-3′) | Amplicon Size, bp | |
|---|---|---|---|
| Forward | Reverse | ||
| Oxidative stress | |||
| GPX3 | TAGCCACCCTCAAGTATGTTCGA | CCCATTCACATCGCCTTTCT | 80 |
| GPX1 | CCCCTGCAACCAGTTTGG | CGCCTGGTCGGACGTACTT | 80 |
| SOD1 | CACGATGGTGGTCCATGAAA | TTCCAGCGTTGCCAGTCTTT | 80 |
| CAT | TCCAAGGCGAAGGTGTTTG | CCCGATTCTCCAGCAACAGT | 80 |
| MT1A | CCCACTGGCGGCTCCT | ACTTGGCACAGCCCACAG | 80 |
| MT2A | CACCGCGGGTGAATCCT | GAGCAGCAGCTCTTCTTGCA | 80 |
| MGST3 | TTTTTTCTAGCTGTCGGAGGTGTT | AGGACTCGTCCAACGATCCA | 80 |
| NFE2L2 | CAAGTTTGGGAGGAACTATTATCCA | GTACTAGTCTCAGCCAGCTTGTCATT | 80 |
| Metabolism | |||
| PC | CAAAGCAGGTGGGCTACGAGAAC | CAGGTCCACATCTGTGATCTCCTC | 137 |
| PCK1 | CCTGTTGGTGTCCCTCTGGTCTAC | CATGATGACTTTGCCCTTGTACTCC | 117 |
| IGF1R | TTAAAATGGCCAGAACCTGAG | ATTATAACCAAGCCTCCCAC | 314 |
| SLC2A4 | CTCTTCTGGAGCAGGAAGTGAAA | CCTCTGTGGCCCTCAGTCAT | 80 |
| ACADVL | ATCATTGCTAAGGCGGTGGAT | TCCTGGATCAGCCCAAAGTT | 80 |
| ACSL1 | CAACCCCAAAGGAGCAATGAT | ACGTGTTCTCTGTCATTTTCACAAA | 80 |
| CPT1A | GAGGAATGTCAGGAGGTCATTGA | AAGTGCGGAATGGGAAGGA | 80 |
| PPARA | CCCATAACGCGATTCGTTTT | CATGCTCACACGTAAGGATTTCTG | 80 |
| Negative energy balance | |||
| FGF21 | CGGATCGCTGCACTTTGAC | CCGACTGGTAGACGTTGTATCCA | 80 |
| KLB | CGCTTGGCATGGGTATGG | TCGAATGAGCCTTGATCAGGTT | 80 |
| Housekeeping gene | |||
| GAPDH | GCCGATGCCCCCATGT | CAGGAGGCATTGCTGACAATC | 80 |
| ACTB | GGCCGAGCGGAAATCG | GCCATCTCCTGCTCGAAGTC | 80 |
| RPL27 | GCCCGACGAGAGGCAA | AACCGCAGCTTCTGGAAGAA | 80 |
a) GPX3; glutathione peroxidase 3, GPX1; glutathione peroxidase 1, SOD1; superoxide dismutase 1, CAT; catalase, MT1A; metallothionein 1A, MT2A; metallothionein 2A, MGST3; microsomal glutathione S-transferase 3, NFE2L2; nuclear factor E2-related factor 2, PC; pyruvate carboxylase, PCK1; cytosolic phosphoenolpyruvate carboxykinase, IGF1R; insulin like growth factor 1 receptor, SLC2A4; solute carrier family 2 member 4, ACADVL; acyl-CoA dehydrogenase very long chain, ACSL1; acyl-CoA synthetase long-chain family member 1, CPT1A; carnitine-palmitoyl-transferase 1A, PPARA; peroxisome proliferator-activated receptor α, FGF21; fibroblast growth factor 21, KLB; klotho beta, GAPDH; glyceraldehyde-3-phosphate dehydrogenase, ACTB; β-actin, RPL27; ribosomal protein L27.
Statistical analyses
Outliners were excluded by the outliner test (ROUT method, Q=1.0%). Data were assessed for normality using the Shapiro–Wilk test. Significant differences between the SARA and non-SARA groups at the same sampling time were evaluated using the unpaired t-test for normally distributed variables and Mann–Whitney U-test for non-normallly distributed variables. A mixed-model repeated measures analysis of variance, using time as a fixed effect, followed by Dunnett’s multiple comparison method, was used to determine within-group differences. Correlation coefficients were calculated using Pearson’s correlation coefficient analysis for normally distributed variables and Spearman’s correlation coefficient analysis for non-normally distributed variables. A heatmap was constructed using Prism software (version 8.21; GraphPad Software Inc., La Jolla, CA, USA) based on the Pearson’s or Spearman’s correlation coefficient. Additionally, all numerical data were analyzed using Prism software (version 8.21). A P value <0.05 was considered significant.
RESULTS
DMI and milk yield
DMI increased significantly (P<0.05) at 1–6 weeks postpartum compared with 3 weeks prepartum in both groups. Milk yield increased significantly (P<0.05) at 1–6 weeks postpartum compared with 0 week postpartum in both groups. No significant difference was observed in DMI or milk yield between the SARA and non-SARA groups during the periparturient period. For example, at 4 weeks postpartum, DMI in the SARA and non-SARA group were 25.7 kg and 23.6 kg, and milk yield in the SARA and non-SARA group were 45.1 kg and 45.9 kg, respectively.
Biochemical components
Mean values at 0–6 weeks postpartum in the SARA and non-SARA group, TG (mg/dl) was 2.4–6.5 and 3.3–6.4, T-CHO (mg/dl) was 67.0–175.1 and 65.4–174.0, TP (g/dl) was 6.4–6.9 and 6.4–7.4, ALB (g/dl) was 4.5–5.2 and 4.1–4.6, BUN (mg/dl) was 9.6–12.5 and 9.5–11.7, AST (IU/l) was 76.8–99.1 and 66.8–100.3, GGT (IU/l) was 22.6–27.4 and 18.6–26.9, Ca (mg/dl) was 8.4–10.0 and 8.5–9.4, IP (mg/dl) was 2.8–3.4 and 1.8–2.9, GLU (mg/dl) was 57.8–68.3 and 55.2–81.5, NEFA (µmol/l) was 143–549 and 160–569, T-KB (µmol/l) was 639–806 and 640–922, respectively. T-CHO and NEFA levels, as well as AST activity, increased significantly (P<0.05), and TG concentrations decreased significantly (P<0.05) after parturition in both groups. GLU concentration significantly (P<0.05) decreased after parturition in the SARA group, and T-KB concentration increased significantly (P<0.05) after parturition in the non-SARA group. TG concentration at 0 weeks and T-KB concentration at 2 weeks postpartum were significantly lower (P<0.05) in the SARA group compared with the non-SARA group.
Oxidative stress parameters
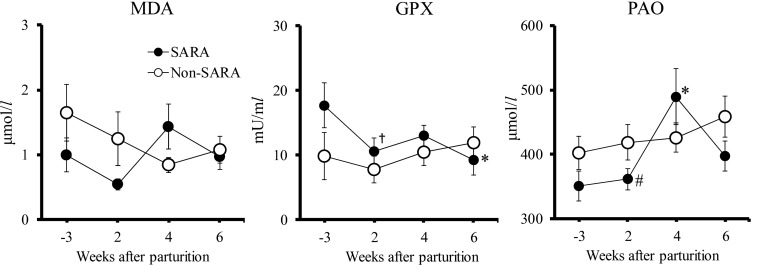
The MDA level did not change significantly (P<0.05) at any point during the periparturient period in the SARA and non-SARA groups (P=0.103 and 0.253, Fig. 1). GPX activity tended to decrease (P<0.1) 2 weeks postpartum, and decreased significantly (P<0.05) 6 weeks postpartum compared with 3 weeks prepartum in the SARA group. No significant differences were observed in MDA level or GPX activity between the SARA and non-SARA groups during the periparturient period. PAO level increased significantly (P<0.05) 4 weeks postpartum compared with 3 weeks prepartum in the SARA group. The SARA group tend to have a lower PAO level (P=0.090) 2 weeks postpartum than that of the non-SARA group.
Fig. 1.
Changes in malondialdehyde (MDA), glutathione peroxidase (GPX), and potential antioxidant capacity (PAO) levels in Holstein cows with subacute ruminal acidosis (SARA group, n=8) and without SARA (non-SARA group, n=8) during the periparturient period. *, † Denote significant (P<0.05) and tendency (P<0.1) within-group differences compared to 3 weeks prepartum. # Denotes a tendency (P<0.1) difference between the SARA and non-SARA groups. Values represent mean ± SE.
Relative gene expression
In the SARA group, glutathione peroxidase 1 (GPX1) and microsomal glutathione S-transferase 3 (MGST3) expression levels decreased were significantly (P<0.05) after parturition and glutathione peroxidase 3 (GPX3) expression 2 weeks postpartum and metallothionein 2A (MT2A) expression 6 weeks postpartum increased significantly (P<0.05) (Fig. 2). In the non-SARA group, superoxide dismutase 1 (SOD1) and MGST3 expression levels were significantly (P<0.05) lower 2 weeks postpartum. Expression of metallothionein 1A (MT1A) tended to be higher 2 and 6 weeks postpartum in the SARA group than that in the non-SARA group (P=0.054, and P=0.055, respectively). Pyruvate carboxylase (PC) and cytosolic phosphoenolpyruvate carboxykinase (PCK1) expression levels increased significantly (P<0.05) after parturition in both groups. Expression of solute carrier family 2 member 4 (SLC2A4) in both groups and insulin like growth factor 1 receptor (IGF1R) in the SARA group decreased significantly (P<0.05) after parturition. Acyl-CoA dehydrogenase very long chain (ACADVL) and carnitine-palmitoyl-transferase 1A (CPT1A) expression levels in the SARA group, and ACADVL and acyl-CoA synthetase long-chain family member 1 (ACSL1) expression levels in the non-SARA group were significantly higher (P<0.05) 2 weeks postpartum. Expression of IGF1R and ACADVL was significantly (P<0.05) lower 6 weeks postpartum in the SARA group compared with the non-SARA group.
Fig. 2.
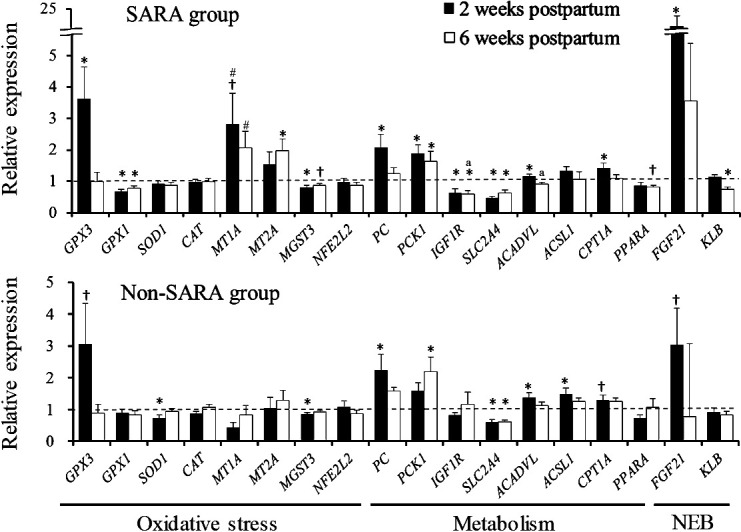
Relative gene expression involved in oxidative stress, metabolism, and negative energy balance (NEB) relative to 3 weeks prepartum in Holstein cows with subacute ruminal acidosis (SARA group, n=9) and without SARA (non-SARA group, n=9) during the periparturient period. *, † Denote significant (P<0.05) and tendency (P<0.1) within-group differences compared to 3 weeks prepartum. a, # Denote significant (P<0.05) and tendency (P<0.1) differences between the SARA and non-SARA groups. Values represent mean ± SE.
Correlation analyses of relative gene expression
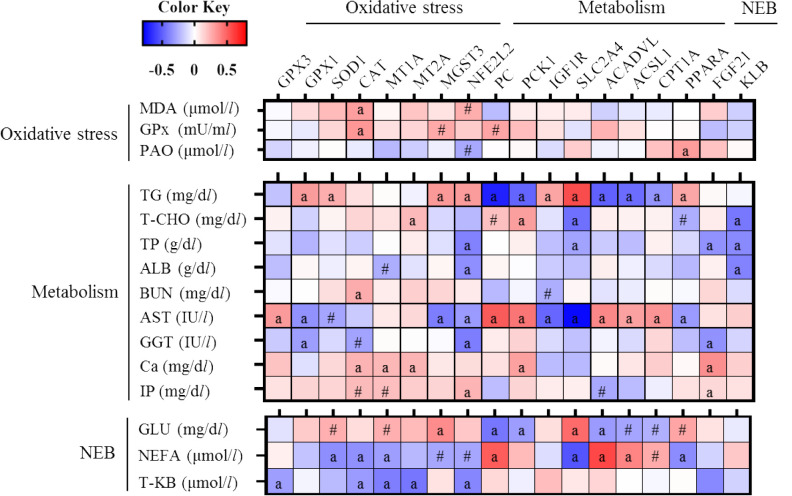
The expression levels of SOD1, catalase (CAT), and MT1A were negatively correlated (P<0.05; r= −0.356, −0.350, and −0.305, respectively) with NEFA level (Fig. 3). The expression levels of GPX3, CAT, MT1A, MT2A, and NFE2L2 were negatively correlated (P<0.05; r= −0.305, −0.317, −0.407, −0.432, and −0.380, respectively) with T-KB concentration. The expression levels of GPX1, MGST3, and NFE2L2 were negatively correlated (P<0.05; r= −0.344, −0.410, and −0.316, respectively) with AST level. The expression levels of PC, PCK1, ACADVL, ACSL1, and CPT1A were positively correlated with AST (r=0.689, 0.575, 0.497, 0.391, and 0.445, respectively) and NEFA levels (r=0.510, 0.215, 0.577, 0.385, and 0.263, respectively), and negatively correlated with TG (r= −0.697, −0.488, −0.497, −0.464, and −0.338, respectively) and GLU levels (r= −0.431, −0.310, −0.314, −0.283, and −0.248, respectively). By contrast, the expression levels of IGF1R and SLC2A4 were positively correlated with TG (r=0.374 and 0.743) and GLU levels (r=0.101 and 0.467), and negatively correlated with AST (r= −0.479 and −0.773) and NEFA levels (r= −0.109 and −0.534).
Fig. 3.
Heatmaps of the correlation coefficients for Holstein cows during the periparturient period. Cells are colored based on Pearson’s or Spearman’s correlation coefficient analyses. Blue and red represent negative and positive correlations, respectively. a, # Denote significant (P<0.05) and tendency (P<0.1) correlations.
DISCUSSION
Previous studies have shown that peripheral blood oxidative stress parameters increase in SARA-induced cows fed a high-grain diet [1, 13]. Plasma MDA levels increase and GPX activity decreases in SARA-induced cows compared with those in non-SARA cows [1]. However, in the present study, MDA level and GPX activity were not different at any point between the SARA and non-SARA groups. Abaker et al. [1] compared SARA and non-SARA cows after an 18 week long-term SARA induced challenge, which differed in the duration of SARA from our present study. Therefore, the lack of significant differences in MDA level and GPX activity between the groups was likely due to the lack of a severe long-term challenge compared to the previous report [1]. GPX activity decreased gradually after parturition in the SARA group. In addition, the PAO level was lower than that in the non-SARA group 2 weeks postpartum. Therefore, antioxidant capacity decreased after parturition in the SARA group. The PAO levels increased significantly, and the MDA level and GPX activity increased numerically at 4 weeks postpartum in the SARA group compared with that prepartum. Bernabucci et al. [5] reported that the antioxidant enzyme transiently increases during the early stage of the oxidative stress response in dairy cows during the periparturient period. Therefore, we assumed that the PAO level increased because of the compensatory effects of the antioxidants accompanying increased oxidative stress. However, it was not possible to clarify why oxidative stress increased 4 weeks postpartum in the SARA group.
Oxidative stress is associated with high NEFA values and hyperketonemia, and NEFAs are mobilized from adipose tissue and increase β-oxidation in the liver during NEB, enhancing the production of ROS [3, 29]. High NEFA and β-hydroxybutyrate levels increase ROS production in hepatocytes, leading to increased oxidative stress [27, 28]. In the present study, AST and NEFA increased after parturition in both groups, and GLU decreased in the SARA group, suggesting that NEB occurred, and β-oxidation of NEFA in the liver was enhanced after parturition in both groups. In addition, the expression of many of the hepatic antioxidative genes was negatively correlated with AST, GGT, NEFA, and T-KB levels, and positively correlated with the GLU level. Therefore, reduced hepatic antioxidative gene expression was associated with NEB, such as increases in NEFA and T-KB and decreases in GLU and liver function by lipid β-oxidation in periparturient dairy cows.
Expression of the hepatic NFE2L2 target gene with antioxidative properties is enhanced during the transition period [11] when oxidative stress increases [3, 29]. In addition, the expression of several hepatic NFE2L2 target genes decrease with increased blood MDA and decreased blood GPX in SARA-induced cows [1]. These reports [1, 11] suggest that hepatic gene expression related to oxidative stress is upregulated or downregulated in response to increased oxidative stress. In the present study, GPX1 and MGST3 expression levels decreased, GPX3 and MT2A expression levels increased after parturition in the SARA group, and SOD1 and MGST3 decreased 2 weeks postpartum in the non-SARA group. In addition, significant intragroup differences were identified 2 and 6 weeks postpartum in the SARA group, but only at 2 weeks postpartum in the non-SARA group. These results suggest that the different hepatic reactions to oxidative stress are induced and show greater and more longitudinal fluctuations in hepatic gene expression related to oxidative stress in the SARA group than those in the non-SARA group.
MT1A encodes the metallothionein 1A protein, which controls oxidative stress, inflammation, and hormone signaling [17]. Furthermore, MT1A is one of the NFE2L2 target genes [11], and relative NFE2L2 mRNA and protein expression decreased in SARA-induced cows [1]. MT1A expression was higher in the SARA group 2 and 6 weeks postpartum compared with the non-SARA group. These results suggest that the expression of MT1A might be associated with the response to oxidative stress in SARA cows. NFE2L2 controls the transcription of genes related to antioxidative functions [11]. In the present study, NEF2L2 expression did not change significantly after parturition in either group, but was positively correlated with blood MDA level and negatively correlated with blood PAO level. In addition, CAT expression was positively correlated with MDA and expression of many of the hepatic NFE2L2 target genes was positively correlated with MDA and negatively correlated with PAO, although not statistically significant. These results suggest that changes in peripheral blood oxidative stress markers might be associated with the status of hepatic gene expression related to oxidative stress.
PC and PCK1 are involved in the gluconeogenic pathway [26], and ACSL, CPT1A, and ACADVL are involved in β-oxidation of lipids [19, 25, 31]. In the present study, expression of these genes increased after parturition in both groups and was positively correlated with NEFA and AST, and negatively correlated with GLU, suggesting that NEFA increased and GLU decreased after parturition in response to NEB [29], and gluconeogenesis and β-oxidation of lipids in the liver were enhanced. In addition, FGF21 expression increased at 2 weeks and 6 weeks postpartum in the SARA group compared with the non-SARA group. FGF21 expression increases with NEB development, and enhances fatty acid oxidation [9, 14]. Therefore, these results suggested that SARA group might respond more strongly to NEB than non-SARA group. Further, ACADVL expression decreased 6 weeks postpartum in the SARA group compared with the non-SARA group. ACADVL expresses an enzyme that converts acyl CoA to acetyl CoA in mitochondria during β-oxidation of lipids [31]. Therefore, we assumed that while it was necessary to enhance fatty acid oxidation, as shown by the increased expression of FGF21, β-oxidation of lipids in the liver was suppressed 6 weeks postpartum in the SARA group compared to the non-SARA group.
IGF1R expression decreased after parturition in the SARA group, and SLC2A4 expression decreased after parturition in both groups. In contrast to the correlations between blood metabolites and gene expression related to gluconeogenesis and β-oxidation of lipids, IGF1R and SLC2A4 expression levels were positively correlated with GLU, and negatively correlated with NEFA. IGF1R and SLC2A4 are involved in insulin function [8, 10]. These results suggest that the insulin response decreased in both groups accompanying NEB after parturition. Insulin sensitivity and responsiveness are attenuated during the early lactation period [4], and the expression of hepatic IGF1 mRNA decreases [24]. The action of IGF1 is mainly controlled by IGF1 receptor signaling [8]. In the present study, the expression of IGF1R, which encodes the IGF1 receptor, decreased 6 weeks postpartum in the SARA group compared with the non-SARA group. The peripheral tissue response to insulin remains low during the early lactation period but gradually recovers [4]. We speculated that recovery of the hepatic insulin response might be delayed in the SARA group compared to the non-SARA group. SARA induces metabolic endotoxemia and systemic inflammation [1, 12], and metabolic endotoxemia results in insulin resistance [7]. Therefore, it has been suggested that SARA may cause insulin resistance [20], as supported by our results.
In conclusion, hepatic antioxidative gene expression decreased in association with an increase of NEFA and enhanced β-oxidation of lipids accompanied by NEB in Holstein cows during the periparturient period. In addition, the decrease in peripheral blood GPX activity and fluctuations in hepatic gene expression related to oxidative stress were larger in the SARA group than those in the non-SARA group. The SARA group was considered high oxidative stress status. Moreover, SARA cows might develop glycometabolic changes such as suppressed β-oxidation in the liver and a reduced insulin reaction compared to non-SARA cows.
REFERENCES
- 1.Abaker J. A., Xu T. L., Jin D., Chang G. J., Zhang K., Shen X. Z.2017. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 100: 666–678. doi: 10.3168/jds.2016-10871 [DOI] [PubMed] [Google Scholar]
- 2.Abuelo Á., Hernández J., Benedito J. L., Castillo C.2015. The connexion between serum redox balance and concentration of lactic acid enantiomers in dairy cows around the time of calving. Comp. Clin. Pathol. 24: 465–468. doi: 10.1007/s00580-014-1975-x [DOI] [Google Scholar]
- 3.Abuelo A., Hernández J., Benedito J. L., Castillo C.2019. Redox biology in transition periods of dairy cattle: role in the health of periparturient and neonatal animals. Antioxidants 8: 20. doi: 10.3390/antiox8010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell A. W., Bauman D. E.1997. Adaptations of glucose metabolism during pregnancy and lactation. J. Mammary Gland Biol. Neoplasia 2: 265–278. doi: 10.1023/A:1026336505343 [DOI] [PubMed] [Google Scholar]
- 5.Bernabucci U., Ronchi B., Lacetera N., Nardone A.2005. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 88: 2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2 [DOI] [PubMed] [Google Scholar]
- 6.Botsoglou N. A., Fletouris D. J., Papageorgiou G. E., Vassilopoulos V. N., Mantis A. J., Trakatellis A. G.1994. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 42: 1931–1937. doi: 10.1021/jf00045a019 [DOI] [Google Scholar]
- 7.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., Waget A., Delmée E., Cousin B., Sulpice T., Chamontin B., Ferrières J., Tanti J. F., Gibson G. R., Casteilla L., Delzenne N. M., Alessi M. C., Burcelin R.2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772. doi: 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 8.Coyne G. S., Kenny D. A., Waters S. M.2011. Effect of dietary n-3 polyunsaturated fatty acid supplementation on bovine uterine endometrial and hepatic gene expression of the insulin-like growth factor system. Theriogenology 75: 500–512. doi: 10.1016/j.theriogenology.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 9.Domouzoglou E. M., Maratos-Flier E.2011. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am. J. Clin. Nutr. 93: 901S–5. doi: 10.3945/ajcn.110.001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duehlmeier R., Sammet K., Widdel A., von Engelhardt W., Wernery U., Kinne J., Sallmann H. P.2007. Distribution patterns of the glucose transporters GLUT4 and GLUT1 in skeletal muscles of rats (Rattus norvegicus), pigs (Sus scrofa), cows (Bos taurus), adult goats, goat kids (Capra hircus), and camels (Camelus dromedarius). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146: 274–282. doi: 10.1016/j.cbpa.2006.10.029 [DOI] [PubMed] [Google Scholar]
- 11.Gessner D. K., Schlegel G., Keller J., Schwarz F. J., Ringseis R., Eder K.2013. Expression of target genes of nuclear factor E2-related factor 2 in the liver of dairy cows in the transition period and at different stages of lactation. J. Dairy Sci. 96: 1038–1043. doi: 10.3168/jds.2012-5967 [DOI] [PubMed] [Google Scholar]
- 12.Gozho G. N., Plaizier J. C., Krause D. O., Kennedy A. D., Wittenberg K. M.2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88: 1399–1403. doi: 10.3168/jds.S0022-0302(05)72807-1 [DOI] [PubMed] [Google Scholar]
- 13.Guo Y., Xu X., Zou Y., Yang Z., Li S., Cao Z.2013. Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with subacute ruminal acidosis and its regulation with pelleted beet pulp. J. Anim. Sci. Biotechnol. 4: 31. doi: 10.1186/2049-1891-4-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M. J., Jacometo C. B., Graugnard D. E., Corrêa M. N., Schmitt E., Cardoso F., Loor J. J.2014. Overfeeding dairy cattle during late-pregnancy alters hepatic PPARα-regulated pathways including hepatokines: impact on metabolism and peripheral insulin sensitivity. Gene Regul. Syst. Bio. 8: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y. H., Toji N., Kizaki K., Kushibiki S., Ichijo T., Sato S.2016. Effects of dietary forage and calf starter on ruminal pH and transcriptomic adaptation of the rumen epithelium in Holstein calves during the weaning transition. Physiol. Genomics 48: 803–809. doi: 10.1152/physiolgenomics.00086.2016 [DOI] [PubMed] [Google Scholar]
- 16.Kleen J. L., Hooijer G. A., Rehage J., Noordhuizen J. P. T. M.2003. Subacute ruminal acidosis (SARA): a review. J. Vet. Med. A Physiol. Pathol. Clin. Med. 50: 406–414. doi: 10.1046/j.1439-0442.2003.00569.x [DOI] [PubMed] [Google Scholar]
- 17.Lappas M.2018. Expression and regulation of metallothioneins in myometrium and fetal membranes. Am. J. Reprod. Immunol. 80: e13040. doi: 10.1111/aji.13040 [DOI] [PubMed] [Google Scholar]
- 18.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 19.Lopes-Marques M., Cunha I., Reis-Henriques M. A., Santos M. M., Castro L. F.2013. Diversity and history of the long-chain acyl-CoA synthetase (Acsl) gene family in vertebrates. BMC Evol. Biol. 13: 271. doi: 10.1186/1471-2148-13-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda M., Kawasumi K., Sato S., Arai T.2019. Evaluation of blood adiponectin levels as an index for subacute ruminal acidosis in cows: a preliminary study. Vet. Res. Commun. 43: 215–224. doi: 10.1007/s11259-019-09760-0 [DOI] [PubMed] [Google Scholar]
- 21.Mavangira V., Sordillo L. M.2018. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 116: 4–14. doi: 10.1016/j.rvsc.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 22.Plaizier J. C., Krause D. O., Gozho G. N., McBride B. W.2008. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet. J. 176: 21–31. doi: 10.1016/j.tvjl.2007.12.016 [DOI] [PubMed] [Google Scholar]
- 23.Pregel P., Bollo E., Cannizzo F. T., Biolatti B., Contato E., Biolatti P. G.2005. Antioxidant capacity as a reliable marker of stress in dairy calves transported by road. Vet. Rec. 156: 53–54. doi: 10.1136/vr.156.2.53 [DOI] [PubMed] [Google Scholar]
- 24.Radcliff R. P., McCormack B. L., Crooker B. A., Lucy M. C.2003. Plasma hormones and expression of growth hormone receptor and insulin-like growth factor-I mRNA in hepatic tissue of periparturient dairy cows. J. Dairy Sci. 86: 3920–3926. doi: 10.3168/jds.S0022-0302(03)74000-4 [DOI] [PubMed] [Google Scholar]
- 25.Saini-Chohan H. K., Mitchell R. W., Vaz F. M., Zelinski T., Hatch G. M.2012. Delineating the role of alterations in lipid metabolism to the pathogenesis of inherited skeletal and cardiac muscle disorders: Thematic Review Series: Genetics of Human Lipid Diseases. J. Lipid Res. 53: 4–27. doi: 10.1194/jlr.R012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selim S., Salin S., Taponen J., Vanhatalo A., Kokkonen T., Elo K. T.2014. Prepartal dietary energy alters transcriptional adaptations of the liver and subcutaneous adipose tissue of dairy cows during the transition period. Physiol. Genomics 46: 328–337. doi: 10.1152/physiolgenomics.00115.2013 [DOI] [PubMed] [Google Scholar]
- 27.Shi X., Li D., Deng Q., Li Y., Sun G., Yuan X., Song Y., Wang Z., Li X., Li X., Liu G.2015. NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes. J. Steroid Biochem. Mol. Biol. 145: 103–112. doi: 10.1016/j.jsbmb.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 28.Song Y., Li N., Gu J., Fu S., Peng Z., Zhao C., Zhang Y., Li X., Wang Z., Li X., Liu G.2016. β-Hydroxybutyrate induces bovine hepatocyte apoptosis via an ROS-p38 signaling pathway. J. Dairy Sci. 99: 9184–9198. doi: 10.3168/jds.2016-11219 [DOI] [PubMed] [Google Scholar]
- 29.Sordillo L. M., Raphael W.2013. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. North Am. Food Anim. Pract. 29: 267–278. doi: 10.1016/j.cvfa.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya Y., Chiba E., Sugino T., Kawashima K., Hasunuma T., Kushibiki S., Kim Y. H., Sato S.2020. Changes in rumen fermentation, bacterial community, and predicted functional pathway in Holstein cows with and without subacute ruminal acidosis during the periparturient period. J. Dairy Sci. 103: 4702–4716. doi: 10.3168/jds.2019-17546 [DOI] [PubMed] [Google Scholar]
- 31.Tucci S., Herebian D., Sturm M., Seibt A., Spiekerkoetter U.2012. Tissue-specific strategies of the very-long chain acyl-CoA dehydrogenase-deficient (VLCAD-/-) mouse to compensate a defective fatty acid β-oxidation. PLoS One 7: e45429. doi: 10.1371/journal.pone.0045429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., Telser J.2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39: 44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]