Abstract
A 3-month-old male Scottish Fold kitten with pleural fluid and low ratio of albumin to globulin (A/G ratio) was brought to our small animal hospital. Since RNA from the type I feline coronavirus (FCoV) were detected in drained pleural fluid, the cat was tentatively diagnosed with effusive feline infectious peritonitis (FIP). Following the administration of itraconazole and prednisolone, the A/G ratio increased, and the pleural fluid mostly disappeared. The fecal FCoV levels temporarily decreased. However, the cat showed neurological manifestations and was eventually euthanized due to status epilepticus after 38 days of treatment. In conclusion, itraconazole partly exerted a beneficial effect in a cat with FIP. However, further investigation of a possible role of itraconazole in FIP treatment is warranted.
Keywords: feline infectious peritonitis, itraconazole, neurological manifestation, pleural fluid, type I feline coronavirus
Feline infectious peritonitis (FIP) is a highly fatal disease caused by systemic infection by the feline coronavirus (FCoV). FCoV is serologically classified into two types: type I (dominant serotype worldwide) and type II (which arises by recombination between type I FCoV and canine coronavirus). FCoV is also divided into two different biotypes: feline enteric coronavirus and the FIP virus. FCoV replicates in monocytes and macrophages which leads to systemic infection. There are two common pathological forms of FIP: the effusive form, which is characterized by an accumulation of abdominal, pleural and/or pericardial fluid and the non-effusive form, which is characterized by granulomatous inflammation in several organs. In some cases, neurological FIP also occurs; manifestations include seizures and ataxia [4]. An anti-inflammatory agent, prednisolone (Pred) was historically used to support cats with FIP, but in recent reports of successful FIP treatment it has been replaced by non-steroidal anti-inflammatory drugs (NSAIDs).
Itraconazole (ICZ), a triazole antifungal agent, inhibits the biosynthesis of cholesterol via binding to the fungal cytochrome P450 [3]. It is reported that cholesterol may be an essential cofactor in type I FCoV infection [19]. Moreover, in vitro studies using Felis catus whole fetus (fcwf)-4 cells revealed that ICZ inhibited type I FCoV infection [18]. Although ICZ is expected to play an inhibitory role against FCoV infection, its effects on the progression of spontaneous FIP are unknown. We evaluated the clinical efficacy of combination therapy of ICZ (10 mg/kg q12 hr) and Pred (1 mg/kg q24 hr) in the treatment of a cat with spontaneous FIP.
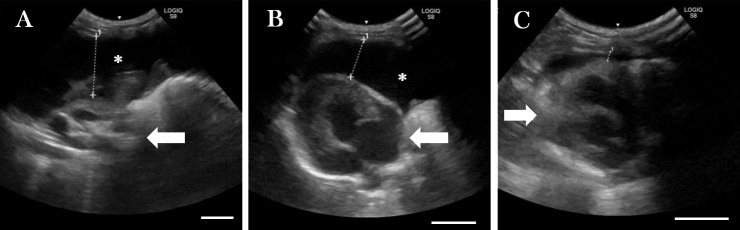
A 3-month-old entire male Scottish Fold kitten, suspected of having an accumulation of pleural fluid, was referred to Kitasato University Small Animal Medical Center. The cat presented on Day 0 with mouth breathing (respiratory rate: 84 breaths per minute). His blood test showed a low ratio of albumin to globulin (A/G ratio; the results of blood chemistry and the reference interval are shown in Table 1). The presence of pleural fluid was confirmed by ultrasonography and then drained. While the respiratory condition improved, the drainage was stopped at 82 ml because his physical activity increased, and the rest of the fluid was undrained (Fig. 1A). The characteristics of the pleural fluid were as follows: yellow-colored and viscous; levels of total protein, 5.4 g/dl; the number of total nucleated cells (mainly non-degenerative neutrophils and macrophages), 1,920 cells/µl; specific gravity, 1.029. In addition, type I FCoV RNA in the pleural fluid were detected by using semi-nested reverse transcription-polymerase chain reaction (RT-PCR) [1]. The cat was strongly suspected to be affected with effusive FIP [13]. On Day 1, we started the administration of ICZ after obtaining informed consent from the owner. ICZ (10 mg/kg q12 hr) and Pred (1 mg/kg q24 hr; rest period: Day 18–21) were administered to the cat. The dosage of ICZ was determined according to the results of an in vitro [18] and an in vivo [2] study. Pred was prescribed to inhibit excessive inflammation. Although the cat showed respiratory signs, including sneezing and purulent rhinitis from Day 8 onward, these symptoms improved after treatment with doxycycline (5 mg/kg q12 hr), nebulization (saline, 15–20 ml; bromhexine hydrochloride, 1 mg; gentamicin, 4 mg) and levofloxacin eye drops (q12 hr). The volume of remaining pleural fluid was unchanged for 7 days after the start of the treatment. However, the fluid steadily decreased and finally almost disappeared on days 14 and 21, respectively (Fig. 1). Moreover, the A/G ratio increased in association with the decreased concentration of globulin. The administration of Pred had been stopped to prevent the inhibition of T cell-mediated immunity since Day 18. However, since the cat showed neurological manifestations, including an ataxic gait and tremor, the administration of Pred was resumed on Day 22. In addition, forced oral feeding and fluid therapy were initiated due to the gradual loss of appetite. On Day 35, the cat’s blood test showed a remarkable increase in the activities of the following enzymes: aspartate transaminase, alanine aminotransferase (ALT) and alkaline phosphatase. Blood levels of total bilirubin (T-Bil) also increased. Two days later (Day 37), the cat presented with clonic seizures, gyration, and head pressing. On Day 38, the cat was euthanized due to status epilepticus after obtaining the owner’s consent and then necropsy was performed.
Table 1. Changes in blood biochemical and hematological parameters and body weight during the treatment.
| Day 0 | Day 3 | Day 6 | Day 9 | Day 14 | Day 21 | Day 28 | Day 35 | Day 38 | RI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Biochemistry | ||||||||||
| TP (g/dl) | 8.3 | 8.2 | 7.5 | 7.9 | 8.2 | 7.7 | 7.9 | 7.2 | 7.2 | 6.6–8.6 |
| ALB (g/dl) | 2.4 | 2.5 | 2.5 | 2.7 | 2.9 | 2.7 | 2.8 | 2.4 | 2.4 | 2.8–3.9 |
| GLO (g/dl) | 5.9 | 5.7 | 5.0 | 5.2 | 5.3 | 5.0 | 5.1 | 4.8 | 4.8 | 2.7–4.5 |
| A/G ratio | 0.41 | 0.44 | 0.50 | 0.52 | 0.55 | 0.54 | 0.55 | 0.50 | 0.50 | 0.60–0.90 |
| AST (U/l) | 39 | 33 | 38 | 35 | 76 | 54 | 122 | 440 | 564 | 13–65 |
| ALT (U/l) | 78 | 62 | 81 | 74 | 104 | 118 | 150 | 428 | 451 | 19–190 |
| ALP (U/l) | 118 | 92 | 89 | 77 | 24 | 26 | 33 | 130 | 150 | 14–153 |
| GGT (U/l) | 0.1 | - | - | - | - | 3.6 | 2.1 | 2.0 | 0.2 | 0.0–5.0 |
| T-Bil (mg/dl) | ND | 0.01 | - | 0.02 | 0.03 | 0.05 | 0.10 | 1.04 | 2.62 | 0.00–0.09 |
| Ammonia (µg/dl) | - | - | - | - | - | - | - | - | 57.9 | 0.1–30.1 |
| Hematology | ||||||||||
| RBC (106/µl) | 7.00 | - | - | - | - | 5.63 | - | - | 5.12 | 6.54–12.20 |
| Hematocrit (%) | 29.2 | - | - | - | - | 24.2 | - | - | 19.7 | 30.3–52.3 |
| Hemoglobin (g/dl) | 9.3 | - | - | - | - | 7.6 | - | - | 7.0 | 9.8–16.2 |
| MCV (fl) | 41.7 | - | - | - | - | 43.0 | - | - | 38.3 | 35.9–53.1 |
| MCH (pg) | 13.3 | - | - | - | - | 13.5 | - | - | 13.6 | 11.8–17.3 |
| MCHC (g/dl) | 31.8 | - | - | - | - | 31.4 | - | - | 35.5 | 28.1–35.8 |
| Reticulocytes (/ml) | 14,700 | - | - | - | - | 9,000 | - | - | 5,700 | 3,000–50,000 |
| WBC (/µl) | 10,540 | - | - | - | - | 4,530 | - | - | 480 | 2,870–17,020 |
| Neutrophils (/µl) | 8,810 | - | - | - | - | 3,340 | - | - | 120 | 1,480–10,290 |
| Lymphocytes (/µl) | 1,250 | - | - | - | - | 1,020 | - | - | 220 | 920–6,880 |
| Monocytes (/µl) | 450 | - | - | - | - | 160 | - | - | 130 | 50–670 |
| Eosinophils (/µl) | 10 | - | - | - | - | 10 | - | - | 0 | 170–1,570 |
| Basophils (/µl) | 20 | - | - | - | - | 0 | - | - | 10 | 10–260 |
| Platelets (103/µl) | 276 | - | - | - | - | 130 | - | - | 34 | 151–600 |
| Body weight (kg) | 1.06 | 1.08 | 1.12 | 1.08 | 0.98 | 1.08 | 0.92 | 0.88 | 0.84 | |
RI: reference interval, TP: total protein, ALB: albumin, GLO: globulin, A/G ratio: albumin to globulin ratio, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, GGT: γ-glutamyltransferase, T-Bil: total bilirubin, RBC: red blood cell, MCV; mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, WBC: white blood cell, ND: not-detected.
Fig. 1.
The volume of pleural fluid (asterisk) observed by ultrasonography on Day 0 (A, remained fluid after drainage), (B) Day 14, and (C) Day 21. Arrows indicate heart. Bar=10 mm.
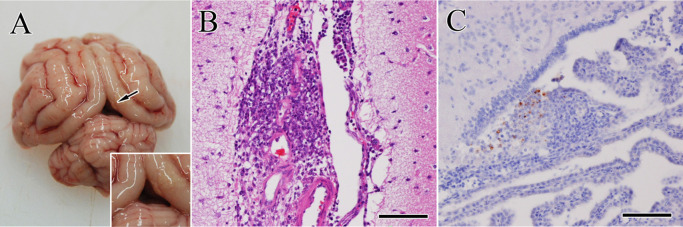
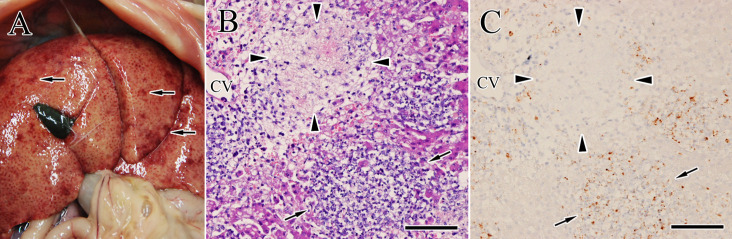
During necropsy, a small quantity of a gelatinous yellow fluid was observed in the thoracic cavity. A small number of whitish-yellow nodules were found on the surface of visceral organs including the liver, kidneys, spleen, and lungs. In the brain, these nodules were found on the leptomeninges of the cerebrum (Fig. 2A) and choroid plexus. All tissues were fixed in 10% neutral buffered formalin and 3 µm-thick tissue sections were stained with hematoxylin and eosin. Microscopically, the nodules on the visceral organs and brain (Fig. 2B) consisted of neutrophils, macrophages, lymphocytes and cell debris indicating pyogranuloma. For immunohistochemistry, selected sections were incubated with primary antibodies against FCoV antigens (mixed monoclonal antibodies against FCoV S protein 6-4-2 as previously reported [9], as well as N protein E22-2 and M protein F18-2 as previously reported [10]). FCoV antigens were scattered in the pyogranuloma of the brain (Fig. 2C) and visceral organs including the liver (Fig. 3C; arrows). A centrilobular necrosis and congestion (includes microvascular rupture and obstruction) were also found in the hypertrophied liver (Fig. 3A and 3B), which was surrounded by FCoV-positive macrophages (Fig. 3C; arrowheads). On the other hand, characteristic lesions of portosystemic shunts, toxoplasmosis or hydrocephalus were not observed.
Fig. 2.
Gross findings of the brain. (A) Whitish-yellow pyogranulomatous nodules (arrow) are observed in the leptomeninges of the cerebral cortex. Inset: higher magnification. (B) The leptomeningeal nodules consisted of neutrophils, macrophages, lymphocytes and cell debris. Hematoxylin and eosin (HE) stain. Bar=100 µm. (C) Viral antigens (stained red-brown) are scattered in the pyogranuloma in the choroid plexus of the forth ventricle. Immunohistochemistry. Bar=100 µm.
Fig. 3.
Gross findings of the liver at necropsy. (A) Marked enlargement of the liver and many white to dark reddish nodules (arrows) on the liver are seen. (B) The lesions are characterized by multifocal pyogranuloma (arrows) and necrotizing granuloma with hemorrhage and fibrinous materials (arrowheads), especially near the central vein (CV). Hematoxylin and eosin (HE) stain. Bar=200 µm. (C) Immunohistochemical image of the lesion same as B. Scattered viral antigens (stained red-brown) are observed in the pyogranuloma (arrows) and around the necrotizing granuloma (arrowheads). Bar=200 µm.
To evaluate an anti-viral effect of ICZ on FCoV replicating in this cat, the FCoV viral RNA in the feces were examined by using a quantitative RT-PCR (RT-qPCR). RNA extraction from 10% fecal emulsion and RT-qPCR were performed as described previously [5]. The fecal FCoV levels gradually decreased until Day 16 (Day 3: 1.30 × 106 copies/ml, Day 16: 4.12 × 102 copies/ml; Fig. 4). However, it had then increased again. On the other hand, FCoV viral RNA was also detected in the cerebrospinal fluid collected after euthanasia (3.99 × 105 copies/ml).
Fig. 4.
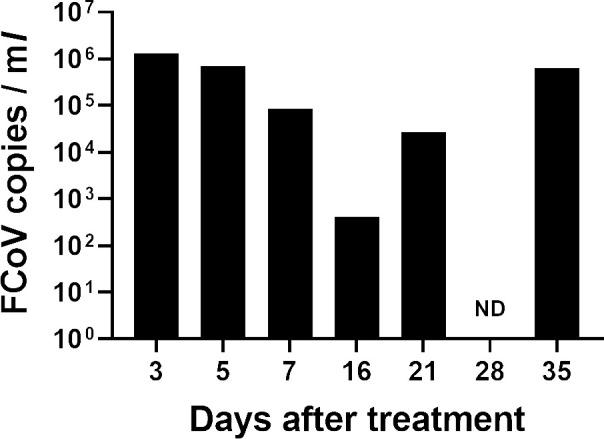
Changes in the copy number of feline coronavirus (FCoV) shedded in the feces. RNA extraction from 10% fecal emulsion and a quantitative reverse transcription-polymerase chain reaction were performed as described previously [5]. ND: not-detected.
FIP is a lethal and–until recently–incurable viral disease in cats [15]. Notably, the survival time of effusive and/or young FIP cats is shorter than that of non-effusive and/or older cats. Ritz et al. (2007) reported that the median survival time of FIP cats (36 out of 37 FIP cats were effusive; median age: 0.5 years) treated with interferon-ω and Pred or Pred alone (the Control group) was 9 or 8 days, respectively [17]. It has also been reported by Tsai et al. (2011) that the mean survival time of cats with effusive FIP was 21.3 ± 19.9 days (survival range: 1–99 days) [21]. The 3-month-old kitten described here was tentatively diagnosed with effusive FIP based on the positive results of semi-nested RT-PCR for type I FCoV in the pleural fluid, which was finally confirmed by the histopathological examination. The cat received combination therapy of ICZ and Pred and survived for 38 days until humane euthanasia was performed due to status epilepticus. Re-accumulation of the pleural fluid was prevented, and the remaining fluid had mostly disappeared (Fig. 1). In addition, the decreased A/G ratio had improved as a result of the decrease in the globulin concentration.
According to a recent in vitro study, ICZ reduced the replication of type I FCoV in fcwf-4 cells [18]. U18666A, an inhibitor of intracellular cholesterol transport, is reported to inhibit the replication of FCoV type I but not type II [19]. These results indicate that type I FCoV requires cholesterol for its replication. ICZ also inhibits cholesterol transport by binding to the Niemann-Pick C1 protein [20]. In this case, the fecal FCoV levels were temporarily decreased from beginning of ICZ treatment (Fig. 4), which was correlated with the decreased pleural fluid. Therefore, ICZ may slow the development of FIP through decreased replication of type I FCoV possibly via the inhibition of cholesterol transport. Per the results of the post mortem examination, however, the FCoV antigen-positive area was localized to the pyogranulomatous lesion found on the lungs and abdominal organs including the liver and kidneys. These findings are representative of the pathological changes that occur during FIP, suggesting that ICZ prescribed in this case (10 mg/kg, q12 hr) was not able to reduce the number of FCoV copies increasing during FIP development completely. In fact, the number of fecal FCoV copies had increased since Day 21 (Fig. 4). FCoV might replicate in his whole body, which possibly deteriorated his condition. Further investigations are needed to reveal why fecal FCoV levels increased again.
During FIP, the accumulation of pleural fluid results from an immune-mediated vasculitis (type III hypersensitivity reaction). FCoV infection-induced activation of monocytes causes its adhesion to vascular endothelial cells via the increased expression of inflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-1β, which results in FIP vasculitis [11]. It has been reported that ICZ inhibits the upregulation of mRNA levels of TNF-α and IL-1β in the kidneys of sepsis mice [12]. In addition, the anti-inflammatory agent Pred was used in combination with ICZ. The strong anti-inflammatory activity of Pred might play an inhibitory role against the FIP development possibly via inhibition of the vasculitis, but not FCoV replication. Thus, Pred and/or ICZ might reduce the accumulation of pleural fluid in the cat. The anti-inflammatory effects of these drugs may also involve the increase in the A/G ratio through the reduction of immunoglobulins. However, the risk of long-term use of NSAIDs such as meloxicam is relatively lower than that of Pred. NSAIDs might have to be used for the inhibition of inflammatory conditions associated with FIP.
ICZ is usually used as an antifungal agent. Hepatotoxicity is one of the adverse effects associated with ICZ use. It has been reported that ICZ (12.5–26.3 mg/kg/day) increased activity of ALT in the treatment of cryptococcosis in cats [14]. On the other hand, increased activity of hepatic enzymes and T-Bil levels are also observed in cats with FIP [8]. In this case, those parameters were remarkably increased on Day 35. In the liver section, the centrilobular necrosis was found (Fig. 3B; arrowheads). FCoV infection-induced vasculitis caused microvascular rupture and obstruction, which led to the augmentation of local hypoxia followed by necrosis in the centrilobular region of the liver. FCoV-positive macrophages surrounded the lesion (Fig. 3C; arrowheads). The necrotic lesion can be found in liver tissues of FIP cat without ICZ administration [7]. In addition, Boothe et al. (1997) reported that ICZ (10 mg/kg q12 hr, 6 weeks) had no adverse effects on clinical laboratory tests in cats [2]. Therefore, it is possible that the hepatic disorder induced by FCoV infection, but not ICZ-induced hepatotoxicity, might cause the changes in the blood biochemical parameters. On Day 38, the blood ammonia level was increased possibly due to the hepatic disorder. The hepatic encephalopathy might be related to neurological manifestations described below.
Neurological complications of FIP are a common cause of neurological dysfunction in cats and tend to be associated with the non-effusive form compared with the effusive form [4]. In this case, the cat showed neurological manifestations including ataxic gait, tremor, and clonic seizures. The cat’s brain was infected with FCoV, which caused granulomatous meningitis (Fig. 2). It is speculated that FCoV hematogenously invaded the meninges and caused severe inflammation. The pathological type of the brain lesion corresponds to ‘diffuse leptomeningitis with superficial encephalitis’ of the categories in Rissi’s report [16]. While the concentration of ICZ in the cerebrospinal fluid is rapidly elevated after the drug is administered, its half-life in the brain is shorter than that in the plasma because of the quick efflux of ICZ from the brain via P-glycoprotein [6]. Thus, ICZ might have failed to inhibit the infection and replication of FCoV in the brain. In a clinical trial evaluating the effects of GC376, a 3C-like protease inhibitor, on the development of FIP, 8 out of 20 FIP cats showed severe neurological signs including incoordination and tonic/clonic seizures as shown in this case. Increased dosage of GC376 could partly ameliorate those neurological signs possibly via an increase in the amount of blood-brain barrier penetration [15]. The higher dosage of ICZ (>10 mg/kg) may possibly play protective roles against neurologic FIP.
This is the first case report evaluating the clinical efficacy of the combination therapy of ICZ and Pred for treating effusive FIP. In the present case, the cat treated with both ICZ and Pred had a relatively long survival time, experienced a decrease in the accumulation of pleural fluid, and an increase in the A/G ratio. In addition, the fecal FCoV levels temporarily decreased. However, since the clinical information obtained from this case is restrictive, further clinical investigations regarding the effects of ICZ in cats with FIP are required.
Acknowledgments
ACKNOWLEDGMENTS. We wish to thank the staff and students involved in the care of this patient, and greatly appreciate all the helpful comments and suggestions from the reviewers. The authors received no financial support for the preparation of this case report.
REFERENCES
- 1.Benetka V., Kübber-Heiss A., Kolodziejek J., Nowotny N., Hofmann-Parisot M., Möstl K.2004. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Vet. Microbiol. 99: 31–42. doi: 10.1016/j.vetmic.2003.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boothe D. M., Herring I., Calvin J., Way N., Dvorak J.1997. Itraconazole disposition after single oral and intravenous and multiple oral dosing in healthy cats. Am. J. Vet. Res. 58: 872–877. [PubMed] [Google Scholar]
- 3.De Beule K., Van Gestel J.2001. Pharmacology of itraconazole. Drugs 61 Suppl 1: 27–37. doi: 10.2165/00003495-200161001-00003 [DOI] [PubMed] [Google Scholar]
- 4.Diaz J. V., Poma R.2009. Diagnosis and clinical signs of feline infectious peritonitis in the central nervous system. Can. Vet. J. 50: 1091–1093. [PMC free article] [PubMed] [Google Scholar]
- 5.Doki T., Tarusawa T., Hohdatsu T., Takano T.2020. In vivo antiviral effects of U18666A against type I feline infectious peritonitis virus. Pathogens 9: 67. doi: 10.3390/pathogens9010067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felton T., Troke P. F., Hope W. W.2014. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 27: 68–88. doi: 10.1128/CMR.00046-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaonkar P., Halmare N., Jamdade S., Kurkure N.2019. Feline infectious peritonitis in a male Persian Cat. Int. J. Curr. Microbiol. Appl. Sci. 8: 1446–1453. doi: 10.20546/ijcmas.2019.801.154 [DOI] [Google Scholar]
- 8.Hartmann K.2005. Feline infectious peritonitis. Vet. Clin. North Am. Small Anim. Pract. 35: 39–79, vi. doi: 10.1016/j.cvsm.2004.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohdatsu T., Okada S., Koyama H.1991. Characterization of monoclonal antibodies against feline infectious peritonitis virus type II and antigenic relationship between feline, porcine, and canine coronaviruses. Arch. Virol. 117: 85–95. doi: 10.1007/BF01310494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohdatsu T., Sasamoto T., Okada S., Koyama H.1991. Antigenic analysis of feline coronaviruses with monoclonal antibodies (MAbs): preparation of MAbs which discriminate between FIPV strain 79-1146 and FECV strain 79-1683. Vet. Microbiol. 28: 13–24. doi: 10.1016/0378-1135(91)90096-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kipar A., May H., Menger S., Weber M., Leukert W., Reinacher M.2005. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 42: 321–330. doi: 10.1354/vp.42-3-321 [DOI] [PubMed] [Google Scholar]
- 12.Li X. Y., Zhang Y. Q., Xu G., Li S. H., Li H.2018. miR-124/MCP-1 signaling pathway modulates the protective effect of itraconazole on acute kidney injury in a mouse model of disseminated candidiasis. Int. J. Mol. Med. 41: 3468–3476. [DOI] [PubMed] [Google Scholar]
- 13.Longstaff L., Porter E., Crossley V. J., Hayhow S. E., Helps C. R., Tasker S.2017. Feline coronavirus quantitative reverse transcriptase polymerase chain reaction on effusion samples in cats with and without feline infectious peritonitis. J. Feline Med. Surg. 19: 240–245. doi: 10.1177/1098612X15606957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medleau L., Jacobs G. J., Marks M. A.1995. Itraconazole for the treatment of cryptococcosis in cats. J. Vet. Intern. Med. 9: 39–42. doi: 10.1111/j.1939-1676.1995.tb03270.x [DOI] [PubMed] [Google Scholar]
- 15.Pedersen N. C., Kim Y., Liu H., Galasiti Kankanamalage A. C., Eckstrand C., Groutas W. C., Bannasch M., Meadows J. M., Chang K. O.2018. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 20: 378–392. doi: 10.1177/1098612X17729626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rissi D. R.2018. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J. Vet. Diagn. Invest. 30: 392–399. doi: 10.1177/1040638718755833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz S., Egberink H., Hartmann K.2007. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 21: 1193–1197. doi: 10.1111/j.1939-1676.2007.tb01937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano T., Akiyama M., Doki T., Hohdatsu T.2019. Antiviral activity of itraconazole against type I feline coronavirus infection. Vet. Res. (Faisalabad) 50: 5. doi: 10.1186/s13567-019-0625-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takano T., Endoh M., Fukatsu H., Sakurada H., Doki T., Hohdatsu T.2017. The cholesterol transport inhibitor U18666A inhibits type I feline coronavirus infection. Antiviral Res. 145: 96–102. doi: 10.1016/j.antiviral.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trinh M. N., Lu F., Li X., Das A., Liang Q., De Brabander J. K., Brown M. S., Goldstein J. L.2017. Triazoles inhibit cholesterol export from lysosomes by binding to NPC1. Proc. Natl. Acad. Sci. USA 114: 89–94. doi: 10.1073/pnas.1619571114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai H. Y., Chueh L. L., Lin C. N., Su B. L.2011. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in Taiwan. J. Feline Med. Surg. 13: 74–80. doi: 10.1016/j.jfms.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]