Abstract
Severe fibrotic and thrombotic events permeate the healthcare system, causing suffering for millions of patients with inflammatory disorders. As late-state consequences of chronic inflammation, fibrosis and thrombosis are the culmination of pathological interactions of activated endothelium, neutrophils and platelets after vessel injury. Coupling of these three cell types ensures a pro-coagulant, cytokine-rich environment that promotes the capture, activation and proliferation of circulating immune cells and recruitment of key pro-fibrotic cell types such as myofibroblasts. As the first responders to sterile inflammatory injury, it is important to understand how endothelial cells, neutrophils and platelets help create this environment. There has been a growing interest in this intersection over the past decade that has helped shape the development of therapeutics to target these processes. Here, we review recent insights into how neutrophils, platelets and endothelial cells guide the development of pathological vessel repair that can also result in underlying tissue fibrosis. We further discuss recent efforts that have been made to translate this knowledge into therapeutics and provide perspective as to how a compound or combination therapeutics may be most efficacious when tackling fibrosis and thrombosis that is brought upon by chronic inflammation.
Keywords: neutrophil, platelet, endothelial dysfunction, inflammation, fibrosis, glycocalyx
1. Introduction
The peripheral vasculature is a complex continuum made up in part by a collagenous tunica externa, an elastic and smooth muscle-lined tunica media and an endothelial monolayer making up the tunica intima, or inner vessel lining. This structure differs slightly in the capillaries, which are composed of a tunica intima and basement membrane. Vascular integrity relies on coordinated homeostasis of these dynamic components. As the inner lining of the blood vessel, the endothelium provides an interface between circulating blood components and the adjacent tissues and plays a particularly pivotal role in tissue damage and fibrosis. In response to vascular damage, metabolic disorders or inflammation, endothelial cells prompt the recruitment of key inflammatory cells: platelets and neutrophils. Changes to endothelial morphology, chemokine production and surface proteins foster a pro-inflammatory environment that mediates the mobilization of circulating immune cells such as polymorphonuclear neutrophils and platelets. In many circumstances, interactions between recruited neutrophils, platelets and the endothelium lead to the resolution of vessel damage; indeed, inflammatory events are vital for proper wound healing [1]. However, if inflammatory stimuli are persistent these interactions can tip the balance toward fibrotic healing.
The fibrotic response is the culmination of many chronic inflammatory diseases. Fibrosis is characterized as the accumulation of excess extracellular matrix (ECM) proteins, such as collagen and fibronectin. Although ECM deposition is a vital and largely self-restorative part of wound healing, repetitive or severe damage can cause pathological dysregulation of this process leading to fibrosis. Inflammation mediated by the innate immune response is a critical trigger of pathological fibrosis [2]. In response to vascular damage or systemic and local inflammation, endothelium transitions from a quiescent state to a state called endothelial dysfunction, which in its severe form causes the exposure of underlying ECM such as collagen [3]. Soluble pro-inflammatory factors such as reactive oxygen species (ROS) and matrix metalloproteinases (MMPs) are released by the endothelium [4,5], recruiting circulating neutrophils to the scene. Neutrophils and circulating platelets tether to upregulated cell adhesion molecules that are expressed on inflamed endothelium, such as E-selectin, P-selectin and von Willebrand Factor (vWF). This tethering initiates an inflammatory cascade that, if persistent, culminates as thrombotic vessel occlusion or fibrotic myofibroblast proliferation and ECM deposition. Left unchecked, excessive ECM deposition can result in impaired organ function and, in some cases, end-stage organ failure and death.
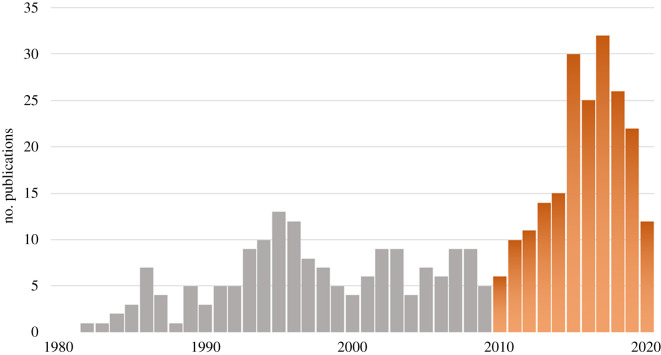
Given the influential roles endothelial cells, neutrophils and platelets play in aberrant inflammation, thrombosis and fibrosis, many studies have focused on elucidating mechanisms behind pro-fibrotic and prothrombotic events in order to develop targeted therapeutics that interfere with these processes. These cell types have been of growing interest over the past decade, as is evidenced by the steady increase in the relevant literature (figure 1). In this review, we detail how disruptions to the endothelium and its protective ECM layer, the glycocalyx, contribute to endothelial cell dysfunction, neutrophil and platelet dysregulation, thrombosis, and fibrosis. We discuss the promise and limitations of therapeutic strategies for limiting neutrophil and platelet-mediated fibrosis.
Figure 1.
PubMed search results since 1980. A PubMed literature search for the years 1980–2020 was conducted for the following key terms: neutrophil, platelet, endothelial and thrombosis or fibrosis. Results were further narrowed down to the years 2010–2020, during which the number of publications per year steadily increased. Publications were screened for titles relevant to topics discussed in this review.
1.1. Endothelial dysfunction within the scope of inflammation
The blood vessels are a conduit for immune cell trafficking in response to tissue damage. Amid differences in vessel composition, elasticity, size and stiffness, all blood vessels are lined by an inner layer of endothelium. The vascular endothelium is made up of a heterogeneous continuum of individual endothelial cells that reside on a bed of collagen-rich ECM [6]. As its most basic function, the endothelium acts as a physical barrier between circulating fluids and the surrounding tissues. For many years, this was believed to be the sole purpose of the endothelium. It is now well accepted that the endothelium is a complex, bioactive cell layer that regulates immune cell function and tissue access, vasoreactivity, and the extravasation of macromolecules, solutes, hormones and fluids [7]. Endothelial cell health plays a precarious role in chronic inflammatory and thrombogenic diseases, including diabetes [8], atherosclerosis [4,9], acute respiratory distress syndrome [10], cystic fibrosis [11] and liver cirrhosis [12]. Damage to the endothelium initiates and sustains inflammation and thrombogenesis [7], with endothelial cells themselves providing a platform to fast-track thrombosis during dysregulated haemostasis, and fibrosis during pathological wound repair.
The endothelium is lined by a thin, glycosaminoglycan-rich barrier called the endothelial glycocalyx. The glycocalyx is a dynamic participant in endothelial cell barrier function and immune regulation [13]. Structurally, the glycocalyx ranges from nanometres to several micrometres thick, depending on the vessel type, location within a vessel, conditions and the imaging technique used [14,15]. Even within one vessel, the glycocalyx is a heterogeneous structure [16]. The glycocalyx is comprised a glycoprotein or proteoglycan core protein anchored to underlying actin cytoskeletal filaments at the endothelial surface [13,17]. While the luminal portion of a glycoprotein is decorated with small sugar residues, the proteoglycan core, primarily from the syndecan and glypican families [16,18], extends into the vessel lumen and is decorated with glycosaminoglycans (GAGs) such as heparan sulfate, chondroitin sulfate, hyaluronan and dermatan sulfate. GAG components, particularly heparan sulfate and dermatan sulfate, possess anticoagulant and anti-thrombotic qualities [19,20] that have been used as therapeutics [21–24]. Though heparan sulfate makes the greatest contribution to glycocalyx thickness, heterogeneity within the glycocalyx suggests that each GAG contributes to glycocalyx function and vessel permeability [25].
Under healthy conditions, the glycocalyx exists in dynamic equilibrium with circulating blood, changing its composition and thickness in response to haemodynamic forces and cues from soluble factors [26] in order to maintain equilibrium between hydrostatic and oncotic forces in the vessel [27]. Changes to the rheological environment are detected by the glycocalyx [28] and transduced to the underlying endothelium via actin anchoring. The actin cytoskeleton and the glycocalyx exist in a dynamic equilibrium with one another, each being modified in response to changes in the other to control endothelial barrier properties [29,30]. Indeed, mechanical stress has been shown to alter GAG and proteoglycan synthesis and remodelling in vascular endothelial cells [31,32], lending insight into how pathological stretch and disturbed flow can influence endothelial cell behaviour and function.
After physical, local or systemic inflammatory insult, damaged endothelium exhibit signs of endothelial dysfunction. Endothelial dysfunction is a complex phenomenon involving heightened ROS production, altered nitric oxide (NO) production and disruptions to vascular tone; production of MMPs and pro-inflammatory cytokines; and upregulation of cell adhesion molecules such as E-selectin, P-selectin and intracellular adhesion molecule-1 (ICAM-1) [4,5,33,34]. MMPs and ROS facilitate glycocalyx degradation and shedding, causing impaired mechanosensing and altering cell behaviour [22,28,35] to enable immune cell capture and trafficking [26,36–39]. Compromised mechanosensing can in turn perpetuate reductions in shear-sensitive NO secretion and augment vascular permeability. Paired with the release of sequestered chemokines and the exposure of immune cell adhesion molecules along the endothelial surface, glycocalyx degeneration facilitates inflammation, thrombosis and eventual fibrosis by promoting immune cell migration into the underlying tissue [40,41].
Dysfunctional endothelium plays an interesting role in perpetual inflammation. While in a dysfunctional state, endothelial cell senescence is accelerated and migration is hindered [31,42]. In damaged vessels, the inability to replenish the endothelial layer with healthy cells results in a prolonged inflammatory attack. Even when re-endothelialization can occur, newly formed glycocalyx is fragile and less responsive to shear [26], and readily concedes to the inflammatory state. Further exacerbating the dysfunctional state, circulating platelets bind to collagen, which normally resides below the surface of the endothelium [6,43], but is exposed between contracting cells [44] or is revealed after vessel denudation [45]. In some cases, adherent platelets induce a pro-fibrotic cascade involving platelet-mediated leucocyte recruitment, tissue factor secretion and fibrin(ogen) deposition [46,47].
2. Drivers of platelet–neutrophil aggregation, endothelial dysfunction and fibrosis
Inflammatory stimulus and vessel choice influence the relative contributions of platelets, neutrophils and endothelial cells to inflammation, thrombosis and fibrosis [48] (table 1). Here, we discuss recent insights into how these three cell types collaborate to exacerbate inflammation, thrombosis and fibrosis.
Table 1.
Endothelial injury methods and outcomes. (HUVEC, human umbilical vein endothelial cells; PSGL-1, P-selectin glycoprotein ligand-1; Par4, protease-activated receptor 4; TNF-α, tumour necrosis factor alpha; IFN-γ, interferon gamma; CCL2, C-C motif chemokine ligand 2; IL-1β, interleukin-1 beta; ApoE, apolipoprotein E; TF, tissue factor.)
| vessel or cell type | injury method | key observations | references |
|---|---|---|---|
| cremaster arteriole | laser activation | laser activated endothelial cells trigger thrombus formation; neutrophil slow rolling on thrombus mediated by P-selectin-PSGL-1 | [49] |
| cremaster arteriole and HUVEC | laser activation | endothelial activation precedes platelet accumulation; normal fibrin formation observed in Par4-/- mice | [50] |
| cremaster arteriole | laser activation | prothrombinase found on activated endothelial cells | [51] |
| cremaster arteriole | laser activation | neutrophils contain and express tissue factor at the site of laser injury; neutrophils accumulated before platelets | [52] |
| venule | TNF-α | TNF-α activated endothelial cells recruit neutrophils; platelets bind adherent neutrophils rather than endothelium | [49] |
| artery and HUVEC | TNF-α and IFN-γ | fractalkine causes degranulation, activation, and expression of platelet P-selectin on adherent platelets, mediating neutrophil recruitment | [53] |
| HUVEC | TNF-α | endothelial TF drives fibrin deposition and coagulation; upregulated ICAM can be targeted for delivering recombinant thrombomodulin to inflamed cells | [47] |
| cremaster arteriole | CCL2, TNF-α, IL-1β, or IFN-γ | platelets guide neutrophils to extravasation points via P-selectin-PSGL-1 and CD40/CD40 L | [54] |
| artery and HUVEC | ApoE-/- mice | increased endothelial stiffness causes enhanced leucocyte transendothelial migration | [55] |
| artery | ApoE-/- mice | reduced glycocalyx thickness and increased platelet adhesion occur at bifurcation point | [56] |
| artery | ApoE-/- mice | endothelial dysfunction and glycocalyx impairment coincide with endothelial-dependent vasodilation, permeability, and increases in atherosclerotic biomarkers | [4] |
2.1. Endothelial cells
As the lining of the conduits of blood components, metabolites and immune cells, the vascular endothelium is often on the front line in an inflammatory insult. A major source of endothelial inflammation is the overproduction of ROS, whose aetiology can range from diet, to hormone dysregulation, to cellular by-products such as those produced during macrophage digestion of apoptotic debris and foreign substances [34]. Excess ROS inactivates NO production—a known mediator of vascular tone. Reduced NO enhances vessel stiffness and contractility, both of which contribute to endothelial dysfunction [4]. Paradoxically, NO overproduction can likewise induce cellular inflammation and apoptosis [57], highlighting the importance of maintaining tight regulation of homeostasis.
Endothelial cytoskeletal structure and stiffness play a critical role in leucocyte recruitment and fibrosis. Tumour necrosis factor alpha (TNF-α) stimulated endothelial cells present a cortical stiffness gradient to slow-rolling neutrophils, guiding them to transmigration sites [55]. This effect is augmented on stiff matrices [55,58], consistent with neutrophil recruitment and extravasation in atherosclerotic vessels [9]. Pro-fibrotic cellular pathways such as the autotaxin/lysophosphatidic acid axis likewise stimulate endothelial actin rearrangement and cell contractility, causing a vascular leak and aiding in the migration of fibroblasts in the underlying tissue [59]. Structural alterations of the cytoskeleton affect glycocalyx distribution and function [29], further exacerbating endothelial dysfunction and vascular damage.
Dysfunctional endothelial cells are known initiators of fibrosis. In response to noxious stimuli such as excess ROS, lipopolysaccharide (LPS), bleomycin or physical damage, dysfunctional endothelial cells secrete a milieu of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 [2]. TNF-α initiates a signalling cascade that can lead to endothelial apoptosis or necrosis [60]. Surviving endothelial cells begin to shed their glycocalyx, unmasking upregulated selectins and integrin-binding ligands embedded within the cell membrane [61]. In the venules, selectins act as the initial point of neutrophil capture [62], which can then act as secondary capture sites for circulating platelets [49]. Bleomycin-induced pulmonary fibrosis exemplifies the potential pathological response once the endothelium becomes inflamed. In one representative study, bleomycin-induced pulmonary endothelial inflammation resulted in a peak in endothelial inflammation at 7 days post-bleomycin instillation; and enhanced vWF, plasminogen activator inhibitor-1, MMP-12 and NO that led to increased collagen deposition, and pulmonary fibrosis peaking at day 21, clearly delineating the link between endothelial dysfunction and fibrosis [57].
Similarly, studies using alternative stimuli have evidenced endothelial cells as sources of tissue factor (TF) [47,50,63], a protein that is primarily secreted by activated monocytes to initiate platelet deposition and thrombin formation [64] and thereby actuate thrombogenesis and tissue fibrosis [65,66]. Endothelial-derived TF has been reported in response to TNF-α [47,63] and laser activation [50,51], a technique which can elicit endothelial activation without vessel denudation that has been used as a model of vascular thrombosis and atherosclerosis [67]. Endothelial TF has been shown to cause fibrin deposition, upregulation of ICAM-1 and vascular cell adhesion molecule-1, and increased platelet binding. Atkinson et al. demonstrated that endothelial activation, calcium mobilization and granule secretion precede platelet accumulation in cremaster arterioles [50]. Notably, fibrin production persisted on activated human umbilical vein endothelial cells (HUVECs) upon treatment with platelet depleted plasma, further validating endothelial cells as a source of TF. Ivanciu and colleagues later observed enhanced prothrombinase activity on similarly activated endothelium, reinforcing the idea that activated endothelium forms a pro-coagulant surface that supports thrombus formation after injury [51].
2.1.1. Link to the inflammatory triangle
Despite similar methods of endothelial activation, there is a lack of consensus regarding the main source of TF produced in response to vessel damage. Contrary to the results described above, leucocytes have been demonstrated as the first responders to endothelial activation. Adherent leucocytes express TF and create a platform for platelet binding [52]. In a separate study, neutrophil rolling and adhesion was observed only after platelet thrombi had formed, with rolling mediated by platelet P-selectin and neutrophil P-selectin glycoprotein ligand-1 (PSGL-1) [49], suggesting multiple factors are probably at play, orchestrating the timing and sequence of events.
While it is clear all three cell types work in coordination to address vessel damage, more studies are needed to elucidate mechanisms behind thrombo-inflammation in models of cytokine, bacterial and physical damage. Understanding these mechanisms may allow for the development of more targeted therapeutics to combat vascular inflammation and fibrosis, for example targeting endothelial—but not leucocyte or platelet-derived TF, or selectively upregulated cell adhesion molecules. Further investigation into these mechanisms from the perspective of neutrophils and platelets follows.
2.2. Neutrophils
Neutrophils play a paradoxical role in inflammation. The neutrophil recruitment cascade is a conserved process that is integral to the resolution of inflammation, infection and wound healing [68]. However, the pathological activation of neutrophils perpetuates acute and chronic inflammatory diseases. Pathological neutrophilia in response to cytokine storm is observed in rheumatic diseases, as well as infectious diseases such as coronavirus pneumonia seen in severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and coronavirus disease 2019 (COVID-19) [69,70], leading to irreversible tissue fibrosis or necrosis.
We have known for decades that neutrophil engagement exacerbates endothelial activation [71] and collagen synthesis [72]. Neutrophils are recruited to the endothelial surface in response to endothelial cues after cellular activation. As previously described, endothelial cells release a milieu of pro-inflammatory stimuli upon damage such as ROS, TNF-α, IL-1β and MMPs that degrade the endothelial glycocalyx and recruit circulating neutrophils to the damaged area. Tethered neutrophils roll along the endothelial surface, stabilized by shear-strengthening, transient catch bonds between endothelial E/P-selectin, CD44 and neutrophil PSGL-1 [61,73]. E/P-selectin engagement directs neutrophil signalling by stimulating an intracellular neutrophil calcium burst [74,75] and augments chemokine signalling to activate neutrophil β2 integrins, leading to cell arrest, migration into the underlying tissue, and further neutrophil recruitment.
With the exception of the pulmonary system, in which cell recruitment occurs at the capillaries [76,77], veins and post-capillary venules act as the primary capture sites for neutrophils during inflammatory damage, though arterial recruitment has been observed after physical vessel damage by laser activation [49–52,78]. This unequal involvement is perhaps owing to differences in junctional proteins that result in increased intrinsic leakiness within the venules, selectin and ICAM-1 expression, shear stress and flow mechanics, and gene expression [7,79]. In the venules, TNF-α and LPS activation quickly result in neutrophil adhesion to the vessel wall [48,49,80,81]. Adherent and activated neutrophils secrete endothelial barrier disrupting molecules such as ROS and release of neutrophil degranulation products including myeloperoxidase, elastase and metalloproteases [82], facilitating migration through the endothelium.
Further damage can be caused by neutrophil extracellular trap (NET) production. Coordinated PSGL-1 [83] and CXCR2 [73] signalling leads to enhanced neutrophil adhesion, NET formation and flow restriction. While their primary function is to trap pathogens, NETs have been shown to enhance inflammation and endothelial permeability and have been implicated in several inflammatory diseases [82]. Further, NETs are known to trap platelets, red blood cells and fibrin, fostering the growth of pathological thrombi. NETs are drivers of vascular damage, venous thrombosis [73,84] and virus-induced organ damage and mortality, as is seen in severe COVID-19 [69,85].
2.2.1. Link to the inflammatory triangle
Neutrophil-mediated fibrin deposition and thrombosis can occur even in the absence of exogenous inflammatory stimuli or direct endothelial cell damage, such as in a murine stenosis model of deep vein thrombosis (DVT). In this model, the endothelium remains intact and the underlying collagen-rich ECM unexposed, but altered blood flow by partial vessel occlusion is enough to cause cooperative signalling by neutrophils at the endothelial surface that initiates fibrin formation and propagates venous thrombosis [73,84]. Once bound to the endothelium, neutrophils physically alter the rheological environment within the vasculature by creating an altered flow pattern near the vessel wall [48]. Drag forces grab circulating platelets and bring them towards the surface of the neutrophil, allowing molecular interactions between platelet and neutrophil. Interestingly, NETs have also been implicated in DVT and probably exacerbate neutrophil-mediated thrombosis [86].
This relationship between rheological haemostasis and thrombosis aligns with atherosusceptible vascular regions such as vessel branches, where blood flow patterns are disturbed [87]. However, rather than disturbed flow causing endothelial activation, activated neutrophils cause rheological changes that promote platelet adhesion and vessel activation. It is evident that the interplay between neutrophil, endothelial and platelet activation cannot be reduced to a single initiator of thrombosis, vessel stenosis or tissue fibrosis. Therefore, while blocking neutrophil-vessel interactions has shown therapeutic promise in reducing neutrophil-mediated vessel damage [81,88–92], pro-inflammatory neutrophil–platelet interactions are a compelling target for emerging therapeutics [93].
2.3. Platelets
Platelets are cell fragments of megakaryocytes that govern the haemostatic resolution of vascular wounds [94,95]. Thrombocytopenia and platelet depletion caused by genetic deficiencies or anticoagulants can provoke disorders such as haemophilia and interfere with the proper resolution of infection [96] and inflammation [97–99]. More recently, platelets have been recognized as drivers of inflammatory damage, working alongside circulating neutrophils to perpetuate inflammation [93,100,101]. During severe vessel damage, endothelial cell death [102] or vessel denudation [45] causes the exposure of underlying ECM. Circulating platelets readily bind to and activate on exposed collagen-rich ECM that lies beneath the endothelial layer to create a platelet plug.
While platelet plugs are vital for maintaining haemostasis within an injured vessel [103], in some cases, the haemostatic response can quickly become imbalanced by infiltrating immune cells, shifting the plug from haemostatic to thrombotic. In a haemostatic platelet plug, densely packed P-selectin positive platelets and a fibrin network form an ultra-dense thrombus core surrounded by more loosely packed P-selectin negative, minimally activated platelets at the luminal surface [103,104]. Once the healing cascade is compromised and thrombus formation becomes pathogenic, smaller vessels such as arterioles, capillaries and post-capillary venules are at a greater risk of occlusion and resultant tissue ischemia owing to thrombi taking up a larger relative proportion of the vessel [7,46,105]. Platelet thrombi formed after a traumatic or ischemia reperfusion injury can release the α-granule chemokine neutrophil-activating peptide 2 (NAP-2) to recruit circulating neutrophils to the injury site. These thrombi then act as migration points for circulating neutrophils [106], driving tissue fibrosis.
2.3.1. Link to the inflammatory triangle
Adherent platelets have also been shown to guide neutrophils to inflammatory sites [54,107] or act as anchor points for secondary capture of circulating neutrophils [49,108–110]. This is especially apparent in arteries, where adherent platelets may be necessary for neutrophil recruitment [53], perhaps owing to high shear forces in the artery [7]. In addition to facilitating capture, activated platelets and platelet-derived soluble factors such as extracellular vesicles [111] can act as drivers of neutrophil and endothelial activation [83,102,110,112–114] and affect their migration behaviour [115]. Platelet–neutrophil–endothelial cell interactions are influenced by several factors, including the P-selectin-PSGL-1 axis [49,53,73,114,116–118] via phosphodiesterase type-4 [109] or Src family kinases [110,119], and the vWF-glycoprotein Ibα axis [48,84]. Similar to endothelial cells and neutrophils, shear stress seems to be a critical factor in achieving physiologically relevant platelet activation [120].
Upon activation, endothelial cells release Weibel-Palade bodies to the vascular surface. Weibel-Palade bodies are secretory granules that store platelet adhesion ligands P-selectin and vWF. Now available, P-selectin and vWF recruit additional circulating neutrophils and platelets [118], causing greater platelet and neutrophil recruitment and further aggravating the endothelium. In regions of endothelial denudation, this recruitment can be beneficial for wound healing [99]; however, when uncontrolled it can also lead to irreversible fibrotic damage.
2.4. Implications in tissue fibrosis
Paradoxically, immune cells recruited for repair can potentiate damage if the inflammatory stimulus is not resolved. Activated leucocytes, platelets and leucocyte-platelet complexes have been implicated as drivers of pulmonary and cardiovascular fibrotic diseases [121,122]. While not typically considered centre stage in fibrosis, dysregulated platelet and neutrophil accumulation play a critical role in aberrant tissue repair [123]. Platelet activation and degranulation are associated with myofibroblast proliferation and the overactive deposition of ECM components that results in tissue fibrosis [124]. Furthermore, activated platelets secrete large quantities of chemokines that are chemotactic for neutrophils and monocytes, triggering immune cells to migrate, activate and produce additional cytokines and enzymes that stimulate the production of transforming growth factor-β (TGF-β), a key mediator of myofibroblast formation, collagen deposition and fibrosis [125,126].
Neutrophil migration along activated endothelium is one of the first steps towards the resolution of acute inflammation; however, without proper clearance, neutrophil accumulation causes sustained, chronic inflammation. Extravasating neutrophils are activated by ECM proteins, leading to injury and remodelling of the surrounding tissue [127], as is seen in chronic obstructive pulmonary disease and atherosclerosis [128]. Excessive neutrophil activation, degranulation and respiratory burst activity damages surrounding tissues [129], in part by the release of matrix-degrading MMPs, toxic mediators such as ROS and reactive nitrogen species [2], and pro-inflammatory cytokines that activate and recruit other immune cells such as macrophages and T-lymphocytes. The reparative action by myofibroblasts in response to matrix degradation leads to the formation of a dense, disorganized fibrotic tissue [124].
3. Clinical manifestations
Several pro-fibrotic diseases have been linked to perturbations in the proposed inflammatory triangle. Select disease states are briefly discussed below.
3.1. Lung disease
The lungs are a particularly interesting platform for studying aberrant neutrophil–platelet interactions. Platelets have been shown to support recruitment and activation of neutrophils in the pulmonary capillaries in abdominal sepsis [124], acute lung injury [125], acute respiratory distress syndrome [125,130,131] and allergic inflammation [126]. Elevated platelet activation indices have been reported in idiopathic pulmonary fibrosis (IPF) patients [132], with anti-platelet drugs showing promise in alleviating IPF by reducing platelet activation and platelet-mediated neutrophil infiltration [133]. Recent insights into IPF have suggested that imbalanced endothelial activation plays a vital role in disease pathogenesis. Activated endothelium has been shown to secrete microparticles [134] and chemokines such as IL-8 into circulation, augmenting neutrophil recruitment and activation [135]. While the mechanisms underlying IPF are still unknown, vascular contributors such as these are an encouraging target.
3.2. Neointimal hyperplasia
Endothelial denudation, as occurs after balloon angioplasty and severe inflammatory damage [102], presents a unique environment for platelet–neutrophil interactions. Under these conditions, circulating platelets bind to and activate on exposed collagen [44,46,109,136,137] and release extracellular vesicles that have been shown to influence neutrophil activation states, causing the upregulation of platelet receptor CD41 [111]. Intimal hyperplasia, or fibrosis within the artery itself, has been clearly linked with increased inflammation [138]. This, in conjunction with studies relating platelet-mediated neutrophil binding and activation with thrombosis [49,54,102,108,109], suggests that more complex or combinatorial therapeutics are needed to reduce platelet- and neutrophil-mediated tissue damage.
3.3. COVID-19
Vascular inflammation and associated cytokine storm are known contributors to COVID-19 morbidities. These events are in part characterized by immune cell infiltration, NET formation [85] and dysregulated platelet activation [139], leading to hypercoagulopathy and vascular complications. Despite their role in hypercoagulopathy and the formation of pathogenic platelet–neutrophil complexes in this disease, platelets have paradoxically been shown to preserve endothelial integrity in severe COVID-19, with thrombocytopenia being associated with the impairment of platelet-dependent endothelium-protective mechanisms [140,141]. Therefore, as we learn more about this disease, it will be important to distinguish between restorative and noxious immune cell activation states when developing treatments.
Ventilator-induced damage likewise imbalances the inflammatory triangle. Severe COVID-19 patients that have developed acute respiratory distress syndrome require mechanical ventilation; while a necessary therapy, mechanical ventilation has been associated with exacerbated lung damage, referred to as ventilator-induced lung injury (VILI) [132,133]. Neutrophil infiltration and NET formation are elevated in patients with VILI [132], in part owing to platelet–endothelial interactions. Platelets have been reported to contribute to neutrophil recruitment in VILI by presenting leucocyte-binding proteins at the endothelial surface [142,143]. It is likely that VILI and inflammation-induced damage go hand-in-hand in these patients, with platelet–neutrophil interactions exacerbating cytokine storm-related morbidities.
4. Therapeutic advances
As initiators of the fibrotic cascade, neutrophils, platelets and activated endothelium have been investigated as potential therapeutic targets to combat vascular injury and occlusion and prevent the progression of fibrosis. For other vascular indications, established therapeutics such as rosuvastatin (statin; slows cholesterol production) [144] and ticagrelor (blood thinner; anti-platelet medication) [145] have exhibited a capacity to limit neutrophil binding, platelet aggregation and neutrophil–platelet aggregates and are discussed elsewhere [145–148]. Here, we focus on efforts to protect or regenerate the glycocalyx, and select mediators of endothelial, neutrophil and platelet-induced inflammatory damage.
4.1. Glycocalyx protection
While essential for healing processes, the inflammatory response can be devastating for damaged endothelium and underlying tissue if not kept under control. A pathological inflammatory response can lead to immune cell influx, altered vascular and tissue remodelling, and irreversible fibrotic damage, as is seen in ischemia reperfusion injury [149] and acute lung injury [150]. As a regulator of endothelial health, the inflammatory response and vascular permeability to macromolecules and immune cells, the endothelial glycocalyx presents a promising therapeutic target for a wide range of diseases without sacrificing the healing component of inflammation [151]. Here, we discuss recent efforts at preserving the glycocalyx that have shown promise in reducing vascular and underlying tissue damage. For a comprehensive review on glycocalyx preserving molecules, we refer the reader elsewhere [152,153].
Established drugs have been repurposed to combat glycocalyx damage and subsequent leucocyte and platelet adhesion, as is the case with the general anaesthetic sevoflurane. This drug is thought to function in part through upregulation of sialyltranferase, which in turn catalyses the transfer of sialic acid, an important mediator of oxidative stress [38,154]. Sevoflurane has shown promise in glycocalyx preservation in animal models of pulmonary ischemia reperfusion during lung transplant [155], cardiac ischemia reperfusion [156] and aortic damage by H2O2 [154], although limited efficacy has been shown in humans [157,158].
GAGs and proteoglycans such as heparin and heparan sulfate have shown promise as glycocalyx protecting therapeutics for many years [159]. Unfractionated and low molecular weight heparin are best known for their anticoagulant properties and use in treating or preventing thrombotic events by activating anti-thrombin III [160]. However, heparin has further been shown to possess anti-inflammatory properties, lending to improved vascular outcomes after inflammatory damage by limiting vascular permeability [161], neutrophil adherence and migration [63,162], and platelet adhesion [163], fibrin deposition [63], and thrombus formation [92,164,165]. As one of the oldest anticoagulants in clinical medicine, heparin and heparin-like products have been heavily studied [165–169]. More recently, low molecular weight heparin has been used to treat coagulopathy seen in severe COVID-19 patients [97,170,171], and nebulized unfractionated heparin is currently being evaluated in clinical trials for acute lung injury [171] and COVID-19 (ACCORD 2: A Multicentre, Seamless, Phase 2 Adaptive Randomisation Platform Study to Assess the Efficacy and Safety of Multiple Candidate Agents for the Treatment of COVID 19 in Hospitalised Patients, EudraCT number 2020-001736-95) [171]. While risks of thrombocytopenia and haemorrhagic shock limit the clinical use of this drug for inflammatory indications in its standard form, non-anticoagulant mechanisms of heparin are a promising pivot point for heparin-derived therapeutics [172].
Other promising molecules include sphingosine-1-phosphate and sulodexide. The signalling triggered by sphingolipid sphingosine-1-phosphate has been described as glycocalyx protective [173]. When paired with heparan sulfate, sphingosine-1-phosphate has been shown to regenerate the glycocalyx and restore inter-endothelial communication [21]. Sphingosine-1-phosphate analogues and receptor modulators have been tested clinically for a range of autoimmune and inflammatory diseases, including multiple sclerosis, psoriasis, acute stroke and inflammatory bowel disease [174,175]. However, prolonged exposure to sphingosine-1-phosphate has also been shown to enhance vascular leak and fibrosis after lung injury [176], therefore caution should be taken before employing sphingosine-1-phosphate modulating drugs to different organ systems and disease indications.
Sulodexide is a decades-old glycosaminoglycan mixture made up of 80% fast-moving heparin (iduronylglycosaminoglycan) and 20% dermatan sulfate that mitigates the bleeding risk of heparin-only therapeutics while maintaining anti-thrombotic potential [177,178]. Originally used for cardiac indications such as myocardial infarction [179], sulodexide has since been extended to other vascular disorders that manifest as endothelial dysfunction and glycocalyx damage, such as in type-2 diabetes mellitus patients [180] and in patients with venous ulcers [181]. Sulodexide has been shown to promote glycocalyx regeneration and improve animal survival after severe sepsis [22] and reduce the levels of collagen degrading MMP9 in patients with chronic vascular disease [24].
Taken together, the benefits seen with this class of molecules with respect to decreased neutrophil and platelet adhesion further suggest that restoring the function of the critical endothelial glycocalyx barrier can improve outcomes for fibrotic and thrombotic diseases that result from overexuberant interactions between endothelial cells, platelets and neutrophils.
4.2. Cytokine inhibitors
Recombinant human cytokines and cytokine receptors have been used as therapeutic targets to modulate inflammatory activity, driven in part by the pain patients experience as a result of acute and chronic inflammatory diseases [182,183]. Three core pro-inflammatory cytokines have been of particular interest in this realm: TNF-α, IL-1β, and IL-6. These cytokines act as key inducers of endothelial activation, neutrophil accumulation and activation, and platelet adhesion and degranulation, suggesting a potential benefit of inhibitors in regulating endothelial activation and the downstream consequences of endothelial dysfunction. There are currently several Food and Drug Administration (FDA) approved TNF-α, IL-1 and IL-6 inhibitors for clinical indications such as rheumatic diseases, Crohn's disease and cryopyrin-associated periodic syndromes (CAPS) [184].
TNF-α blockers have been successfully employed to combat fibrotic indications such rheumatoid arthritis and psoriasis [185,186]; unfortunately, these benefits are not ubiquitous for all fibrotic diseases, as was evidenced by their ineffectiveness against idiopathic pulmonary fibrosis [187]. Anti-TNF-α therapies for chronic heart failure have likewise been tested in clinical trials with little success [188], and potential beneficial effects on endothelial function in patients with inflammatory arthropathies are inconsistent [189].
IL-1β antagonists have shown greater potential in vascular inflammatory disorders, perhaps in part owing to the integral role of IL-1 in leucocyte and endothelial signalling. The FDA approved IL-1 agonists canakinumab (originally approved for CAPS), anakinra (originally approved for rheumatoid arthritis) and rilonacept (originally approved for CAPS) have shown promise in clinical trials of cardiovascular conditions such as pericarditis and recurrent ischemic events after myocardial infarction [190,191], but thus far none have been approved for these indications.
IL-6 is known to promote endothelial cell dysfunction and regulate leucocyte recruitment to the vascular wall [192]; therefore, it is unsurprising that FDA approved IL-6 blockers for arthritic conditions are of interest for treating off-label inflammatory conditions such as systemic sclerosis. Clinical trials of tocilizumab and sarilumab have recently begun as a treatment for ‘cytokine storm'-related morbidities in severe SARS-CoV-2 patients [70,193]. The anti-IL-6 receptor tocilizumab is particularly promising, as it has been shown to cause transient neutropenia without impairing host defence [194].
While cytokine inhibitors and cytokine receptor blockers may be beneficial in certain inflammatory conditions, the mixed results of this class of molecules suggest the need for combination therapies, or therapies more directed at the key cellular interactions.
4.3. Collagen protection
Severe endothelial damage can cause the exposure of the underlying collagen matrix, prompting rapid platelet adhesion. As described in this review, activated endothelium and collagen-adherent platelets provide an adhesive surface for neutrophil recruitment, eventually leading to tissue fibrosis; therefore, targeting exposed collagen could enhance anti-neutrophil–platelet therapies.
Several groups have designed collagen-targeting therapeutics to discourage platelet and/or neutrophil accumulation. Paderi and colleagues designed a proteoglycan mimetic consisting of collagen-binding peptides conjugated to a dermatan sulfate backbone that bound collagen, but not endothelium, of denuded arteries after balloon angioplasty [45]. Their therapeutic reduced in vivo platelet-induced vasospasm in the femoral artery as well as whole blood and platelet binding in vitro. Further, their studies showed that reduced platelet binding to the arterial wall correlated with reduced fibrosis or neointimal hyperplasia in vivo.
Similarly, McMasters et al. designed a thermoresponsive collagen-binding nanoparticle for effective systemic delivery of a mitogen-activated protein kinase-activated protein kinase 2 (MK2) anti-inflammatory peptide [43]. The authors show that nanoparticle delivery of the MK2 inhibitor in vitro reduced cellular binding to collagen surfaces, IL-6 levels in endothelial cells and smooth muscle cells, and platelet activation on a collagen matrix. Additional nanostructures have been developed to aid in drug delivery to exposed collagen. Collagen IV targeting nanoburrs [195] and nanofibres [196,197] have been designed to target angioplasty injured vasculature [195] and atherosclerotic plaques [197].
4.4. P- and E-selectin
Rather than targeting platelet deposition on denuded endothelium, Totani et al. have focused on preventing neutrophil deposition on adherent platelets [109], thereby reducing the likelihood of thrombosis within the vessel. Blockade of phosphodiesterase type-4 by rolipram, originally an anti-depressant drug, caused a reduction in neutrophil binding to fixed, activated platelets in vitro as well as adherent neutrophils along the denuded femoral artery in vivo. Adhesion was likewise lost on untreated P-selectin deficient platelets, further suggesting P-selectin as a mediator of platelet–neutrophil interactions.
P-selectin has been a focal point in anti-inflammatory therapeutics owing to the substantial role it plays in endothelial signalling, neutrophil and platelet recruitment to inflamed endothelium, and the formation of platelet-leucocyte aggregates. Competitive inhibitors of P- and E-selectin and their binding partners, PSGL-1, CD44 and E-selectin ligand 1, have been investigated as antagonists to neutrophil and platelet-mediated vascular damage. Monoclonal antibody therapy to P- and E-selectin have shown promise in combating platelet and neutrophil-mediated injury [198–200], as is seen with the FDA approved P-selectin antibody crizanlizumab, designed to address vaso-occlusive crises in sickle cell anaemia [201]. However, clinical trials of Inclacumab, the monoclonal antibody designed to bind P-selectin, evidenced that a major limitation of antibody therapy is the need for high doses for therapeutic effects [90,202], leading to high production costs [203,204].
Because of the role E- and P-selectin play in vaso-occlusive processes, small molecule inhibitors of these ligands are also under various stages of development. The pan-selectin antagonist Rivepansel (GMI-1070) was designed to combat vaso-occlusive crises in severe sickle cell anaemia by preventing the interaction of leucocytes and endothelium [205], but unfortunately failed to meet its phase 3 clinical trial endpoints. Clinical trials for GMI-1271, a small molecule inhibitor of E-selectin, is currently underway for treating venous thrombosis [206]. Exogenous recombinant human vimentin, a cytoskeletal structural protein and CD44-binding partner [207], has been shown to act as a competitive inhibitor of neutrophil binding to platelets and endothelial cells in a P-selectin-dependent manner [88]. Treatment with recombinant human vimentin reduced neutrophilia and acute lung injury scores in mice treated with sub-lethal doses of LPS. Clinical trials assessing the role of vimentin in sepsis, rheumatoid arthritis and renal transplant are currently underway. However, given that both increases and deficiencies of this protein can lead to vascular abnormalities [207,208], the effects of exogenous systemic administration will need to be extensively studied before vimentin is used as a therapeutic.
Selectins have been used as targeting modalities for glycan and polysaccharide-based molecules. Glycomimetics designed to bind selectins or selectin tetrasaccharide sialyl-Lewisx are a new perspective on anti-inflammatory therapeutics [209], in part because of the anti-inflammatory nature of polysaccharides, glycosaminoglycans and proteoglycans. Recently, a selectin-targeting dermatan sulfate conjugate has been reported as reducing neutrophil and platelet interactions with inflamed endothelial cells in vitro and reduced thrombus formation in vivo [91,164]. Similarly, glycopeptide analogues of PSGL-1 effectively reduced neutrophil–platelet aggregates [80].
Polymer microcapsules coated with fucoidan, a complex polysaccharide that has been shown to slow blood clotting, have been proposed as a drug delivery tool targeted to inflamed vessels expressing P-selectin under high shear [210]. Polymer, glycosaminoglycan and polysaccharide-based therapeutics present indirect benefits in addition to competing for selectin binding. Bulky therapeutics could provide a steric boundary limiting neutrophil and platelet interactions with inflamed endothelium and exposed collagen. Dual targeting to inflamed endothelium and exposed collagen could likewise prevent the pathological accumulation of neutrophils, platelets and neutrophil–platelet aggregates in damaged vessels and thereby reduce immunothrombosis and tissue fibrosis.
As the initial points of capture for both neutrophils and platelets, selectin inhibition may provide a way to modulate thrombosis and fibrosis early on in disease progression. Therapeutics that blanket the adhesive endothelium, such as polymer-based molecules and glycoconjugates that are targeted towards upregulated selectins, may overcome limitations of monoclonal antibodies and recombinant proteins. Nonetheless, therapeutics will need to be studied on a case-by-case basis to maximize their efficacy as anti-inflammatory, anti-thrombotic or anti-fibrotic therapies.
5. Conclusion and perspective
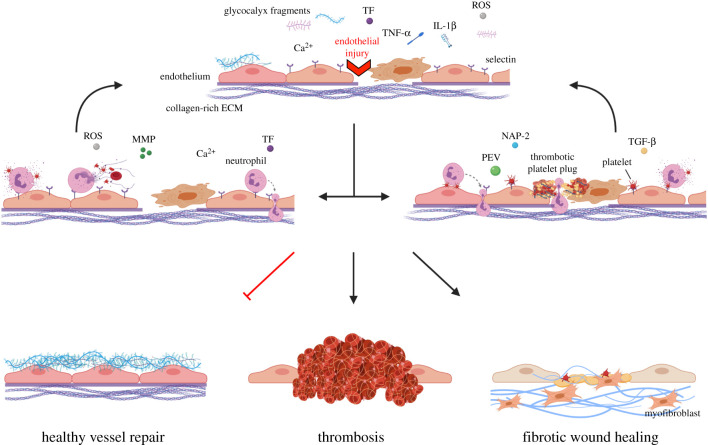
Neutrophils, platelets and activated endothelial cells each make substantial contributions to the initiation and perpetuation of vascular dysfunction. As such, many studies have been conducted to establish the relative contribution each of these makes to downstream thrombosis and fibrosis. When physiological redundancies are considered, it is likely that the relative contributions shift depending on the vascular environment in a coordinated effort to resolve an inflammatory stimulus (figure 2). Endothelial activation initiates the secretion of pro-inflammatory cytokines and shedding of glycocalyx components, prompting the recruitment of circulating immune cells. Upregulated endothelial E- and P-selectin capture circulating platelets and neutrophils. Adhesion of these inflammatory regulators propels further recruitment, eventually overwhelming the vessel's capacity for self-restoration and shifting the cellular environment towards thrombotic or fibrotic. Captured and rolling neutrophils capitalize on contracting, leaky endothelium, which presents extravasation points that facilitate neutrophil migration into the underlying tissue. Neutrophil activation augments endothelial activation through the release of degranulation products and, in severe cases, NET formation.
Figure 2.
Neutrophil, platelet and endothelial contributions to vessel repair. In response to vessel injury, endothelial cells shed their protective glycosaminoglycan-rich layer, the endothelial glycocalyx, exposing upregulated selectins that have been mobilized to the cell surface. Activated endothelial cells secrete pro-inflammatory cytokines such as TNF-α and IL-1β that recruit circulating neutrophils and platelets to the damaged region. ROS production and dysregulated calcium homeostasis causes reductions in NO, enhanced cell contraction, vessel leakiness and the exposure of the underlying collagen-rich ECM. In some cases, neutrophils captured by upregulated selectins degranulate, releasing additional ROS and matrix-degrading MMPs. Adherent neutrophils act as secondary capture points for circulating platelets. Neutrophils undergoing NETosis capture red blood cells, platelets and fibrin, facilitating thrombosis development. Neutrophil activation results in further endothelial activation, which can lead to apoptosis of endothelial cells. In other cases, platelets anchor to upregulated vWF and P-selectin on the endothelial surface via GPIIbIIIa and PSGL-1. Adherent platelets secrete factors such as NAP-2, TGF-β and platelet extracellular vesicles (PEVs) that cause further endothelial activation, neutrophil recruitment, and promote the migration and proliferation of collagen-producing myofibroblasts. Platelets form a platelet plug at regions with severe endothelial damage, where denudation has occurred. Neutrophils infiltrate the platelet plug, causing an imbalance in the haemostatic response that shifts plug formation towards thrombotic. Activated platelets help guide crawling neutrophils through the thrombus or endothelium. Persistent or unresolved endothelial damage causes continued recruitment and activation of platelets and neutrophils, overwhelming the vessel and shifting the repair process towards thrombosis and fibrotic wound healing.
Severe endothelial activation exposes the underlying collagen-rich ECM, causing platelet recruitment beyond that triggered by selectin upregulation. Activated adherent platelets express platelet P-selectin, which can act as a congregation point for additional platelets or a secondary capture point for circulating neutrophils. Accumulation of platelets alone or platelet–neutrophil aggregates can result in vessel thrombosis and, in severe cases, vessel occlusion and tissue ischemia. In vessels more susceptible to fibrosis, platelet activation stimulates the production of pro-fibrotic cytokine TGF-β, pathological ECM secretion and downstream fibrosis.
Several therapeutic strategies have been implemented to combat the downstream effects of endothelial dysfunction. Each approach primarily targets one interaction to halt what appears to be a highly coupled group of interactions that, when unbalanced, results in pathological thrombosis and/or fibrosis. Conflicting outcomes suggest that no one therapeutic will be a panacea that resets the balance towards healthy regeneration and healing. Based on our current understanding of the coordination of endothelial cells, neutrophils, and platelets when the endothelium becomes dysfunctional, combination therapeutics or complex therapies that target multiple adhesion axes simultaneously may be most efficacious in treating thrombotic and fibrotic conditions.
Deeper understanding of these complex pathways is needed to develop therapeutic strategies for what is surely a large number of distinct pathological states that result from sterile inflammation, diffuse dysfunction that exists with metabolic syndrome, and the sudden and severe response to cytokine storm, like that seen as a result of severe COVID-19 infections, among others. Key discoveries such as the role of NO and ROS in endothelial vasoreactivity or, more recently, the role of platelets as drivers of thrombosis, inflammation and fibrosis, have been integral in strengthening our understanding of these complex phenomena. Furthermore, elucidating how interactions between neutrophils and endothelial cells can result in NET formation and eventual thrombus formation provides a new foundation from which to innovate and think about disease and healing. The community needs to remain open to the fact that the mechanisms involved in endothelial dysfunction, coupled with platelet and neutrophil interactions, are multipronged; therefore, combinatorial approaches to treatment may well be required. This mode of thinking will expand the potential of targeting not just intercellular signalling cascades, but also cell-cell and cell-ECM interactions to quickly alter the biological response to injury and disease.
Acknowledgements
Figure 2 was created with BioRender.com.
Authors' contributions
T.D. and A.P. wrote and edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a Predoctoral Fellowship from the Tobacco-Related Disease Research Program (TRDRP) T29DT0237.
References
- 1.Eming SA, Krieg T, Davidson JM. 2007. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525. ( 10.1038/sj.jid.5700701) [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Ramalingam TR. 2012. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040. ( 10.1038/nm.2807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woywodt A, Bahlmann FH, de Groot K, Haller H, Haubitz M. 2002. Circulating endothelial cells: life, death, detachment and repair of the endothelial cell layer. Nephrol. Dial. Transplant. 17, 1728–1730. ( 10.1093/ndt/17.10.1728) [DOI] [PubMed] [Google Scholar]
- 4.Bar A, et al. 2019. Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/low-density lipoprotein receptor-deficient mice. J. Am. Heart Assoc. 8, e011171 ( 10.1161/JAHA.118.011171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KH, Park WJ. 2015. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J. Korean Med. Sci. 30, 1213–1225. ( 10.3346/jkms.2015.30.9.1213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Shi G-P. 2014. Vascular wall extracellular matrix proteins and vascular diseases. Biochimica et biophysica acta 1842, 2106–2119. ( 10.1016/j.bbadis.2014.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krüger-Genge A, Blocki A, Franke R-P, Jung F. 2019. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 20, 4411 ( 10.3390/ijms20184411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altabas V. 2015. Diabetes, endothelial dysfunction, and vascular repair: what should a diabetologist keep his eye on? Int. J. Endocrinol. 2015, 848272 ( 10.1155/2015/848272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. 2011. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Trans. Med. 3, 112ra22 ( 10.1126/scitranslmed.3002761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanner A, Mendes ES. 2010. Airway endothelial dysfunction in asthma and chronic obstructive pulmonary disease. Am. J. Res. Critical Care Med. 182, 1344–1351. ( 10.1164/rccm.201001-0038PP) [DOI] [PubMed] [Google Scholar]
- 11.Totani L, et al. 2017. Mechanisms of endothelial cell dysfunction in cystic fibrosis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1863, 3243–3253. ( 10.1016/j.bbadis.2017.08.011) [DOI] [PubMed] [Google Scholar]
- 12.Stoy S, Patel VC, Sturgeon JP, Manakkat Vijay GK, Lisman T, Bernal W, Shawcross DL. 2018. Platelet-leucocyte aggregation is augmented in cirrhosis and further increased by platelet transfusion. Aliment Pharmacol. Ther. 47, 1375–1386. ( 10.1111/apt.14600) [DOI] [PubMed] [Google Scholar]
- 13.Weinbaum S, Tarbell JM, Damiano ER. 2007. The structure and function of the endothelial glycocalyx layer. Ann. Rev. Biomed. Eng. 9, 121–167. ( 10.1146/annurev.bioeng.9.060906.151959) [DOI] [PubMed] [Google Scholar]
- 14.Barker AL, Konopatskaya O, Neal CR, Macpherson JV, Whatmore JL, Winlove CP, Unwin PR, Shore AC. 2004. Observation and characterisation of the glycocalyx of viable human endothelial cells using confocal laser scanning microscopy. Phys. Chem. Chem. Phys. 6, 1006–1011. ( 10.1039/B312189E) [DOI] [Google Scholar]
- 15.Cao RN, Tang L, Xia ZY, Xia R. 2019. Endothelial glycocalyx as a potential theriapeutic target in organ injuries. Chin. Med. J. (Engl.) 132, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curry FE. 2018. The molecular structure of the endothelial glycocalyx layer (EGL) and surface layers (ESL) modulation of transvascular exchange. Adv. Exp. Med. Biol. 1097, 29–49. ( 10.1007/978-3-319-96445-4_2) [DOI] [PubMed] [Google Scholar]
- 17.Kundra P, Goswami S. 2019. Endothelial glycocalyx: role in body fluid homeostasis and fluid management. Indian J. Anaesth. 63, 6–14. ( 10.4103/ija.IJA_751_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, Satchell SC, Bates DO, Salmon AHJ. 2017. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 595, 5015–5035. ( 10.1113/JP274167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trowbridge JM, Gallo RL. 2002. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology 12, 117R–125R. ( 10.1093/glycob/cwf066) [DOI] [PubMed] [Google Scholar]
- 20.Tovar AMF, de Mattos DA, Stelling MP, Sarcinelli-Luz BSL, Nazareth RA, Mourão PAS. 2005. Dermatan sulfate is the predominant antithrombotic glycosaminoglycan in vessel walls: implications for a possible physiological function of heparin cofactor II. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1740, 45–53. ( 10.1016/j.bbadis.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 21.Mensah SA, Cheng MJ, Homayoni H, Plouffe BD, Coury AJ, Ebong EE. 2017. Regeneration of glycocalyx by heparan sulfate and sphingosine 1-phosphate restores inter-endothelial communication. PLoS ONE 12, e0186116 ( 10.1371/journal.pone.0186116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song JW, Zullo JA, Liveris D, Dragovich M, Zhang XF, Goligorsky MS. 2017. Therapeutic restoration of endothelial glycocalyx in sepsis. J. Pharmacol. Exp. Ther. 361, 115–121. ( 10.1124/jpet.116.239509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipowsky HH, Lescanic A. 2017. Inhibition of inflammation induced shedding of the endothelial glycocalyx with low molecular weight heparin. Microvasc. Res. 112, 72–78. ( 10.1016/j.mvr.2017.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannello F, Medda V, Ligi D, Raffetto JD. 2013. Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase secretion and activity: possible pharmacological role against collagen degradation in vascular chronic diseases. Curr. Vasc. Pharmacol. 11, 354–365 ( 10.2174/1570161111311030010) [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Lipowsky HH. 2010. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc. Res. 80, 394–401. ( 10.1016/j.mvr.2010.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai K, Wang W. 2014. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech. Model Mechanobiol. 13, 303–311. ( 10.1007/s10237-013-0502-3) [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Adamson RH, Liu B, Curry FE, Weinbaum S. 2000. Starling forces that oppose filtration after tissue oncotic pressure is increased. Am. J. Physiol. Heart Circ. Physiol. 279, H1724–H1H36 ( 10.1152/ajpheart.2000.279.4.H1724) [DOI] [PubMed] [Google Scholar]
- 28.Harding IC, Mitra R, Mensah SA, Nersesyan A, Bal NN, Ebong EE. 2019. Endothelial barrier reinforcement relies on flow-regulated glycocalyx, a potential therapeutic target. Biorheology 56, 131–149. ( 10.3233/BIR-180205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Wang W. 2018. Structural alteration of the endothelial glycocalyx: contribution of the actin cytoskeleton. Biomech. Model. Mechanobiol. 17, 147–158. ( 10.1007/s10237-017-0950-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thi MM, Tarbell JM, Weinbaum S, Spray DC. 2004. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a ‘bumper-car’ model. Proc. Natl Acad. Sci. USA 101, 16 483–16 488. ( 10.1073/pnas.0407474101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo TA, Stoll D, Nader HB, Dreyfuss JL. 2018. Mechanical stretch implications for vascular endothelial cells: altered extracellular matrix synthesis and remodeling in pathological conditions. Life Sciences 213, 214–225. ( 10.1016/j.lfs.2018.10.030) [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Rabodzey A, Forbes Dewey JC. 2007. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am. J. Physiol. Heart Circ. Physiol. 293, H1023–H1H30. ( 10.1152/ajpheart.00162.2007) [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Kalogeris T, Korthuis RJ. 2019. Reactive species-induced microvascular dysfunction in ischemia/reperfusion. Free Radic. Biol. Med. 135, 182–197. ( 10.1016/j.freeradbiomed.2019.02.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai H, Harrison DG. 2000. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844. ( 10.1161/01.RES.87.10.840) [DOI] [PubMed] [Google Scholar]
- 35.Curry FE, Michel CC. 1980. A fiber matrix model of capillary permeability. Microvasc. Res. 20, 96–99. ( 10.1016/0026-2862(80)90024-2) [DOI] [PubMed] [Google Scholar]
- 36.Uchimido R, Schmidt EP, Shapiro NI. 2019. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Critical Care 23, 16 ( 10.1186/s13054-018-2292-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehm M, et al. 2007. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 116, 1896–1906. ( 10.1161/CIRCULATIONAHA.106.684852) [DOI] [PubMed] [Google Scholar]
- 38.Chappell D, Heindl B, Jacob M, Annecke T, Chen C, Rehm M, Conzen P, Becker BF. 2011. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 115, 483–491. ( 10.1097/ALN.0b013e3182289988) [DOI] [PubMed] [Google Scholar]
- 39.McDonald KK, Cooper S, Danielzak L, Leask RL. 2016. Glycocalyx degradation induces a proinflammatory phenotype and increased leukocyte adhesion in cultured endothelial cells under flow. PLoS ONE 11, e0167576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L. 2014. Modulation of endothelial glycocalyx structure under inflammatory conditions. Med. Inflamm. 2014, 694312 ( 10.1155/2014/694312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker BF, Jacob M, Leipert S, Salmon AHJ, Chappell D. 2015. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br. J. Clin. Pharmacol. 80, 389–402. ( 10.1111/bcp.12629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buijs J, Musters M, Verrips T, Post JA, Braam B, Riel N. 2004. Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am. J. Physiol. Heart Circ. Physiol. 287, H2651–H26H8 ( 10.1152/ajpheart.00332.2004) [DOI] [PubMed] [Google Scholar]
- 43.McMasters J, Panitch A. 2017. Collagen-binding nanoparticles for extracellular anti-inflammatory peptide delivery decrease platelet activation, promote endothelial migration, and suppress inflammation. Acta Biomaterialia 49, 78–88. ( 10.1016/j.actbio.2016.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouns SLN, Provenzale I, van Geffen JP, van der Meijden PEJ, Heemskerk JWM. 2020. Localized endothelial-based control of platelet aggregation and coagulation under flow: a proof-of-principle vessel-on-a-chip study. J. Thromb. Haemost. 18, 931–941. ( 10.1111/jth.14719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paderi JE, Stuart K, Sturek M, Park K, Panitch A. 2011. The inhibition of platelet adhesion and activation on collagen during balloon angioplasty by collagen-binding peptidoglycans. Biomaterials 32, 2516–2523. ( 10.1016/j.biomaterials.2010.12.025) [DOI] [PubMed] [Google Scholar]
- 46.Kuijpers MJ, Munnix IC, Cosemans JM, Vlijmen BV, Reutelingsperger CP, Egbrink MO, Heemskerk JWM. 2008. Key role of platelet procoagulant activity in tissue factor-and collagen-dependent thrombus formation in arterioles and venules in vivo differential sensitivity to thrombin inhibition. Microcirculation 15, 269–282. ( 10.1080/10739680701653517) [DOI] [PubMed] [Google Scholar]
- 47.Greineder CF, Johnston IH, Villa CH, Gollomp K, Esmon CT, Cines DB, Poncz M, Muzykantov VR. 2017. ICAM-1-targeted thrombomodulin mitigates tissue factor-driven inflammatory thrombosis in a human endothelialized microfluidic model. Blood Adv. 1, 1452–1465. ( 10.1182/bloodadvances.2017007229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puhr-Westerheide D, et al. 2019. Neutrophils promote venular thrombosis by shaping the rheological environment for platelet aggregation. Sci. Rep. 9, 15932 ( 10.1038/s41598-019-52041-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim KH, Barazia A, Cho J. 2013. Real-time imaging of heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus formation in live mice. J. Vis. Exp. 74, 50329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkinson BT, Jasuja R, Chen VM, Nandivada P, Furie B, Furie BC. 2010. Laser-induced endothelial cell activation supports fibrin formation. Blood 116, 4675–4683. ( 10.1182/blood-2010-05-283986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanciu L, Krishnaswamy S, Camire RM. 2014. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood 124, 1705–1714. ( 10.1182/blood-2014-03-565010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darbousset R, et al. 2012. Tissue factor–positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 120, 2133–2143. ( 10.1182/blood-2012-06-437772) [DOI] [PubMed] [Google Scholar]
- 53.Schulz C, et al. 2007. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood. Circulation 116, 764–773. ( 10.1161/CIRCULATIONAHA.107.695189) [DOI] [PubMed] [Google Scholar]
- 54.Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pörnbacher M, Lauber K, Krombach F, Reichel CA. 2016. Platelets guide leukocytes to their sites of extravasation. PLOS Biol. 14, e1002459 ( 10.1371/journal.pbio.1002459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaefer A, et al. 2014. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J. Cell Sci. 127, 4470–4482. ( 10.1242/jcs.154708) [DOI] [PubMed] [Google Scholar]
- 56.Reitsma S, Oude Egbrink MG, Heijnen VV, Megens RT, Engels W, Vink H, Slaaf DW, van Zandvoort M. 2011. Endothelial glycocalyx thickness and platelet-vessel wall interactions during atherogenesis. Thromb. Haemost. 106, 939–946. ( 10.1160/TH11-02-0133) [DOI] [PubMed] [Google Scholar]
- 57.Kato S, Inui N, Hakamata A, Suzuki Y, Enomoto N, Fujisawa T, Nakamura Y, Watanabe H, Suda T. 2018. Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis. Respir. Res. 19, 127 ( 10.1186/s12931-018-0831-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stroka KM, Aranda-Espinoza H. 2011. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 118, 1632–1640. ( 10.1182/blood-2010-11-321125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakai N, et al. 2019. The involvement of autotaxin in renal interstitial fibrosis through regulation of fibroblast functions and induction of vascular leakage. Sci. Rep. 9, 7414 ( 10.1038/s41598-019-43576-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Idriss HT, Naismith JH. 2000. TNFα and the TNF receptor superfamily: structure-function relationship(s). Microsc. Res. Tech. 50, 184–195. () [DOI] [PubMed] [Google Scholar]
- 61.Morikis VA, Simon SI. 2018. Neutrophil mechanosignaling promotes integrin engagement with endothelial cells and motility within inflamed vessels. Front. Immunol. 9, 2774 ( 10.3389/fimmu.2018.02774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678 ( 10.1038/nri2156) [DOI] [PubMed] [Google Scholar]
- 63.Kirchhofer D, Tschopp TB, Hadváry P, Baumgartner HR. 1994. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J. Clin. Invest. 93, 2073–2083. ( 10.1172/JCI117202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grover SP, Mackman N. 2018. Tissue factor. Arterioscler. Thromb. Vasc. Biol. 38, 709–725. ( 10.1161/ATVBAHA.117.309846) [DOI] [PubMed] [Google Scholar]
- 65.Mercer PF, Chambers RC. 2013. Coagulation and coagulation signalling in fibrosis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1832, 1018–1027. ( 10.1016/j.bbadis.2012.12.013) [DOI] [PubMed] [Google Scholar]
- 66.Chrysanthopoulou A, et al. 2011. Tissue factor–thrombin signaling enhances the fibrotic activity of myofibroblasts in systemic sclerosis through up-regulation of endothelin receptor A. Arthritis Rheumatism 63, 3586–3597. ( 10.1002/art.30586) [DOI] [PubMed] [Google Scholar]
- 67.Westrick RJ, Winn ME, Eitzman DT. 2007. Murine models of vascular thrombosis. Arterioscler. Thromb. Vasc. Biol. 27, 2079–2093. ( 10.1161/ATVBAHA.107.142810) [DOI] [PubMed] [Google Scholar]
- 68.Calvente CJ, et al. 2019. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J. Clin. Invest. 129, 4091–4109. ( 10.1172/JCI122258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narasaraju T, Tang B, Herrmann M, Muller S, Chow VTK, Radic M. 2020. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19. Front. Pharmacol. 11, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C, Zhang XR, Ju ZY, He WF. 2020. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 36, E005. [DOI] [PubMed] [Google Scholar]
- 71.Mesri M, Altieri DC. 1998. Endothelial cell activation by leukocyte microparticles. J. Immunol. 161, 4382–4387. [PubMed] [Google Scholar]
- 72.Kay EP, Nimni M, Smith RE. 1984. Modulation of endothelial cell morphology and collagen synthesis by polymorphonuclear leukocytes. Invest. Ophthalmol Visual Sci. 25, 502–512. [PubMed] [Google Scholar]
- 73.Yago T, Zhang N, Zhao L, Abrams CS, McEver RP. 2018. Selectins and chemokines use shared and distinct signals to activate beta2 integrins in neutrophils. Blood Adv. 2, 731–744. ( 10.1182/bloodadvances.2017015602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. 2008. Calcium flux in neutrophils synchronizes β2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann. Biomed. Eng. 36, 632–646. ( 10.1007/s10439-008-9453-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang B, Ling Y, Lin J, Du X, Fang Y, Wu J. 2017. Force-dependent calcium signaling and its pathway of human neutrophils on P-selectin in flow. Protein Cell 8, 103–113. ( 10.1007/s13238-016-0364-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Downey GP, Worthen GS, Henson PM, Hyde DM. 1993. Neutrophil sequestration and migration in localized pulmonary inflammation. Capillary localization and migration across the interalveolar septum. Am. Rev. Respir. Dis. 147, 168–176. ( 10.1164/ajrccm/147.1.168) [DOI] [PubMed] [Google Scholar]
- 77.Doerschuk CM. 2001. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 8, 71–88. ( 10.1111/j.1549-8719.2001.tb00159.x) [DOI] [PubMed] [Google Scholar]
- 78.Park SA, Choe YH, Park E, Hyun Y-M. 2018. Real-time dynamics of neutrophil clustering in response to phototoxicity-induced cell death and tissue damage in mouse ear dermis. Cell Adh. Migr. 12, 424–431. ( 10.1080/19336918.2018.1471322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pober JS, Sessa WC. 2014. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 7, a016345 ( 10.1101/cshperspect.a016345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krishnamurthy VR, et al. 2015. Glycopeptide analogues of PSGL-1 inhibit P-selectin in vitro and in vivo. Nat. Commun. 6, 6387 ( 10.1038/ncomms7387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gong Y, Zhang Y, Feng S, Liu X, Lu S, Long M. 2017. Dynamic contributions of P- and E-selectins to beta2-integrin-induced neutrophil transmigration. FASEB J. 31, 212–223. ( 10.1096/fj.201600398rrr) [DOI] [PubMed] [Google Scholar]
- 82.Ma Y, Yang X, Chatterjee V, Meegan JE, Beard RS, Yuan SY. 2019. Role of neutrophil extracellular traps and vesicles in regulating vascular endothelial permeability. Front. Immunol. 10, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. 2015. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 126, 242–246. ( 10.1182/blood-2015-01-624023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Brühl M-L, et al. 2012. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835. ( 10.1084/jem.20112322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnes BJ, et al. 2020. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 217, e20200652 ( 10.1084/jem.20200652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuchs TA, Brill A, Wagner DD. 2012. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 32, 1777–1783. ( 10.1161/ATVBAHA.111.242859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Green JP, Souilhol C, Xanthis I, Martinez-Campesino L, Bowden NP, Evans PC, Wilson HL. 2018. Atheroprone flow activates inflammation via endothelial ATP-dependent P2X7-p38 signalling. Cardiovasc. Res. 114, 324–335. ( 10.1093/cvr/cvx213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lam FW, Da Q, Guillory B, Cruz MA. 2018. Recombinant human vimentin binds to P-selectin and blocks neutrophil capture and rolling on platelets and endothelium. J. Immunol. 200, 1718–1726. ( 10.4049/jimmunol.1700784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Telen MJ, et al. 2015. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood 125, 2656–2664. ( 10.1182/blood-2014-06-583351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stahli BE, et al. 2016. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention according to timing of infusion: insights from the SELECT-ACS trial. J. Am. Heart Assoc. 5, e004255 ( 10.1161/JAHA.116.004255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wodicka JR, Morikis VA, Dehghani T, Simon SI, Panitch A. 2019. Selectin-targeting peptide-glycosaminoglycan conjugates modulate neutrophil-endothelial interactions. Cell. Mol. Bioeng. 12, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Culmer DL, Dunbar ML, Hawley AE, Sood S, Sigler RE, Henke PK, Wakefield TW, Magnani JL, Myers DD. 2017. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb. Haemost. 117, 1171–1181. ( 10.1160/TH16-04-0323) [DOI] [PubMed] [Google Scholar]
- 93.Ramirez GA, Manfredi AA, Maugeri N. 2019. Misunderstandings between platelets and neutrophils build in chronic inflammation. Front. Immunol. 10, 2491 ( 10.3389/fimmu.2019.02491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baimukanova G, et al. 2016. Platelets regulate vascular endothelial stability: assessing the storage lesion and donor variability of apheresis platelets. Transfusion 56(Suppl 1), S65–S75. ( 10.1111/trf.13532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghoshal K, Bhattacharyya M. 2014. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. Scientific World Journal. 2014, 781857 ( 10.1155/2014/781857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amison RT, O'Shaughnessy BG, Arnold S, Cleary SJ, Nandi M, Pitchford SC, Bragonzi A, Page CP. 2018. Platelet depletion impairs host defense to pulmonary infection with pseudomonas aeruginosa in mice. Am. J. Res. Cell Mol. Biol. 58, 331–340. ( 10.1165/rcmb.2017-0083OC) [DOI] [PubMed] [Google Scholar]
- 97.Zhou X, Li Y, Yang Q. 2020. Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: implications from clinical features to pathological findings. Circulation 141, 1736–1738. [DOI] [PubMed] [Google Scholar]
- 98.Gros A, Syvannarath V, Lamrani L, Ollivier V, Loyau S, Goerge T, Nieswandt B, Jandrot-Perrus M, Ho-Tin-Noé B. 2015. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood 126, 1017–1026. ( 10.1182/blood-2014-12-617159) [DOI] [PubMed] [Google Scholar]
- 99.Slaba I, Wang J, Kolaczkowska E, McDonald B, Lee W-Y, Kubes P. 2015. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology 62, 1593–1605. ( 10.1002/hep.28003) [DOI] [PubMed] [Google Scholar]
- 100.Gros A, Ollivier V, Ho-Tin-Noe B. 2014. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front. Immunol. 5, 678 ( 10.3389/fimmu.2014.00678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lindberg U, Svensson L, Hellmark T, Segelmark M, Shannon O. 2018. Increased platelet activation occurs in cystic fibrosis patients and correlates to clinical status. Thromb. Res. 162, 32–37. ( 10.1016/j.thromres.2017.12.012) [DOI] [PubMed] [Google Scholar]
- 102.Maugeri N, Rovere-Querini P, Baldini M, Baldissera E, Sabbadini MG, Bianchi ME, Manfredi AA. 2013. Oxidative stress elicits platelet/leukocyte inflammatory interactions via HMGB1: a candidate for microvessel injury in sytemic sclerosis. Antioxidants Redox Signal. 20, 1060–1074. ( 10.1089/ars.2013.5298) [DOI] [PubMed] [Google Scholar]
- 103.Tomaiuolo M, Matzko CN, Poventud-Fuentes I, Weisel JW, Brass LF, Stalker TJ. 2019. Interrelationships between structure and function during the hemostatic response to injury. Proc. Natl Acad. Sci. USA 116, 2243–2252. ( 10.1073/pnas.1813642116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, , Brass LF. 2013. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 121, 1875–1885. ( 10.1182/blood-2012-09-457739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Welsh JD, Poventud-Fuentes I, Sampietro S, Diamond SL, Stalker TJ, Brass LF. 2017. Hierarchical organization of the hemostatic response to penetrating injuries in the mouse macrovasculature. J. Thromb. Haemost. 15, 526–537. ( 10.1111/jth.13600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghasemzadeh M, et al. 2013. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood 121, 4555–4566. ( 10.1182/blood-2012-09-459636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pitchford S, Pan D, Welch HCE. 2017. Platelets in neutrophil recruitment to sites of inflammation. Curr. Opin. Hematol. 24, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zwaginga JJ, Torres HIG, Lammers J-WJ, Sixma JJ, Koenderman L, Kuijper PHM. 1999. Minimal platelet deposition and activation in models of injured vessel wall ensure optimal neutrophil adhesion under flow conditions. Aterioscler. Thromb. Vasc. Biol. 19, 1549–1554. ( 10.1161/01.ATV.19.6.1549) [DOI] [PubMed] [Google Scholar]
- 109.Totani L, et al. 2014. Phosphodiesterase type 4 blockade prevents platelet-mediated neutrophil recruitment at the site of vascular injury. Arterioscler. Thromb. Vasc. Biol. 34, 1689–1696. ( 10.1161/ATVBAHA.114.303939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Evangelista V, et al. 2007. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood 109, 2461–2469. ( 10.1182/blood-2006-06-029082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuravi SJ, Harrison P, Rainger GE, Nash GB. 2019. Ability of platelet-derived extracellular vesicles to promote neutrophil-endothelial cell interactions. Inflammation 42, 290–305. ( 10.1007/s10753-018-0893-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. 2005. Activated platelets induce Weibel-Palade–body secretion and leukocyte rolling in vivo: role of P-selectin. Blood 106, 2334–2339. ( 10.1182/blood-2005-04-1530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petri B, et al. 2010. von Willebrand factor promotes leukocyte extravasation. Blood 116, 4712–4719. ( 10.1182/blood-2010-03-276311) [DOI] [PubMed] [Google Scholar]
- 114.Abdulla A, Awla D, Hartman H, Weiber H, Jeppsson B, Regnér S, Thorlacius H. 2012. Platelets regulate P-selectin expression and leukocyte rolling in inflamed venules of the pancreas. Eur. J. Pharmacol. 682, 153–160. ( 10.1016/j.ejphar.2012.02.014) [DOI] [PubMed] [Google Scholar]
- 115.Frydman GH, Le A, Ellett F, Jorgensen J, Fox JG, Tompkins RG, Irimia D. 2017. Technical advance: changes in neutrophil migration patterns upon contact with platelets in a microfluidic assay. J. Leukoc. Biol. 101, 797–806. ( 10.1189/jlb.1TA1115-517RR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maugeri N, et al. 2009. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood 113, 5254–5265. ( 10.1182/blood-2008-09-180794) [DOI] [PubMed] [Google Scholar]
- 117.Braun OÖ, Slotta JE, Menger MD, Erlinge D, Thorlacius H. 2008. Primary and secondary capture of platelets onto inflamed femoral artery endothelium is dependent on P-selectin and PSGL-1. Eur. J. Pharmacol. 592, 128–132. ( 10.1016/j.ejphar.2008.06.102) [DOI] [PubMed] [Google Scholar]
- 118.Gremmel T, Koppensteiner R, Kaider A, Eichelberger B, Mannhalter C, Panzer S. 2015. Impact of variables of the P-selectin - P-selectin glycoprotein ligand-1 axis on leukocyte-platelet interactions in cardiovascular disease. Thromb. Haemost. 113, 806–812. ( 10.1160/TH14-08-0690) [DOI] [PubMed] [Google Scholar]
- 119.Xu T, Zhang L, Geng ZH, Wang H-B, Wang J-T, Chen M, Geng J-G. 2007. P-selectin cross-links PSGL-1 and enhances neutrophil adhesion to fibrinogen and ICAM-1 in a Src kinase-dependent, but GPCR-independent mechanism. Cell Adh. Migr. 1, 115–123. ( 10.4161/cam.1.3.4984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Delaney MK, Liu J, Kim K, Shen B, Stojanovic-Terpo A, Zheng Y, Cho J, Du X. 2014. Agonist-induced platelet procoagulant activity requires shear and a Rac1-dependent signaling mechanism. Blood 124, 1957–1967. ( 10.1182/blood-2014-03-560821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fahim A, Crooks MG, Morice AH, Hart SP. 2014. Increased platelet binding to circulating monocytes in idiopathic pulmonary fibrosis. Lung 192, 277–284. ( 10.1007/s00408-013-9546-5) [DOI] [PubMed] [Google Scholar]
- 122.Raker V, Haub J, Stojanovic A, Cerwenka A, Schuppan D, Steinbrink K. 2017. Early inflammatory players in cutaneous fibrosis. J. Dermatol. Sci. 87, 228–235. [DOI] [PubMed] [Google Scholar]
- 123.Jia LX, Qi GM, Liu O, Li TT, Yang M, Cui W, Zhang W-M, Qi Y-F, Du J. 2013. Inhibition of platelet activation by clopidogrel prevents hypertension-induced cardiac inflammation and fibrosis. Cardiovasc. Drugs Ther. 27, 521–530. ( 10.1007/s10557-013-6471-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dees C, et al. 2011. Platelet-derived serotonin links vascular disease and tissue fibrosis. J. Exp. Med. 208, 961–972. ( 10.1084/jem.20101629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cooley BC, et al. 2014. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Trans Med. 6, 227ra34–227ra34 ( 10.1126/scitranslmed.3006927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weiskirchen R, Weiskirchen S, Tacke F. 2019. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol. Aspects Med. 65, 2–15. ( 10.1016/j.mam.2018.06.003) [DOI] [PubMed] [Google Scholar]
- 127.Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. 2018. Update on neutrophil function in severe inflammation. Front. Immunol. 9, 2171 ( 10.3389/fimmu.2018.02171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Padmanabhan J, Gonzalez AL. 2012. The effects of extracellular matrix proteins on neutrophil-endothelial interaction: a roadway to multiple therapeutic opportunities. Yale J. Biol. Med. 85, 167–185. [PMC free article] [PubMed] [Google Scholar]
- 129.Hoenderdos K, et al. 2016. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 71, 1030–1038. ( 10.1136/thoraxjnl-2015-207604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morales-Ortíz J, et al. 2018. Platelet-derived TLT-1 is a prognostic indicator in ALI/ARDS and prevents tissue damage in the lungs in a mouse model. Blood 132, 2495–2505. ( 10.1182/blood-2018-03-841593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen C-M, Lu H-C, Tung Y-T, Chen W. 2020. Antiplatelet therapy for acute respiratory distress syndrome. Biomedicines 8, 230 ( 10.3390/biomedicines8070230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li H, et al. 2017. Neutrophil extracellular traps are pathogenic in ventilator-induced lung injury and partially dependent on TLR4. BioMed Res. Int. 2017, 8272504 ( 10.1155/2017/8272504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Curley GF, Laffey JG, Zhang H, Slutsky AS. 2016. Biotrauma and ventilator-induced lung injury: clinical implications. Chest 150, 1109–1117. ( 10.1016/j.chest.2016.07.019) [DOI] [PubMed] [Google Scholar]
- 134.Bacha NC, et al. 2018. Endothelial microparticles are associated to pathogenesis of idiopathic pulmonary fibrosis. Stem Cell Rev. Rep. 14, 223–235. ( 10.1007/s12015-017-9778-5) [DOI] [PubMed] [Google Scholar]
- 135.Blandinières A, et al. 2019. Interleukin-8 release by endothelial colony-forming cells isolated from idiopathic pulmonary fibrosis patients might contribute to their pathogenicity. Angiogenesis 22, 325–339. ( 10.1007/s10456-018-09659-5) [DOI] [PubMed] [Google Scholar]
- 136.Chan TR, Stahl PJ, Yu SM. 2011. Matrix-bound VEGF mimetic peptides: design and endothelial cell activation in collagen scaffolds. Adv. Funct. Mater. 21, 4252–4262. ( 10.1002/adfm.201101163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Swieringa F, et al. 2016. Platelet control of fibrin distribution and microelasticity in thrombus formation under flow. Arterioscler. Thromb. Vasc. Biol. 36, 692–699. ( 10.1161/ATVBAHA.115.306537) [DOI] [PubMed] [Google Scholar]
- 138.Tanguay J-F, Hammoud T, Geoffroy P, Merhi Y. 2003. Chronic platelet and neutrophil adhesion: a causal role for neointimal hyperplasia in in-stent restenosis. J. Endovasc. Therapy 10, 968–977. ( 10.1177/152660280301000521) [DOI] [PubMed] [Google Scholar]
- 139.Varga Z, et al. 2020. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 395, 1417–1418. ( 10.1016/S0140-6736(20)30937-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smeda M, Chlopicki S. 2020. Endothelial barrier integrity in COVID-19-dependent hyperinflammation: does the protective facet of platelet function matter? Cardiovasc. Res. 116, e118–ee21. ( 10.1093/cvr/cvaa190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lippi G, Plebani M, Henry BM. 2020. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 506, 145–148. ( 10.1016/j.cca.2020.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yadav H, Kor DJ. 2015. Platelets in the pathogenesis of acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L915–L923. ( 10.1152/ajplung.00266.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yiming MT, Lederer DJ, Sun L, Huertas A, Issekutz AC, Bhattacharya S. 2008. Platelets enhance endothelial adhesiveness in high tidal volume ventilation. Am. J. Respir. Cell Mol. Biol. 39, 569–575. ( 10.1165/rcmb.2007-0332OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sexton TR, Wallace EL, Macaulay TE, Charnigo RJ, Evangelista V, Campbell CL, Bailey AL, Smyth SS. 2015. The effect of rosuvastatin on thromboinflammation in the setting of acute coronary syndrome. J. Thromb. Thromb. 39, 186–195. ( 10.1007/s11239-014-1142-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sexton TR, Zhang G, Macaulay TE, Callahan LA, Charnigo R, Vsevolozhskaya OA, Li Z, Smyth S. 2018. Ticagrelor reduces thromboinflammatory markers in patients with pneumonia. JACC Basic Transl. Sci. 3, 435–449. ( 10.1016/j.jacbts.2018.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kubisa MJ, Jezewski MP, Gasecka A, Siller-Matula JM, Postuła M. 2018. Ticagrelor - toward more efficient platelet inhibition and beyond. Ther. Clin. Risk Manag. 14, 129–140. ( 10.2147/TCRM.S152369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koushki K, et al. 2020. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin. Rev. All. Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Birnbaum Y, Tran D, Chen H, Nylander S, Sampaio L, Ye Y. 2019. Ticagrelor improves remodeling, reduces apoptosis, inflammation and fibrosis and increases the number of progenitor stem cells after myocardial infarction in a rat model of ischemia reperfusion. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 53, 961–981. ( 10.33594/000000189) [DOI] [PubMed] [Google Scholar]
- 149.Yang Q, He GW, Underwood MJ, Yu CM. 2016. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: perspectives and implications for postischemic myocardial protection. Am. J. Transl. Res. 8, 765–777. [PMC free article] [PubMed] [Google Scholar]
- 150.Wolfson RK, Chiang ET, Garcia JGN. 2011. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc. Res. 81, 189–197. ( 10.1016/j.mvr.2010.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J, Wu A, Wu Y. 2017. Endothelial glycocalyx layer: a possible therapeutic target for acute lung injury during lung resection. Biomed. Res. Int. 2017, 5969657 ( 10.1155/2017/5969657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tarbell JM, Cancel LM. 2016. The glycocalyx and its significance in human medicine. J. Intern. Med. 280, 97–113. ( 10.1111/joim.12465) [DOI] [PubMed] [Google Scholar]
- 153.Cao RN, Tang L, Xia ZY, Xia R. 2019. Endothelial glycocalyx as a potential theriapeutic target in organ injuries. Chin. Med. J. (Engl.) 132, 963–975. ( 10.1097/CM9.0000000000000177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kazuma S, Tokinaga Y, Kimizuka M, Azumaguchi R, Hamada K, Yamakage M. 2019. Sevoflurane promotes regeneration of the endothelial glycocalyx by upregulating sialyltransferase. J. Surg. Res. 241, 40–47. ( 10.1016/j.jss.2019.03.018) [DOI] [PubMed] [Google Scholar]
- 155.Casanova J, Simon C, Vara E, Sanchez G, Rancan L, Abubakra S, Calvo A, Gonzalez FJ, Garutti I. 2016. Sevoflurane anesthetic preconditioning protects the lung endothelial glycocalyx from ischemia reperfusion injury in an experimental lung autotransplant model. J Anesth. 30, 755–762. ( 10.1007/s00540-016-2195-0) [DOI] [PubMed] [Google Scholar]
- 156.Becker BF, Chen C, Chappell D, Annecke T, Conzen P, Jacob M, Welsch U, Zwissler B. 2016. Sevoflurane mitigates shedding of hyaluronan from the coronary endothelium, also during ischemia/reperfusion: an ex vivo animal study. Hypoxia (Auckl.) 4, 81–90. ( 10.2147/HP.S98660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nemme J, Krizhanovskii C, Ntika S, Sabelnikovs O, Vanags I, Hahn RG. 2020. Hypervolemia does not cause degradation of the endothelial glycocalyx layer during open hysterectomy performed under sevoflurane or propofol anesthesia. Acta Anaesthesiol. Scand. 64, 538–545. ( 10.1111/aas.13511) [DOI] [PubMed] [Google Scholar]
- 158.Kim HJ, Kim E, Baek SH, Kim HY, Kim JY, Park J, Choi E-J. 2018. Sevoflurane did not show better protective effect on endothelial glycocalyx layer compared to propofol during lung resection surgery with one lung ventilation. J. Thorac. Dis. 10, 1468–1475. ( 10.21037/jtd.2018.03.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. 1998. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J. Clin. Invest. 101, 877–889. ( 10.1172/JCI1509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alquwaizani M, Buckley L, Adams C, Fanikos J. 2013. Anticoagulants: a review of the pharmacology, dosing, and complications. Curr. Emerg. Hosp. Med. Rep. 1, 83–97. ( 10.1007/s40138-013-0014-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schmidt EP, et al. 2012. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223. ( 10.1038/nm.2843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Massena S, Christoffersson G, Hjertström E, Zcharia E, Vlodavsky I, Ausmees N, Rolny C, Li J-P, Phillipson M. 2010. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood 116, 1924–1931. ( 10.1182/blood-2010-01-266072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Riffo-Vasquez Y, Somani A, Man F, Amison R, Pitchford S, Page CP. 2016. A non-anticoagulant fraction of heparin inhibits leukocyte diapedesis into the lung by an effect on platelets. Am. J. Res. Cell Mol. Biol. 55, 554–563. ( 10.1165/rcmb.2015-0172OC) [DOI] [PubMed] [Google Scholar]
- 164.Wodicka J, Chambers A, Sangha G, Goergen C, Panitch A. 2017. Development of a glycosaminoglycan derived, selectin targeting anti-adhesive coating to treat endothelial cell dysfunction. Pharmaceuticals 10, 36 ( 10.3390/ph10020036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. 2015. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv. Pharmacol. Sci. 2015, 507151 ( 10.1155/2015/507151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nutescu EA, Burnett A, Fanikos J, Spinler S, Wittkowsky A. 2016. Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J. Thromb. Thromb. 41, 15–31. ( 10.1007/s11239-015-1314-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aláez-Versón CR, Lantero E, Fernàndez-Busquets X. 2017. Heparin: new life for an old drug. Nanomedicine 12, 1727–1744. ( 10.2217/nnm-2017-0127) [DOI] [PubMed] [Google Scholar]
- 168.Vaidyanathan D, Williams A, Dordick JS, Koffas MAG, Linhardt RJ. 2017. Engineered heparins as new anticoagulant drugs. Bioeng. Transl. Med. 2, 17–30. ( 10.1002/btm2.10042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oduah EI, Linhardt RJ, Sharfstein ST. 2016. Heparin: past, present, and future. Pharmaceuticals (Basel, Switzerland) 9, 38 ( 10.3390/ph9030038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim SY, et al. 2020. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 181, 104873 ( 10.1016/j.antiviral.2020.104873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van Haren FMP, et al. 2020. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Critical Care 24, 454 ( 10.1186/s13054-020-03148-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Buijsers B, Yanginlar C, Maciej-Hulme ML, de Mast Q, van der Vlag J. 2020. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine 59, 102969 ( 10.1016/j.ebiom.2020.102969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang L, Zeng M, Fan J, Tarbell JM, Curry F-RE, Fu BM. 2016. Sphingosine-1-phosphate maintains normal vascular permeability by preserving endothelial surface glycocalyx in intact microvessels. Microcirculation (New York, NY: 1994) 23, 301–310. ( 10.1111/micc.12278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Park S-J, Im D-S. 2017. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther. (Seoul) 25, 80–90. ( 10.4062/biomolther.2016.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. 2017. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmunity Rev. 16, 495–503. ( 10.1016/j.autrev.2017.03.007) [DOI] [PubMed] [Google Scholar]
- 176.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. 2010. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Resp. Cell Mol. Biol. 43, 662–673. ( 10.1165/rcmb.2009-0345OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lauver DA, Lucchesi BR. 2006. Sulodexide: a renewed interest in this glycosaminoglycan. Cardiovasc. Drug Rev. 24, 214–226. ( 10.1111/j.1527-3466.2006.00214.x) [DOI] [PubMed] [Google Scholar]
- 178.Bignamini AA, Matuska J. 2020. Sulodexide for the symptoms and signs of chronic venous disease: a systematic review and meta-analysis. Adv. Ther. 37, 1013–1033. ( 10.1007/s12325-020-01232-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Condorelli M, et al. 1994. IPO-V2: A prospective, multicenter, randomized, comparative clinical investigation of the effects of sulodexide in preventing cardiovascular accidents in the first year after acute myocardial infarction. J. Am. Col. Cardiol. 23, 27–34. ( 10.1016/0735-1097(94)90498-7) [DOI] [PubMed] [Google Scholar]
- 180.Broekhuizen LN, et al. 2010. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 53, 2646–2655. ( 10.1007/s00125-010-1910-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Coccheri S, Mannello F. 2013. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des. Devel. Ther. 8, 49–65. ( 10.2147/DDDT.S6762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vanderwall AG, Milligan ED. 2019. Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front. Immunol. 10, 3009 ( 10.3389/fimmu.2019.03009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Borthwick LA, Wynn TA, Fisher AJ. 2013. Cytokine mediated tissue fibrosis. Biochimica et Biophysica Acta. 1832, 1049–1060. ( 10.1016/j.bbadis.2012.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rider P, Carmi Y, Cohen I. 2016. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int. J. Cell Biol. 2016, 9259646 ( 10.1155/2016/9259646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haraoui B, Bykerk V. 2007. Etanercept in the treatment of rheumatoid arthritis. Ther. Clin. Risk Manag. 3, 99–105. ( 10.2147/tcrm.2007.3.1.99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Campanati A, Giuliodori K, Ganzetti G, Liberati G, Offidani AM. 2010. A patient with psoriasis and vitiligo treated with etanercept. Am. J. Clin. Dermatol. 11(Suppl 1), 46–48. ( 10.2165/1153424-S0-000000000-00000) [DOI] [PubMed] [Google Scholar]
- 187.Raghu G, et al. 2008. Treatment of idiopathic pulmonary fibrosis with etanercept. Am. J. Resp. Critical Care Med. 178, 948–955. ( 10.1164/rccm.200709-1446OC) [DOI] [PubMed] [Google Scholar]
- 188.Anker SD, Coats AJS. 2002. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int. J. Cardiol. 86, 123–130. ( 10.1016/S0167-5273(02)00470-9) [DOI] [PubMed] [Google Scholar]
- 189.Nguyen T, Wu JJ. 2014. Relationship between tumor necrosis factor-α inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 18, 49–54. ( 10.7812/TPP/13-092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. 2020. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 126, 1260–1280. ( 10.1161/CIRCRESAHA.120.315937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Szekely Y, Arbel Y. 2018. A review of interleukin-1 in heart disease: where do we stand today? Cardiol. Ther. 7, 25–44. ( 10.1007/s40119-018-0104-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Barnes TC, Anderson ME, Moots RJ. 2011. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011, 721608 ( 10.1155/2011/721608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.U.S. National Library of Medicine. 2020. Anti-il6 treatment of serious COVID-19 disease with threatening respiratory failure. See https://ClinicalTrials.gov/show/NCT04322773. [DOI] [PubMed]
- 194.Wright HL, Cross AL, Edwards SW, Moots RJ. 2014. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology 53, 1321–1331. ( 10.1093/rheumatology/keu035) [DOI] [PubMed] [Google Scholar]
- 195.Chan JM, et al. 2010. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc. Natl Acad. Sci. USA 107, 2213–2218. ( 10.1073/pnas.0914585107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Moyer TJ, Kassam HA, Bahnson ESM, Morgan CE, Tantakitti F, Chew TL, Kibbe MR, Stupp SI. 2015. Shape-dependent targeting of injured blood vessels by peptide amphiphile supramolecular nanostructures. Small 11, 2750–2755. ( 10.1002/smll.201403429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.So MM, Mansukhani NA, Peters EB, Albaghdadi MS, Wang Z, Rubert Pérez CM, Kibbe MR, Stupp SI. 2018. Peptide amphiphile nanostructures for targeting of atherosclerotic plaque and drug delivery. Adv. Biosyst. 2, 1700123 ( 10.1002/adbi.201700123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nigam A, Kopecky SL. 2002. Therapeutic potential of monoclonal antibodies in myocardial reperfusion injury. Am. J. Cardiovasc. Drugs 2, 367–376. ( 10.2165/00129784-200202060-00002) [DOI] [PubMed] [Google Scholar]
- 199.Weyrich AS, Ma XY, Lefer DJ, Albertine KH, Lefer AM. 1993. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J. Clin. Invest. 91, 2620–2629. ( 10.1172/JCI116501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schmitt C, Abt M, Ciorciaro C, Kling D, Jamois C, Schick E, Benghozi R, Gaudreault J. 2015. First-in-man study with inclacumab, a human monoclonal antibody against P-selectin. J. Cardiovasc. Pharmacol. 65, 611–619. ( 10.1097/FJC.0000000000000233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Riley TR, Riley TT. 2019. Profile of crizanlizumab and its potential in the prevention of pain crises in sickle cell disease: evidence to date. J. Blood Med. 10, 307–311. ( 10.2147/JBM.S191423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Niwa R, Satoh M. 2015. The current status and prospects of antibody engineering for therapeutic use: focus on glycoengineering technology. J. Pharm. Sci. 104, 930–941. ( 10.1002/jps.24316) [DOI] [PubMed] [Google Scholar]
- 203.Tardif JC, et al. 2013. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J. Am. Coll. Cardiol. 61, 2048–2055. ( 10.1016/j.jacc.2013.03.003) [DOI] [PubMed] [Google Scholar]
- 204.Chames P, Van Regenmortel M, Weiss E, Baty D. 2009. Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 157, 220–233. ( 10.1111/j.1476-5381.2009.00190.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.U.S. National Library of Medicine. 2014. Efficacy and safety of Rivipansel (GMI-1070) in the treatment of vaso-occlusive crisis in hospitalized subjects with sickle cell disease. See https://ClinicalTrials.gov/show/NCT02187003. [DOI] [PubMed]
- 206.U.S. National Library of Medicine. 2016. Study to assess safety, tolerability, and efficacy of GMI-1271 in patients with calf-level deep venous thrombosis (DVT). See https://ClinicalTrials.gov/show/NCT02744833. [DOI] [PubMed]
- 207.Langlois B, et al. 2017. Vimentin knockout results in increased expression of sub-endothelial basement membrane components and carotid stiffness in mice. Sci. Rep. 7, 11628 ( 10.1038/s41598-017-12024-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Danielsson F, Peterson MK, Caldeira Araújo H, Lautenschläger F, Gad AKB. 2018. Vimentin diversity in health and disease. Cells. 7, 147 ( 10.3390/cells7100147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moog KE, et al. 2017. Polymeric selectin ligands mimicking complex carbohydrates: from selectin binders to modifiers of macrophage migration. Angewandte Chemie International Edition 56, 1416–1421. ( 10.1002/anie.201610395) [DOI] [PubMed] [Google Scholar]
- 210.Li B, Juenet M, Aid-Launais R, Maire M, Ollivier V, Letourneur D, Chauvierre C. 2017. Development of polymer microcapsules functionalized with fucoidan to target P-selectin overexpressed in cardiovascular diseases. Adv. Healthc Mater. 6, 1601200 ( 10.1002/adhm.201601200) [DOI] [PubMed] [Google Scholar]