Abstract
目的
线粒体DNA 8344 A>G(m.8344A>G)是肌阵挛性癫痫伴破碎红纤维(mitochondrial myoclonus epilepsy with ragged-red fibers,MERRF)综合征的常见致病突变,报道1例罕见的由m.8344A>G突变引起的线粒体脑肌病伴乳酸酸中毒和卒中样发作/肌阵挛性癫痫伴破碎红纤维/Leigh(mitochondrial encephalopathy, lactic acidosis and stroke-like episodes /MERRF/Leigh,MELAS/MERRF/Leigh)重叠综合征。
方法
随访观察1例线粒体病患者,应用聚合酶链反应-限制性片段长度多态性(polymerase chain reaction-restriction fragment length polymorphism,PCR-RFLP)、高通量测序及Sanger测序对其线粒体基因进行分析。
结果
患者男性,25岁,6岁后出现运动耐力差;10岁时因偏侧视野缺损行头颅磁共振成像(magnetic resonance imaging,MRI)示枕叶卒中样病灶,磁共振波谱分析(magnetic resonance spectroscopy,MRS)示病灶内乳酸峰升高;后患者认知功能逐渐减退;12岁出现行走不稳、意向性震颤,以及四肢肌阵挛发作;21岁因意识障碍入院,多次行头颅MRI示双侧壳核后部、丘脑及中脑对称性异常信号且范围逐渐扩大,并出现额叶多发卒中样病灶,经改善代谢、抗癫痫和物理康复治疗后症状逐渐好转;24岁时复查MRI示双侧基底节及丘脑异常信号范围缩小,中脑病灶消退。肌肉病理活检可见破碎红纤维(ragged-red fibers,RRF)。PCR-PFLP检测患者血液DNA发现存在m.8344A>G突变,二代测序显示其突变比例为90%,且未发现其他线粒体DNA位点致病突变。患者母亲的血液亦存在低比例m.8344A>G突变。
结论
报道了m.8344A>G可导致MELAS/MERRF/Leigh重叠综合征,该例患者扩展了m.8344A>G的表型谱。
Keywords: mtDNA 8344 A>G, 线粒体重叠综合征, 线粒体脑肌病伴乳酸酸中毒和卒中样发作, 肌阵挛性癫痫伴破碎红纤维, Leigh综合征
Abstract
Objective
Mitochondrial deoxyribonucleic acid (mtDNA) 8344 A>G (m.8344A>G) mutation is the common mutation associated with mitochondrial myoclonus epilepsy with ragged-red fibers (MERRF) syndrome. Herein we report a rare case with mitochondrial encephalopathy, lactic acidosis and stroke-like episodes/MERRF/Leigh (MELAS/MERRF/Leigh) overlap syndrome caused by m.8344A>G mutation.
Methods
The clinical and imaging data of the patient were collected and an open muscle biopsy was carried out. We further employed molecular genetic analyses to detect mtDNA mutation in the proband and his mother. And then a clinical and neuroimaging follow-up was performed.
Results
This patient was a 25-year-old male, who developed exercise intolerance since the age of 6. At age 10, he suffered from acute episodes of hemianopia, and cranial magnetic resonance imaging (MRI) showed occipital stroke-like lesions and cranial magnetic resonance spectroscopy (MRS) revealed a lactate peak corresponding to the lesion. After that the patient presented slowly progressive psychomotor decline. He had myoclonic seizures and cerebellar ataxia since the age of 12. At age 21, he was admitted to our hospital because of confusion and cranial MRI revealed symmetrical lesions in bilateral posterior putamen, thalami and midbrain. Then repeated MRI showed progression of original lesions and new frontal multiple stroke-like lesions. Symptomatic and rehabilitation treatment relieved his condition. Follow-up cranial MRI at age 24 showed the lesions in basal ganglia and thalami diminished, and the midbrain lesions even completely vanished. Muscle pathology indicated the presence of numerous scattered ragged-red fibers (RRF), suggestive of a mitochondrial disorder. Polymerase chain reaction-restricted fragment length polymorphism (PCR-RFLP) detected the m.8344A>G mutation of the MT-TK gene encoding mitochondrial transfer RNA for lysine in the patient's blood. Next generation sequencing (NGS) of the whole mitochondrial genome identified that the proportion of m.8344A>G was 90%, and no other mtDNA mutation was detected. Sanger sequencing further identified this mutation both in the proband and his mother's blood, although the mutation load was much lower in his mother's blood with approximately 10% heteroplasmy.
Conclusion
The present study is the first to describe a patient with m.8344A>G mutation in association with the MELAS/MERRF/Leigh overlap syndrome, which expands the phenotypic spectrum of the m.8344A>G mutation.
Keywords: MtDNA 8344 A>G; Mitochondrial overlap syndrome; Mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS); myoclonus epilepsy with ragged-red fibers (MERRF); Leigh syndrome
线粒体疾病是因线粒体基因或核基因组中编码线粒体呼吸链相关蛋白的基因突变所致的一组疾病。根据不同临床表现的组合,分为不同的临床综合征[1],其中线粒体脑肌病伴乳酸酸中毒和卒中样发作(mitochondrial encephalopathy, lactic acidosis and stroke-like episodes,MELAS)、肌阵挛性癫痫伴破碎红纤维(clonus epilepsy with ragged-red fibers,MERRF)、Leigh综合征(Leigh syndrome,LS)、Leber遗传性视神经病(Leber’s hereditary optic neuropathy,LHON)、慢性进行性眼外肌瘫痪(chronic progressive external ophthalmoplegia,CPEO)和Kearns-Sayre综合征(Kearns-Sayre syndrome,KSS)等较为常见。MELAS综合征核心症状是以累及大脑皮层为主的卒中样发作,以及痴呆、癫痫、乳酸酸中毒、肌病、头痛、听力下降、糖尿病和身材矮小等,80%的患者为编码线粒体转运RNA(transfer RNA,tRNA)Leu的m.3243A>G突变[2]。MERRF综合征以肌阵挛、全面性癫痫、小脑性共济失调为核心症状,超过80%的患者由编码线粒体tRNALys的m.8344A>G突变所致[3]。Leigh综合征,又称亚急性坏死性脑病,常表现为精神运动发育迟缓或倒退,癫痫发作,以及眼外肌瘫痪、眼球震颤、肌张力障碍或呼吸异常等脑干或基底节受累的症状,影像学以双侧对称的脑干和(或)基底节T2序列局限性高信号为特点[4],分子遗传学研究发现20%的Leigh患者为线粒体DNA突变,80%为核基因突变所致[3]。文献报道部分患者可以表现为两种线粒体综合征的叠加,如MELAS/MERRF[5-6]、MELAS/Leigh[7-8]、MERRF/Leigh[9-10]、MELAS/KSS[11]、MERRF/KSS[12]等两种疾病类型的重叠,但线粒体疾病三者叠加综合征尚少见报道。本文报道1例罕见的m.8344A>G突变引起的MELAS/MERRF/Leigh共3种疾病的重叠综合征。
1. 资料与方法
1.1. 临床资料
随访观察1例2013年12月就诊于北京大学第一医院神经内科通过临床病理检查诊断的线粒体病MELAS/MERRF/Leigh重叠综合征患者。收集患者临床资料,影像学检查随访颅内病灶改变。患者及家属均签署知情同意书。
1.2. 基因突变分析
1.2.1. PCR-RFLP及限制性内切酶酶切反应
提取患者及其母亲的外周血白细胞DNA,应用聚合酶链反应-限制性片段长度多态性(polymerase chain reaction-restriction fragment length polymorphism,PCR-RFLP)对mtDNA常见突变(3243A>G,8344A>G,8993T>C)进行检测。m.8344A>G的正向引物为5′-TACCCTATAGCACCCCCTCT-3′,反向引物为5′-TAGTATTTAGTTGGGGCATTTCACTGTAAAGCCG-TGTTGG-3′。PCR反应体系总体积为25 μL,含DNA模板1.0 μL,引物各0.5 μL,Tag酶12.5 μL和蒸馏水10.5 μL。PCR反应条件为94 ℃预变性5 min,94 ℃变性30 s,58 ℃退火30 s,72 ℃延伸30 s,共35个循环,最后一个循环后于72 ℃延伸7 min,4 ℃冷却。反应结束后取PCR产物10 μL,加入限制性内切酶BgⅡ 0.5 μL,10×酶切缓冲液2 μL,蒸馏水7.5 μL,在37 ℃水浴酶切2 h。最后以2%(质量分数)琼脂糖凝胶电泳,在凝胶成像仪上检测酶切产物的电泳条带。
1.2.2. 高通量测序及数据分析
为排除mtDNA其他位点突变,对患者mtDNA进行二代测序分析。应用mtDNA探针捕获技术,mtDNA经富集后在Illumina HiSeq X-ten平台上进行高通量测序。使用BWA(版本0.7.15, http://bio-bwa.sourceforge.net)将线粒体基因组测序序列与MITOMAP数据库(https://www.mitomap.org/MITOMAP)中的参考序列(NC_012920.1.)进行比对, 平均测序深度为10 000×,超过95.0%的目标区域测序深度大于1 000×。使用VarScan(2.4.0)检测线粒体基因组单核苷酸变异及小片段缺失插入变异,LUMPY(0.2.13)检测大片段结构变异。
1.2.3. Sanger测序
对发现的可疑致病性变异进行Sanger测序验证(患者及其母亲外周血DNA)。
2. 结果
2.1. 临床表现
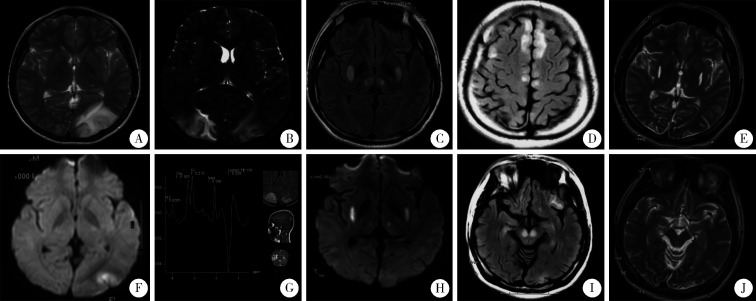
患者男性,25岁,出生后生长发育正常,但6岁后运动耐力差。10岁时突发视物不清, 右侧偏盲,头颅磁共振成像(magnetic resonance imaging, MRI,图 1)示左侧枕叶皮层长T1及长T2信号,DWI高信号(图 1A、1B),头颅磁共振血管成像(magnetic resonance angiography, MRA)未见异常。20 d后又出现左侧偏盲,头颅MRI示双侧枕叶皮层长T1及长T2信号,磁共振波谱成像(magnetic resonance spectroscopy,MRS)示病灶内乳酸峰(lactate peak)升高及N-乙酰天门冬氨酸(N-acetyl aspartate,NAA)峰降低(图 1C、1D)。给予对症治疗后视野缺损逐渐好转,复查头颅MRI异常信号消失。外院行肱二头肌肌肉活检可见破碎红纤维(ragged-red fiber,RRF),诊断为MELAS综合征。此后患者逐渐出现智能倒退。12岁时逐渐出现行走不稳,写字时手抖,四肢时有阵发性快速抽动,予左乙拉西坦抗癫痫治疗,抽动症状逐渐控制。17岁时患者因行走不稳摔倒致左上肢骨折并行骨折固定术。18岁于全身麻醉下行拆除骨折内固定术,术后患者行走不稳及肢体抖动等症状加重。21岁时患者出现反应迟钝,记忆力减退,答非所问,阵发性烦躁,入北京大学第一医院治疗。父母非近亲结婚,家族中无遗传病史,母亲无糖尿病、听力减退及类似疾病史。
图1.
患者头颅MRI表现
Serial cranial MRI of the patient
At age 10 (A to D), left occipital cortex lesion with high signal on T2 and DWI imaging was shown (A, B); repeated MRI 20 days later the first MRI revealed a new lesion in the right occipital cortex with high-T2 signal (C), and magnetic resonance spectroscopy (MRS) showed lactate peak at the same time (D). At age 21(E to H), high-T2 and high-DWI signals were seen in bilateral putamen and thalami (E, F); bilateral new abnormal signals were in frontal cortex and cerebral peduncles when the condition worsened one month later (G, H). At age 24 (I, J), follow-up MRI showed regression of the bilateral putamen and thalami lesions, as well as complete disappearance of bilateral cerebral peduncles lesions (I, J).
入院体格检查:皮肤散在痤疮,呼吸节律正常。精神淡漠,反应迟钝,问话不答,高级皮层功能检查不配合。双侧瞳孔等大、等圆,对光反射灵敏,双眼球各向活动充分,无眼震。双侧额纹、眼裂及鼻唇沟对称。四肢肌力Ⅳ级,肌张力偏低,双手平举及站立时可见四肢不自主震颤,四肢时有阵发性快速抽动,双侧指鼻试验、跟膝胫试验欠稳准,轮替动作笨拙。双上肢腱反射对称减弱,双下肢腱反射消失,双下肢病理征(+)。
2.2. 诊疗经过
自身免疫抗体、铜蓝蛋白、尿代谢筛查未见异常。心电图、超声心动未见异常。血乳酸3.9 mmol/L,丙酮酸116 μmol/L,肌酸激酶164 IU/L。糖耐量试验正常,无低血糖发作。头颅MRI示双侧壳核后部及双侧丘脑对称性长T1长T2信号,DWI高信号(图 1E、1F)。脑电图示δ波为主,双额、右枕、前颞少量不典型三相波单个发放。给予辅酶Q10、左卡尼汀、精氨酸、三磷酸腺苷二钠及多种维生素改善线粒体功能,左乙拉西坦抗癫痫,醋酸泼尼松短期使用。患者精神状态一度逐渐好转,可以与人交流,言语清晰流利,反应尚灵敏。但入院后半月余患者再次出现精神欠佳、反应迟钝、主动语言减少,癫痫发作频率增加。复查头颅MRI示双侧额叶及双侧大脑脚新发长T1及长T2信号,DWI高信号(图 1G、1H),双侧壳核后部及丘脑异常信号范围较前扩大。继续给予改善线粒体能量代谢、清除自由基的药物,加用氯硝西泮控制癫痫,醋酸泼尼松加量,以及营养支持、物理康复等对症治疗。患者病情再次好转,可进行简单交流,可在家人搀扶下站立。听力检查未见异常。
出院后随访,患者生活基本能自理。24岁复查头颅MRI示双侧基底节及丘脑病灶范围较前缩小,大脑皮层及大脑脚病灶消退(图 1I、1J)。
2.3. 基因突变结果
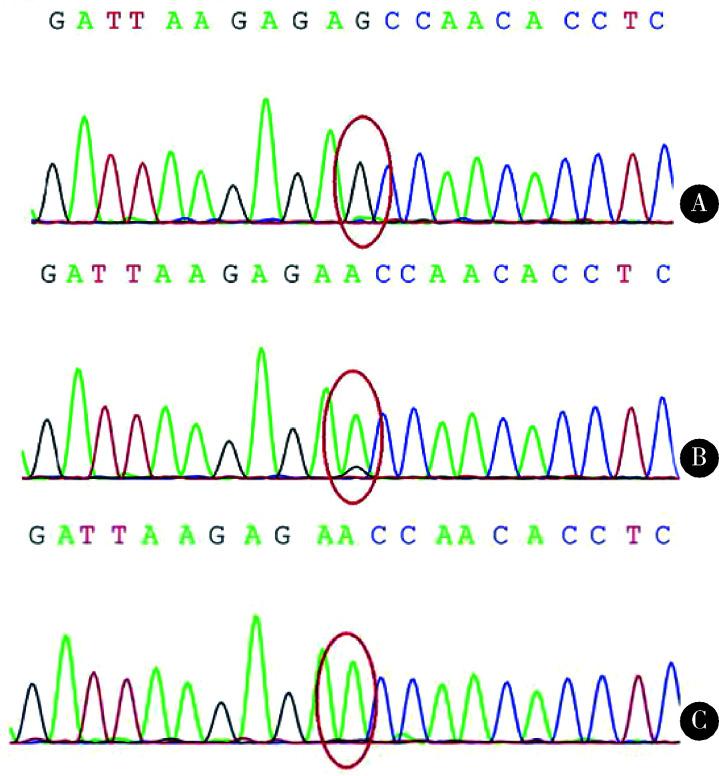
PCR-RFLP示患者血液mtDNA存在m.8344A>G突变。二代测序对患者血液mtDNA全长进行扩增,同样发现m.8344A>G突变,依据测序深度计算突变比例为90%,且未发现其它线粒体位点的突变。Sanger测序验证了m.8344位点A>G的突变。家系验证其母亲血液mtDNA存在相同位点突变,突变比例约为10%(图 2)。
图2.
Sanger测序图示患者(A)及其母亲(B)血液中均可检测到m.8344A>G突变, 患者的突变比例明显高于其母;正常对照组(C)未见m.8344A>G突变
Sanger sequencing revealed m.8344A>G mutation both in the patient (A) and his mother's blood (B), although mutation load was much higher in the patient. And no m.8344A>G mutation was detected in healthy control (C)
3. 讨论
本例患者自幼有运动不耐受,在10岁时出现急性发生的偏盲,头颅MRI显示枕叶皮层病灶且病灶乳酸峰升高,肌肉病理可见破碎红纤维,符合MELAS的诊断;随病情发展,12岁出现肌阵挛性癫痫以及小脑性共济失调,提示出现MERRF的表现;而21岁时又相继出现意识障碍、双侧锥体束征,头颅MRI显示对称性脑干及基底节病变,支持Leigh综合征的临床和影像学特点。患者血液DNA中检测到90%的m.8344A>G突变,进一步肯定了线粒体病的诊断,临床诊断为MELAS/MERRF/Leigh重叠综合征。同时该患者排除了一氧化碳中毒、低血糖、肝豆状核变性、Wernicke脑病和甲基丙二酸血症等其他中毒或代谢性疾病的可能[13]。魏妍平等[14]报道过1例线粒体NADH脱氢酶3(mitochondrially encoded NADH dehydrogenase 3,MT-ND3)基因m.10158T>C突变导致的MELAS/MERRF/Leigh重叠综合征,并与本文病例有相似的临床表现,影像学表现为双侧中脑和四叠体对称性异常信号。
对本例患者的长期随访发现其双侧基底节异常信号范围逐渐缩小,脑干病灶消失。对于Leigh综合征的影像学随访研究,Sofou等[15]发现5/12个病例的病灶减小或完全消退。在Lee等[16]的报道中,6/14个病例的病灶进展,没有病灶完全消退。Bonfante等[4]对13个病例进行随访,3个患者的实质病灶完全消退,7个病灶进展,包括病灶范围的扩大或新病灶的出现,其中有3个最初影像学表现正常的患者,逐渐出现异常病灶。提示Leigh综合征的影像学改变也存在异质性,病灶可随时间进展而发生变化,因其病灶可好转甚至可逆,强调了积极治疗防止其脑损伤的必要性;而早期正常的影像学表现并不能完全排除Leigh综合征,如果临床高度怀疑,需进行密切随访。
有学者报道,线粒体重叠综合征可能是由共存的两种线粒体基因突变所导致,如tRNA基因的m.8356T>C和m.3243A>G双位点致病突变引起的MERRF/MELAS重叠综合征[17];m.3243A>G点突变及mtDNA单一大片段缺失导致的MELAS/KSS重叠综合征[11]。虽然本例患者表现为线粒体脑肌病多个亚型的重叠综合征,但进一步对患者mtDNA进行全长二代测序,未发现m.8344A>G以外的致病突变,因此可以认为m. 8344 A>G导致了该患者的MELAS/MERRF/Leigh重叠综合征。
线粒体DNA为双螺旋的环状分子,含16 569个碱基[18]。线粒体m.8295-m.8364的MT-TK(mitochondrial encoded tRNA lysine)基因编码赖氨酸tRNA。因此,m.8344A>G突变可干扰线粒体呼吸链的组装,从而影响呼吸链酶复合体的功能[19]。临床上,m.8344A>G突变最常引起MERRF综合征,但也可表现出伴或不伴脂肪瘤的肌病[20],肢体远端无力伴呼吸功能不全[21],脊髓小脑变性[22],儿童或成人起病的Leigh综合征[23-24]、MERRF/Leigh重叠综合征[9-10]和中枢神经及周围神经系统的急性脱髓鞘[25]等。本课题组发现该突变导致了线粒体脑肌病MELAS/MERRF/Leigh重叠综合征,进一步扩展了m.8344A>G的临床表型。
本例患者血液中有高达90%的m.8344 A>G的突变比例,尽管患者母亲无临床症状,但对其血液进行Sanger测序,也发现了低比例的相同位点的致病突变[26]。不同于二倍体且遵循孟德尔(Mendelian)遗传规律的核基因突变,线粒体基因突变完全为母系遗传。线粒体DNA多拷贝数的特性导致了其基因突变的异质性,即野生型和突变型mtDNA共存,不同组织可有不同的mtDNA突变水平。只有当突变型mtDNA达到一定比例才引起细胞代谢功能障碍,即阈值效应。各组织对氧化代谢的需求不同,中枢神经系统、心脏、骨骼肌等对能量需求较高,因此在线粒体疾病中受累明显[27]。
综上所述,m.8344A>G可导致MELAS/MERRF/Leigh重叠综合征。
References
- 1.Alston CL, Rocha MC, Lax NZ, et al. The genetics and pathology of mitochondrial disease. J Pathol. 2017;241(2):236–250. doi: 10.1002/path.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Hattab AW, Adesina AM, Jones J, et al. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015;116(1/2):4–12. doi: 10.1016/j.ymgme.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Lake NJ, Compton AG, Rahman S, et al. Leigh syndrome: one disorder, more than 75 monogenic causes. Ann Neurol. 2016;79(2):190–203. doi: 10.1002/ana.24551. [DOI] [PubMed] [Google Scholar]
- 4.Bonfante E, Koenig MK, Adejumo RB, et al. The neuroimaging of Leigh syndrome: case series and review of the literature. Pediatr Radiol. 2016;46(4):443–451. doi: 10.1007/s00247-015-3523-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Zhao H, Ji K, et al. MERRF/MELAS overlap syndrome due to the m.3291T>C mutation. Metab Brain Dis. 2014;29(1):139–144. doi: 10.1007/s11011-013-9464-5. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzoni PJ, Scala RH, Kay CSK, et al. When should MERRF (myoclonus epilepsy associated with ragged-red fibers) be the diagnosis. Arq Neuropsiquiatr. 2014;72(10):803–811. doi: 10.1590/0004-282X20140124. [DOI] [PubMed] [Google Scholar]
- 7.陈 涓涓, 陈 旭辉, 陈 淮菁, et al. m.10158T>C致线粒体脑肌病伴高乳酸血症和卒中样发作叠加Leigh综合征1例临床、病理及基因特点. 中华神经科杂志. 2017;50(6):435–439. [Google Scholar]
- 8.韩 漫夫, 白 润涛, 冯 宏业, et al. 线粒体DNA G13513A突变所致线粒体脑肌病伴高乳酸血症和脑卒中样发作/Leigh重叠综合征. 中华神经科杂志. 2009;42(4):248–252. [Google Scholar]
- 9.Yang H, Lu Q, Cui L. Symmetric thalamic lesions in a patient with a myoclonic epilepsy with ragged red fibers-Leigh spectrum phenotype due to the m.A8344G mutation. Pediatr Neurol. 2014;51(6):e19–20. doi: 10.1016/j.pediatrneurol.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Monden Y, Mori M, Kuwajima M, et al. Late-onset Leigh syndrome with myoclonic epilepsy with ragged-red fibers. Brain Dev. 2013;35(6):582–585. doi: 10.1016/j.braindev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Yu N, Zhang YF, Zhang K, et al. MELAS and Kearns-Sayre overlap syndrome due to the mtDNA m. A3243G mutation and large-scale mtDNA deletions. eNeurologicalSci. 2016;4:15–18. doi: 10.1016/j.ensci.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmanuele V, Silvers DS, Sotiriou E, et al. MERRF and Kearns-Sayre overlap syndrome due to the mitochondrial DNA m.3291T>C mutation. Muscle Nerve. 2011;44(3):448–451. doi: 10.1002/mus.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuccoli G, Yannes MP, Nardone R, et al. Bilateral symmetrical basal ganglia and thalamic lesions in children: an update (2015) Neuroradiology. 2015;57(10):973–989. doi: 10.1007/s00234-015-1568-7. [DOI] [PubMed] [Google Scholar]
- 14.魏 妍平, 崔 丽英, 彭 斌. ND3基因突变导致的线粒体脑肌病重叠综合征二例. 脑与神经疾病杂志. 2016;24(10):626–630. [Google Scholar]
- 15.Sofou K, Steneryd K, Wiklund LM, et al. MRI of the brain in childhood-onset mitochondrial disorders with central nervous system involvement. Mitochondrion. 2013;13(4):364–371. doi: 10.1016/j.mito.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee HF, Tsai CR, Chi CS, et al. Leigh syndrome: clinical and neuroimaging follow-up. Pediatr Neurol. 2009;40(2):88–93. doi: 10.1016/j.pediatrneurol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Yabe I, Sudo A, et al. MERRF/MELAS overlap syndrome: a double pathogenic mutation in mitochondrial tRNA genes. J Med Genet. 2010;47(10):659–664. doi: 10.1136/jmg.2009.072058. [DOI] [PubMed] [Google Scholar]
- 18.Craven L, Alston CL, Taylor RW, et al. Recent advances in mitochondrial disease. Annu Rev Genomics Hum Genet. 2017;18:257–275. doi: 10.1146/annurev-genom-091416-035426. [DOI] [PubMed] [Google Scholar]
- 19.Altmann J, Buchner B, Nadaj-Pakleza A, et al. Expanded phenotypic spectrum of the m.8344A>G "MERRF" mutation: data from the German mitoNET registry. J Neurol. 2016;263(5):961–972. doi: 10.1007/s00415-016-8086-3. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Malaga A, Bautista J, Salazar JA, et al. Lipomatosis, proximal myopathy, and the mitochondrial 8344 mutation. A lipid storage myopathy. Muscle Nerve. 2000;23(4):538–542. doi: 10.1002/(SICI)1097-4598(200004)23:4<538::AID-MUS12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Blakely EL, Alston CL, Lecky B, et al. Distal weakness with respiratory insufficiency caused by the m.8344A > G "MERRF" mutation. Neuromuscul Disord. 2014;24(6):533–536. doi: 10.1016/j.nmd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell N, Kubacka I, Smith R, et al. Association of the mitochondrial 8344 MERRF mutation with maternally inherited spinocerebellar degeneration and Leigh disease. Neurology. 1996;46(1):219–222. doi: 10.1212/wnl.46.1.219. [DOI] [PubMed] [Google Scholar]
- 23.Han JY, Sung JJ, Park HK, et al. Adult onset Leigh syndrome with mitochondrial DNA 8344 A > G mutation. J Clin Neurosci. 2014;21(11):2009–2011. doi: 10.1016/j.jocn.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Tsao CY, Herman G, Boue DR, et al. Leigh disease with mitochondrial DNA A8344G mutation: case report and brief review. J Child Neurol. 2003;18(1):62–64. doi: 10.1177/08830738030180011401. [DOI] [PubMed] [Google Scholar]
- 25.Erol I, Alehan F, Horvath R, et al. Demyelinating disease of central and peripheral nervous systems associated with a A8344G mutation in tRNALys. Neuromuscul Disord. 2009;19(4):275–278. doi: 10.1016/j.nmd.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed ST, Craven L, Russell OM, et al. Diagnosis and treatment of mitochondrial myopathies. Neurotherapeutics. 2018;15(4):943–953. doi: 10.1007/s13311-018-00674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molnar MJ, Kovacs GG. Mitochondrial diseases. Handb Clin Neurol. 2017;145:147–155. doi: 10.1016/B978-0-12-802395-2.00010-9. [DOI] [PubMed] [Google Scholar]