Abstract
目的
通过测定血清Semaphorin 3A(Sema3A)的水平,分析Sema3A与系统性红斑狼疮(systemic lupus erythematosus,SLE)血小板减少的相关性。
方法
应用酶联免疫吸附法检测170例SLE患者、50例干燥综合征(Sjögren’s syndrome,SS)患者、19例脾功能亢进(hypersplenism,HS)患者及150例健康对照(healthy controls,HC)血清中Sema3A的水平,收集患者及健康对照的临床资料及实验室检查,实验室检查主要为患者的血常规及骨髓穿刺活检结果。根据是否合并血小板减少及血小板减少是否缓解,将SLE患者分为SLE合并血小板减少组(41例)、SLE合并血小板减少缓解组(28例)及SLE未合并血小板减少组(101例)。根据是否合并血小板减少,将SS患者分为SS合并血小板减少组(18例)及SS未合并血小板减少组(32例)。将28例进行骨髓穿刺活检的SLE患者,根据骨髓象结果从骨髓增生情况方面将其分为骨髓增生正常组(19例)和增生减低组(9例),从巨核细胞成熟方面将其分为有巨核细胞成熟障碍组(8)和无巨核细胞成熟障碍组(20例)。比较各组Sema3A水平差异,并分析各组患者血清Sema3A水平与血小板的相关性及不同骨髓象间血清Sema3A的水平。
结果
(1) SLE患者血清Sema3A水平较HC显著降低[(3.84±2.76) μg /L vs. (6.96±2.62) μg/L,P < 0.001],SS患者血清Sema3A水平亦较HC显著降低[(4.35±3.57) μg/L vs. (6.96±2.62) μg/L,P < 0.001],HS患者血清Sema3A水平较HC也明显降低[(5.67±2.26) μg/L vs. (6.96±2.62) μg/L,P=0.041]。(2)SLE患者血清Sema3A水平较SS患者降低,但差异无统计学意义[(3.84±2.76) μg/L vs. (4.35±3.57) μg/L,P=0.282],SLE患者血清Sema3A水平与HS患者相比显著降低,差异有统计学意义[(3.84±2.76) μg/L vs. (5.67±2.26) μg/L,P=0.006]。(3)SLE合并血小板减少组Sema3A水平显著低于SLE合并血小板减少缓解组[(1.28±1.06) μg/L vs. (3.83±2.65) μg/L,P < 0.001]和SLE未合并血小板减少组[(1.28±1.06) μg/L vs. (4.87±2.60) μg/L,P < 0.001],而SLE合并血小板减少缓解组与SLE未合并血小板减少组相比,差异无统计学意义[(3.83±2.65) μg/L vs. (4.87±2.60) μg/L,P=0.123]。SLE合并血小板减少组患者Sema3A水平与SS合并血小板减少组患者相比降低,但差异无统计学意义[(1.28±1.06) μg/L vs. (1.68±1.11) μg/L,P=0.189]。(4)相关性分析显示SLE患者的Sema3A水平与血小板显著相关(r=0.600,P < 0.001),SS患者的Sema3A水平与血小板亦呈明显的正相关(r=0.573,P < 0.001),但HS患者与Sema3A却没有表现出明显的相关性(P=0.393)。(5)血清Sema3A在骨髓增生正常和减低的SLE患者,及有和无巨核细胞成熟障碍的SLE患者中的水平差异无统计学意义(P>0.05)。
结论
血清Sema3A在多种合并血小板减少的疾病中水平普遍下降,SLE患者血清Sema3A水平显著降低,并与血小板呈明显正相关,在SS患者中亦可得出类似结论,提示Sema3A与结缔组织病的血小板减少相关。
Keywords: Semaphorin 3A; 红斑狼疮, 系统性; 血小板减少
Abstract
Objective
To measure the level of serum Semaphorin 3A (Sema3A) and to analyze the relationship between serum Sema3A and systemic lupus erythematosus (SLE) with thrombocytopenia.
Methods
The concentration of serum Sema3A was detected by enzyme-linked immuno sorbent assay (ELISA) in 170 SLE patients, 50 Sjögren's syndrome (SS) patients, 19 hypersplenism (HS) patients and 150 healthy controls (HC). Based on the presence of thrombocytopenia and whether the thrombocytopenia was in remission, the SLE patients were divided into three groups: SLE with thrombocytopenia (41 cases), SLE with thrombocytopenia remission (28 cases), and SLE without thrombocytopenia (101 cases). According to whether there was thrombocytopenia, the SS patients were divided into SS with thrombocytopenia (18 cases) and SS without thrombocytopenia (32 cases). The 28 SLE patients who underwent bone marrow aspiration biopsy were divided into two groups from the aspect of whether the bone marrow hyperplasia was normal (19 cases) or low (9 cases), as well as from the aspect of whether the maturity disturbance of megakaryocyte was positive (8 cases) or negative (20 cases). The serum Sema3A levels in SLE, SS, HS with HC were compared, meanwhile, the correlation between serum Sema3A level and platelet (PLT) in the patients with different diseases analyzed.
Results
(1) Serum Sema3A levels in SLE were significantly lower than in HC [(3.84±2.76) μg/L vs. (6.96±2.62) μg/L, P < 0.001], serum Sema3A levels in SS were also obviously lower than in HC [(4.35±3.57) μg/L vs. (6.96±2.62) μg/L, P < 0.001], and in HS it was lower than HC at a certain extant [(5.67±2.26) μg/L vs. (6.96±2.62) μg/L, P=0.041]. (2) Serum Sema3A levels in SLE were slightly lower than in SS, but there was no significant difference [(3.84±2.76) μg/L vs. (4.35±3.57) μg/L, P=0.282]. However, when compared with HS, serum Sema3A levels in SLE were significantly lower [(3.84±2.76) μg/L vs. (5.67±2.26) μg/L, P=0.006]. (3) Serum Sema3A concentration in SLE with thrombocytopenia was significantly lower than in SLE with thrombocytopenia remission [(1.28±1.06) μg/L vs. (3.83±2.65) μg/L, P < 0.001], and in SLE patients without thrombocytopenia [(1.28±1.06) μg/L vs. (4.87±2.60) μg/L, P < 0.001]. There was no significant difference between SLE with thrombocytopenia remission and SLE without thrombocytopenia [(3.83±2.65) μg/L vs. (4.87±2.600 μg/L, P=0.123]. Serum Sema3A concentration in SLE with thrombocytopenia was slightly lower than in SS with thrombocytopenia, but there was no significant difference [(1.28±1.06) μg/L vs. (1.68±1.11) μg/L, P=0.189]. (4) Strong positive correlations were found between serum Sema3A and PLT in SLE (r=0.600, P < 0.001). Positive correlations were also found between serum Sema3A and PLT in SS (r=0.573, P < 0.001). However, there was no such correlation showed in HS patients (P=0.393). (5) There was no significant difference of serum Sema3A concentration in SLE whether the bone marrow hyperplasia was normal or low. And the same situation appeared in the patients whether the maturity disturbance of megakaryocyte was positive or negative (P>0.05).
Conclusion
Serum Sema3A was significantly reduced in SLE patients, and it was highly correlated with the blood damage. Similar conclusions could be drawn in patients with SS. The serum level of Sema3A was generally decreasing in desmosis which merged thrombocytopenia, and was obviously positive correlated with platelet counts.
Keywords: Semaphorin 3A; Lupus erythematosus, systemic; Thrombocytopenia
Semaphorin 3A(Sema3A)又称Collapsin,它是脊椎动物类Semaphorins家族成员中最先被确定的神经轴突导向因子。近年来,有多项研究显示Sema3A参与了机体抑制自身免疫反应的负调控机制,其可能抑制树突状细胞[1]、巨噬细胞[2]、T细胞[3-4]等免疫细胞的功能而在自身免疫性疾病的发病中发挥重要的调控作用。在一项针对32例系统性红斑狼疮(systemic lupus erythematosus,SLE)患者的研究显示,SLE患者血清Sema3A水平较健康对照及类风湿关节炎(rheumatoid arthritis,RA)患者均显著降低,且与疾病活动、肾脏损害和抗心磷脂抗体呈负相关[5]。血小板减少是SLE血液系统损害中最常见的表现[6],有研究显示Sema3A可以抑制血小板的聚集、活化及黏附,提示内皮细胞来源的Sema3A具有维持循环中血小板静息状态的功能,能抑制损伤后血栓的生成[7]。本研究的初期结果显示SLE患者血清Sema3A水平较健康对照显著降低,且在SLE合并血小板减少的患者中这种缺陷更为明显[8]。本研究通过测定Sema3A在系统性红斑狼疮,以及合并血小板减少的疾病中的血清Sema3A水平,为进一步研究Sema3A在血小板减少中的作用机制提供研究基础。
1. 资料与方法
1.1. 研究对象与资料采集
本研究纳入SLE患者170例,均为2011年6月至2013年10月就诊于北京大学人民医院风湿免疫科的门诊及住院患者,所有入选SLE患者均符合1997年美国风湿病学会(American College of Rheumatology,ACR)修订的SLE分类标准。疾病对照为同期于北京大学人民医院风湿免疫科、血液科及肝病科门诊及住院部就诊的50例干燥综合征(Sjögren’s syndrome,SS)及19例除外免疫因素导致的脾功能亢进(hypersplenis, HS)患者,所有入组病例均符合相应的疾病诊断标准,150例健康对照(healthy controls,HC)来自健康体检者。收集患者及健康对照的一般资料(性别、年龄)及实验室检查,实验室指标主要为血常规及骨髓穿刺活检结果。根据是否合并血小板减少及血小板减少是否缓解,将SLE患者分为SLE合并血小板减少组(41例)、SLE合并血小板减少缓解组(28例)及SLE未合并血小板减少组(101例)。根据是否合并血小板减少,将SS患者分为SS合并血小板减少组(18例)及SS未合并血小板减少组(32例)。根据骨髓象结果,将进行了骨髓穿刺活检的28例SLE患者进行分类:根据骨髓增生的情况将其分为骨髓增生正常组(19例)和增生减低组(9例);根据巨核细胞成熟的情况分为有巨核细胞成熟障碍组(8例)和无巨核细胞成熟障碍组(20例)。本研究经北京大学人民医院医学伦理委员会批准(批准号:2015-40),所有研究对象均签署知情同意书。
1.2. 血清Sema3A水平的检测
清晨空腹采集患者和健康对照静脉全血2 mL×2管,4 ℃放置自然凝固后3 000 r/min离心5 min,分离血清,分装后置于-20 ℃低温冰箱保存,应用酶联免疫吸附测定法试剂盒(购自中国武汉Cusabio公司)同批检测血清Sema3A水平,具体操作按说明书进行。
1.3. 统计学分析
所有数据均采用SPSS 13.0统计软件分析。对于正态分布的计量资料用均数±标准差表示,计量资料的组间比较采用t检验或方差分析,多组间的比较用单因素方差法(One-way ANOVA)分析,用Bonferroni方法进行组间比较;对于非正态分布的计量资料采用中位数(四分位间距)表示,两组间比较采用Mann-Whitney U检验,3组以上比较用Kruskal-Wallis检验;计数资料采用Pearson’s χ2检验。两变量间采用Pearson或Spearman法进行相关性分析,P < 0.05为差异有统计学意义。
2. 结果
2.1. 各组研究对象的一般资料及血清Sema3A水平
表 1所示为SLE患者、疾病对照SS患者、HS患者及HC的一般资料及血清Sema3A的水平。SLE患者的血清Sema3A水平较HC显著下降(P < 0.001),SS患者的血清Sema3A水平也显著低于HC(P < 0.001),HS患者的血清Sema3A水平较HC亦明显降低(P=0.041)。在SLE患者与SS及HS患者的比较当中,SLE患者血清Sema3A水平较SS患者降低,但差异无统计学意义(P=0.282),而SLE患者血清Sema3A水平与HS患者相比显著降低,差异具有统计学意义(P=0.006)。
表1.
各组研究对象的一般资料及血清Sema3A水平
General characteristics and serum Sema3A concentration of each group
| Groups | n | Age/years | Male/Female, n | PLT/(×109/L) | Sema3A/(μg/L) | P* |
| Sema3A, Semaphorin 3A; SLE, systemic lupus erythematosus; SS, Sjögren’s syndrome; HS, hypersplenism; HC, healthy controls; PLT, platelet. * the serum Sema3A concentration of each disease group compared with HC. | ||||||
| SLE | 170 | 34.49±14.53 | 22/148 | 163.88±88.61 | 3.84±2.76 | <0.001 |
| SS | 50 | 51.63±13.13 | 6/44 | 125.84±74.90 | 4.35±3.57 | <0.001 |
| HS | 19 | 57.58±11.16 | 11/8 | 51.0(11.0-116.0) | 5.67±2.26 | 0.041 |
| HC | 150 | 42.05±12.82 | 31/119 | 6.96±2.62 | ||
2.2. 3组SLE患者血清Sema3A水平比较
根据是否合并血小板减少及血小板减少是否缓解将SLE患者分为3组,3组血清Sema3A水平差异有统计学意义(F=34.143,P < 0.001),且3组血清Sema3A水平均显著低于HC(P < 0.001)。3组之间比较两两比较,SLE合并血小板减少组[(1.28±1.06) μg/L]明显低于SLE合并血小板减少缓解组[(3.83±2.65) μg/L]和SLE未合并血小板减少组[(4.87±2.60) μg/L],差异具有统计学意义(P < 0.001),而SLE合并血小板减少缓解组与SLE未合并血小板减少组相比,差异无统计学意义(P=0.123)。
2.3. 合并血小板减少的SLE及SS患者血清Sema3A水平比较
SLE合并血小板减少组血清Sema3A水平[(1.28±1.06) μg/L]与SS合并血小板减少组患者的血清Sema3A水平[(1.68±1.11) μg/L]相比降低,但差异无统计学意义(P=0.189)。
2.4. SLE、SS和HS患者血清Sema3A水平与血小板的相关性分析
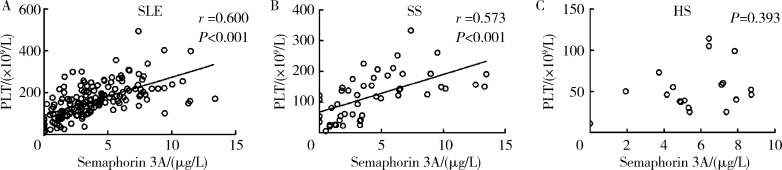
对SLE、SS和HS患者血清Sema3A的水平与血小板进行相关性分析, 结果显示SLE患者的血清Sema3A水平均与血小板呈显著的正相关关系(r=0.600,P < 0.001),SS患者的Sema3A水平与血小板亦呈明显的正相关(r=0.573,P < 0.001),但HS患者的Sema3A表达与血小板则不相关(P=0.393),如图 1。
图1.
SLE、SS和HS患者血清Semaphorin 3A水平与血小板计数的相关性分析
Correlation between serum Semaphorin 3A levels and platelet count in patients with SLE, SS and HS
A, relationship between serum Semaphorin 3A and PLT in SLE; B, relationship between serum Semaphorin 3A and PLT in SS; C, relationship between serum Semaphorin 3A and PLT in HS. SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; HS, hypersplenism; PLT, platelet.
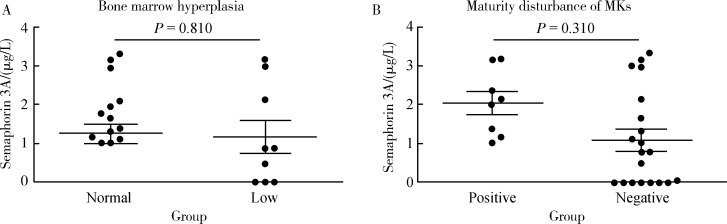
2.5. 不同骨髓象间血清Sema3A的水平
将SLE患者分成骨髓增生正常组和增生减低组,两组之间Sema3A的水平差异无统计学意义(P=0.81);将SLE患者分为有巨核细胞成熟障碍组和无巨核细胞成熟障碍组,两组差异也无统计学意义(P=0.31),见图 2。
图2.
不同骨髓象间血清Semaphorin 3A的水平
Serum Semaphorin 3A levels in different bone marrow
A, level of serum Semaphorin 3A in bone marrow hyperplasia normal group and low group; B, level of serum Semaphorin 3A in maturity disturbance of megakaryocyte positive group and negative group. MKs, megakaryocytes.
3. 讨论
Sema3A是Semaphorins家族中最经典的外分泌蛋白,已有证据表明Sema3A在多种自身免疫病中表达异常,并参与了多种自身免疫性疾病的发生发展,如多发性硬化[9-10]、类风湿关节炎[11]、SLE[5]等。既往研究发现Sema3A对T淋巴细胞的增殖活化具有明显抑制作用, 活化的树突状细胞及T淋巴细胞可分泌Sema3A,Sema3A与其受体Nrp-1的相互作用可通过胞内信号转导,抑制细胞骨架的重排,进而抑制抗CD3/CD28介导的T淋巴细胞的增殖活化及炎症细胞因子的分泌[3]。一项对32例SLE患者的研究显示,SLE患者血清Sema3A水平较健康对照及RA患者均显著降低,且与疾病活动、肾脏损害和抗心磷脂抗体呈负相关[5]。
我们的初期研究结果显示, Sema3A在SLE患者血清Sema3A水平显著低于健康对照,且与SLE患者血小板水平相关[8]。基于以上结果,本课题组进一步深入研究发现,无论是否合并血小板减少,Sema3A在SLE患者外周血中的表达均异常减少,合并血小板减少的SLE患者Sema3A下降幅度更大,提示无论SLE是否合并血液系统损害,外周血Sema3A水平均可出现显著降低,而合并血小板减少后加重了Sema3A的表达缺陷。SLE、SS患者血清Sema3A水平较健康对照显著降低,合并血小板减少的非免疫因素导致的HS患者血清Sema3A水平较健康对照亦有一定程度的降低。但在SLE与SS患者的比较当中,我们发现,无论两组患者是否合并血小板减少,SLE患者的血清Sema3A水平虽较SS患者有所降低,但差异无统计学意义。此外,相关性分析结果显示SLE和SS患者的Sema3A水平与血小板计数显著相关,而HS患者与Sema3A却未表现出相关性。综上,我们可以看出Sema3A降低与SLE合并血小板减少相关,但在SS患者中亦可得出类似结论,提示Sema3A与结缔组织病的血小板减少相关。
血小板减少的原因包括生成障碍、分布异常和破坏增多。HS引起的血小板减少主要是由于血小板在脾内被过分阻滞及过分筛选和吞噬,从而导致血液中血小板分布异常,因此,HS患者循环中的血小板整体水平可能是正常的。这可能是HS患者的Sema3A与血小板没有相关性的原因。在对SLE患者的骨髓象进行分析中,未发现Sema3A的水平与骨髓增生异常及巨核细胞成熟障碍具有相关性,但由于可分析的样本量较小,尚不能得出完全无关的结论,在后续的研究中可进一步扩大样本量进行探讨。
自身免疫性疾病引起的血小板减少的原因主要是自身抗体介导的血小板破坏。本课题前期研究结果发现SLE患者Sema3A阳性组抗核抗体阳性率和抗心磷脂抗体阳性率均显著高于阴性组[8],提示Sema3A的下降可能与自身抗体的产生相关,这可能是Sema3A与血小板减少相关的可能原因之一。近年来,关于特发性血小板减少性紫癜的免疫学研究逐渐从体液免疫扩展到了细胞免疫领域,主要包括T淋巴细胞的克隆性增生[12]、T淋巴细胞的凋亡异常[13]、Th1/Th2比例失衡[14]、调节性T细胞数目减少和/或功能缺陷[15]和细胞毒T淋巴细胞介导的血小板破坏[16]等。Sema3A已被证实具有多重免疫调节作用,而Sema3A是否可通过这些机制对血小板数目进行调节还不得而知。
此外,血小板的信使核糖核酸水平和蛋白水平都已证实存在Sema3A受体Nrp-1和PlexinA1~A3的表达[17]。Kashiwagi等[7]研究了Sema3A对血小板功能的影响,结果发现Sema3A重组蛋白可有效抑制二磷酸腺苷、U46619、抗酒石酸酸性磷酸酶(tartrate-resistant factor acid phosphatase,TRAP)和convulxin诱导的αⅡbβ3活化,并且TRAP诱导血小板扩散至纤维蛋白原的过程在Sema3A存在的情况下也被抑制。除此之外,Sema3A还可减弱TRAP导致的Rac1活化、cofilin磷酸化和纤维状肌动蛋白的生成,提示Sema3A是通过作用于细胞骨架来调节血小板功能的。Kashiwagi等[7]认为内皮细胞源性的Sema3A具有维持循环中血小板保持静息状态的功能,并具有抑制损伤后血栓生成的作用。关于Sema3A在血小板中的作用机制还有很多未知的地方值得我们去探索,如Sema3A是否通过抑制血小板生成从而减少血小板的数量?亦或是Sema3A参与了血小板的破坏过程?血小板是否是外周血Sema3A的主要来源?这些问题都还有待进一步的研究。
既往研究证实了Sema3A与SLE病情活动有关,且对SLE具有一定的诊断价值。本课题组研究报道了血清Sema3A水平与结缔组织病血小板减少的显著相关性,并从多个方面进行了证实,为Sema3A的功能定位提供了新的方向,因此,在未来的研究中,可针对Sema3A在合并血小板减少的免疫性疾病中的免疫调控及血液系统损伤的作用机制进行深入研究,探讨该分子作为特异性诊断和创新性免疫干预的潜能。
Funding Statement
国家自然科学基金(81501396)
Supported by the National Natural Science Foundation of China (81501396)
References
- 1.Sarris M, Andersen KG, Randow F, et al. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28(3):402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji JD, Park-Min KH, Ivashkiv LB. Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages. Hum Immunol. 2009;70(4):211–217. doi: 10.1016/j.humimm.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lepelletier Y, Moura IC, Hadj-Slimane R, et al. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36(7):1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 4.Catalano A, Caprari P, Moretti S, et al. Semaphorin 3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107(8):3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- 5.Vadasz Z, Haj T, Halasz K, et al. Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Res Ther. 2012;14(3):R146. doi: 10.1186/ar3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturrock RD. Hematologic disorders in rheumatic disease. Curr Opin Rheumatol. 1991;3(1):172. doi: 10.1097/00002281-199102000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwagi H, Shiraga M, Kato H, et al. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106(3):913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- 8.高 辉, 马 晓旭, 郭 倩, et al. Sema3A在系统性红斑狼疮患者血清及单个核细胞中的表达. 中华医学杂志. 2017;97(5):370–374. [Google Scholar]
- 9.Okuno T, Nakatsuji Y, Kumanogoh A. The role of immune semaphorins in multiple sclerosis. FEBS Lett. 2011;585(23):3829–3835. doi: 10.1016/j.febslet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Eixarch H, Gutierrez-Franco A, Montalban X, et al. Semaphorins 3A and 7A: potential immune and neuroregenerative targets in multiple sclerosis. Trends Mol Med. 2013;19(3):157–164. doi: 10.1016/j.molmed.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Takagawa S, Nakamura F, Kumagai K, et al. Decreased semaphorin3A expression correlates with disease activity and histological features of rheumatoid arthritis. BMC Musculoskelet Disord. 2013;14:40. doi: 10.1186/1471-2474-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwana M, Kawakami Y, Ikeda Y. Suppression of autoreactive T-cell response to glycoprotein Ⅱb/Ⅲa by blockade of CD40/CD154 interaction: implications for treatment of immune thrombocytopenic purpura. Blood. 2003;101(2):621–623. doi: 10.1182/blood-2002-07-2157. [DOI] [PubMed] [Google Scholar]
- 13.Shenoy S, Mohanakumar T, Chatila T, et al. Defective apoptosis in lymphocytes and the role of IL-2 in autoimmune hematologic cytopenias. Clin Immunol. 2001;99(2):266–275. doi: 10.1006/clim.2001.5017. [DOI] [PubMed] [Google Scholar]
- 14.Panitsas FP, Theodoropoulou M, Kouraklis A, et al. Adult chro-nic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103(7):2645–2647. doi: 10.1182/blood-2003-07-2268. [DOI] [PubMed] [Google Scholar]
- 15.Ling Y, Cao X, Yu Z, et al. Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79(4):310–316. doi: 10.1111/j.1600-0609.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 16.Olsson B, Andersson PO, Jernas M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9(9):1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 17.Wannemacher KM, Wang L, Zhu L, et al. The role of sema-phorins and their receptors in platelets: Lessons learned from neuronal and immune synapses. Platelets. 2011;22(6):461–465. doi: 10.3109/09537104.2011.561891. [DOI] [PMC free article] [PubMed] [Google Scholar]