Abstract
目的
探索非综合征型唇腭裂(non-syndromic cleft lip with or without cleft palate,NSCL/P)这一类常见出生缺陷的可能致病机制,在Hedgehog(HH)通路基因中(PTCH1、PTCH2、SHH、SMO)探索基因多态性对NSCL/P的关联关系以及亲源效应(parent-of-origin effects,PoO)对NSCL/P发病风险的影响。
方法
纳入806个中国非综合征型唇腭裂核心家系,对HH通路基因(PTCH1、PTCH2、SHH、SMO)的83个单核苷酸多态性位点(single nucleotide polymorphisms,SNPs)进行传递不平衡检验(transmission disequilibrium test,TDT),并采用对数线性模型进行亲源效应分析。家系样本来自“唇腭裂基因和交互作用的国际合作研究”项目。采用Plink进行TDT检验;通过R软件中的Haplin v6.2.1软件包开展亲源效应分析。采用Bonferroni法进行多重检验校正。
结果
经过质量控制,共纳入65个SNPs进行分析,Bonferroni显著性水平为7.7×10-4(0.05/65)。未校正P值前,关联分析发现rs4448343与NSCL/P存在关联(P=0.023),6个单体型(rs10512249-rs4448343、rs1461208-rs7786445、rs10512249-rs4448343、rs16909865-rs10512249-rs4448343、rs1461208-rs7786445-rs12698335、rs288756-rs288758-rs1151790)与NSCL/P存在关联(P < 0.05);6个单体型(rs288765-rs1233563、rs12537550-rs11765352、rs872723-rs288765-rs1233563、rs288765-rs1233563-rs288756、rs6459952-rs12537550-rs11765352、rs12537550-rs11765352-rs6971211)具有潜在的PoO效应(P < 0.05)。以上结果经过多重检验校正,均无统计学意义(P>7.7×10-4)。
结论
未发现HH通路基因多态性与NSCL/P的关联,未发现HH通路基因通过PoO效应影响NSCL/P发病风险。
Keywords: 亲源效应, 非综合征型唇腭裂, HH通路基因
Abstract
Objective
Non-syndromic cleft lip with or without cleft palate (NSCL/P) is a common birth defect, affecting 1.4 per 1 000 live births, and multiple genetic and environmental risk factors influencing its risk. All the known genetic risk factors accounted for a small proportion of the heritability. Several authors have suggested parent-of-origin effects (PoO) may play an important role in the etiology of this complex and heterogeneous malformation. To clarify the genetic association between PTCH1, PTCH2, SHH and SMO in hedgehog (HH) pathway and NSCL/P, as well as testing for potential PoO effects in Chinese case-parent trios.
Methods
We tested for transmission disequilibrium tests (TDT) and PoO effects using 83 common single nucleotide polymorphic (SNP) markers of HH pathway genes from 806 NSCL/P case-parent trios. These trios were drawn from an international consortium established for a genome-wide association studies (GWAS) of non-syndromic oral clefts of multiple ethnicities. DNA samples were collected from each trio. Single marker and haplotype based analysis were performed both in TDT tests and PoO effects. SNPs were excluded if they (ⅰ) had a call rate of < 95%, (ⅱ) had a minor allele frequency (MAF) of < 0.05, (ⅲ) had Mendelian errors over all trios of >5%, (ⅳ) had a genotype distribution in the parents that deviated from the Hardy-Weinberg equilibrium (HWE) (P < 0.000 1). The process was done using Plink (version 1.07, http://pngu.mgh.harvard.edu/~purcell/plink/data.shtml). TDT test was performed in Plink v1.07. A log-linear model was used to explore PoO effects using Haplin v6.2.1 as implemented in R package v3.4.2. Significance level was assessed using the Bonferroni correction.
Results
A total of 18 SNPs were dropped due to low MAF, thus leaving 65 SNPs available for the analysis. Thus the Bonferroni threshold was 7.7×10-4 (0.05/65). Nominal significant association with NSCL/P was found at a SNP (rs4448343 in PTCH1, P=0.023) and six haplotypes (rs10512249-rs4448343, rs1461208-rs7786445, rs10512249-rs4448343, rs16909865-rs10512249-rs4448343, rs1461208-rs7786445-rs12698335, and rs288756-rs288758-rs1151790, P < 0.05). A total of six haplotypes (rs288765-rs1233563, rs12537550-rs11765352, rs872723-rs288765-rs1233563, rs288765-rs1233563-rs288756, rs6459952-rs12537550-rs11765352, and rs12537550-rs11765352-rs6971211) showed PoO effect (P < 0.05). None of the results remained significant after the Bonferroni correction (P>7.7×10-4).
Conclusion
Neither significant association between SNPs within HH pathway and the risk of NSCL/P nor PoO effects was seen in this study.
Keywords: Parent-of-origin effects, Non-syndromic cleft lip with or without cleft palate, Hedgehog pathway
非综合征型唇腭裂(non-syndromic cleft lip with or without cleft palate,NSCL/P)是由于胚胎发育过程中颌腭组织发育不全或发育受阻而引起的一组颌面部先天性畸形,中国唇腭裂患病率约为每1 000活产儿1.4人[1]。唇腭裂按解剖学结构可分为单纯唇裂(cleft lip, CL)、单纯腭裂(cleft palate, CP)、唇裂合并腭裂(cleft lip and palate, CLP)。由于单纯唇裂和唇裂合并腭裂具有相似的胚胎起源和流行病学特征,因此CL和CLP通常被合并为唇裂合并或不合并腭裂(cleft lip with or without cleft palate, CL/P)一类[2-3]。CL/P和CP通常被认为是两种不同类型的缺陷,因为它们的胚胎起源不同[4];患病率不同,CL/P患病率高于CP,并且CP患者中女性约为男性的两倍,而CL/P患者男性则是女性的两倍[5]。NSCL/P是多个遗传因素和环境因素共同作用的复杂疾病,全基因组关联研究(genome-wide association study, GWAS)是研究复杂疾病致病基因的高效定位方法。2009年Grant等[6]通过GWAS研究发现8q24与NSCL/P相关,由此开始了探索非综合征型唇腭裂遗传危险因素的GWAS研究时代。GWAS研究不仅验证了既往发现的候选基因,还发现了新的易感基因或区域[7],有研究者发现大部分常见变异组合起来仅能增加较小程度的疾病风险,解释了少部分的遗传度[8],并且这些GWAS研究主要探索患儿易感基因对于疾病风险的影响。因此,探索罕见遗传变异的致病效应、遗传因素的交互作用、遗传-环境交互作用、亲源效应、表观遗传等是当前研究热点。亲源效应(parent-of-origin effects,PoO)是指亲本来源染色体上的等位基因出现差异表达,即两个亲本等位基因中一方表达,另一方沉默,表现为依靠单亲传递某些遗传学性状的现象[9]。大部分已知的具有亲源效应的基因与胚胎发育密切相关,而非综合征型唇腭裂是由于胚胎发育过程中颌腭组织发育不全或发育受阻引起的出生缺陷。因此探索PoO与NSCL/P发病的关联,或可进一步揭开NSCL/P的病因。
Hedgehog(HH)通路在胚胎时期的细胞分化、组织发育及器官形成中发挥重要的作用,尤其与颌面部形态形成密切相关。HH通路基因主要包括分泌型糖蛋白配体基因SHH、跨膜蛋白受体基因PTCH1、PTCH2,跨膜蛋白基因SMO及其下游靶基因。当SHH存在时,解除PTCH对SMO的抑制,从而SMO发挥细胞内信号传导和激活下游靶基因的作用。HH通路基因关系见图 1(来源于KEGG PATHWAY Database,http://www.genome.jp/kegg/pathway.html)。Yu等[10]在中国人群进行的GWAS研究发现,PTCH1基因的rs10512248位点与NSCLP密切相关。目前的研究主要涉及HH通路单个基因多态性与NSCL/P的关联,因此本研究旨在806个中国NSCL/P核心家庭中探索HH通路基因是否通过亲源效应影响NSCL/P的发病风险。
图1.
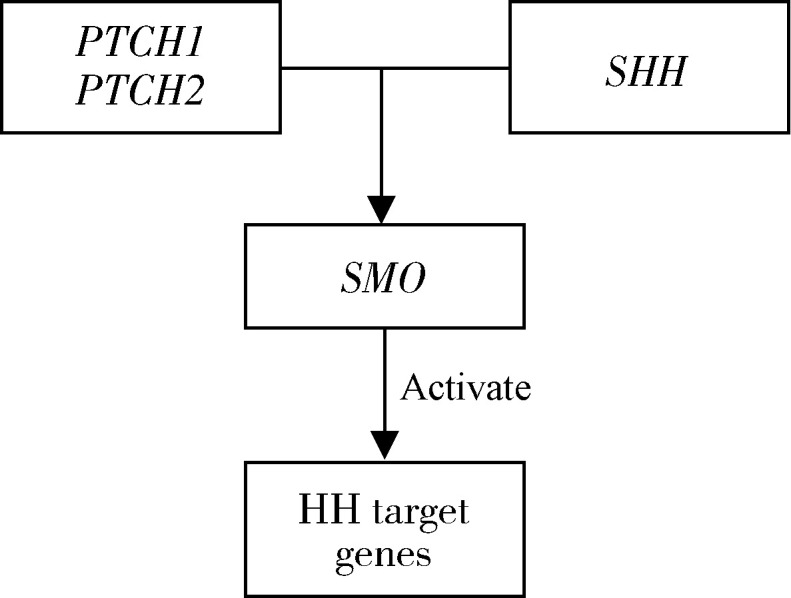
HH通路基因分布情况
The hedgehog pathway genes
1. 资料与方法
1.1. 研究对象
研究对象为806个中国NSCL/P核心家系,通过“唇腭裂基因和交互作用的国际合作研究”项目于2003年至2007年在中国台湾、山东、湖北、四川4个地区募集,合计2 418人。806个家系的地区来源、性别分布见表 1。该项目的研究设计、样本收集、环境暴露信息参见Beaty等[11]的研究,该研究开始前均已获得合作组各单位的伦理委员会批准,患儿唇腭裂病理类型由临床遗传学家或经验丰富的医师确定,确保纳入患儿均为NSCL/P类型,纳入家系的父母均签署知情同意书,通过静脉血、唾液或漱口水等生物标本提取DNA。
表1.
806个中国NSCL/P核心家系分布情况
The distribution of 806 NSCL/P case-parent trios in China
| Site | Male | Female | Total |
| NSCL/P, non-syndromic cleft lip with or without cleft palate. | |||
| Taiwan | 139 | 94 | 233 |
| Shandong | 193 | 81 | 274 |
| Hubei | 132 | 55 | 187 |
| Sichuan | 75 | 37 | 112 |
| Total | 539 | 267 | 806 |
1.2. 基因型测定和数据质量控制
美国约翰霍普金斯大学遗传病研究中心利用Illumina公司Human610-Quad v.1_B芯片完成基因型测定,纳入的位点经过统一的标准进行数据质量控制,排除位点的标准为:(1)基因型缺失率>5%;(2)弱势等位基因频率(minor allele frequency,MAF) < 5%;(3)父母偏离Hardy-Weinberg平衡状态(P < 0.000 1);(4)孟德尔遗传错误率>5%。原始研究纳入4个基因共83个位点,经过数据质量控制,因MAF删除掉18个位点,故研究纳入65个位点。该筛选过程通过Plink(版本号1.07, http://pngu.mgh.harvard.edu/~purcell/plink/data.shtml)软件完成。
1.3. 统计学分析
1.3.1. 关联分析
采用传递不平衡检验(transmission disequilibrium test,TDT)探索基因位点与疾病的关联关系。其无效假设为杂合子父母传递任一等位基因给子代的概率为50%,拒绝无效假设提示该遗传标记位点与潜在致病位点存在连锁并处于连锁不平衡状态。TDT也可用于检测单体型与疾病间的关联。单体型(haplotype)是位于一条染色体特定区域的一组相互关联、倾向于整体遗传给后代的单核苷酸多态性的组合。单体型通过滑窗法构建,分别以2个、3个SNPs作为一个单体型进行TDT检验。采用Plink v1.07进行关联分析。
1.3.2. 亲源效应分析
采用构建对数线性模型的方法分析PoO效应[12],计算PoO效应时,通过最大似然估计方法,比较患儿易感位点来自母亲的相对危险度RRm与来自父亲的相对危险度RRf:
 |
公式1 |
若RRR=1,说明不存在PoO效应[13-14]。Haplin应用EM(expectation maximization)算法,通过最大似然估计确定目标等位基因的父母来源。探索PoO效应采用两种策略:(1)单个位点分析;(2)单体型分析:通过滑窗法构建,分别以2个、3个SNPs作为一个单体型检验PoO效应。研究的显著性水平为7.7×10-4。相关分析采用统计软件R 3.4.2中的Haplin v6.2.1程序包[12]。本研究的统计学分析部分采用Bonferroni法校正多重检验问题,显著性水平设为0.05/65=7.7×10-4。
2. 结果
2.1. 关联分析
纳入65个SNPs进行TDT检验,结果发现rs4448343与NSCL/P的关联有意义(P < 0.05),经过多重检验校正,该关联无统计学意义(P>7.7×10-4)。
进行单体型与NSCL/P的关联研究发现,以2个SNPs为一个单体型,共得到196种单体型,其中3个单体型TDT检验P < 0.05;以3个SNPs为一个单体型,得到单体型269种,其中3个单体型TDT检验P < 0.05。进行Bonferroni校正后,所有单体型与NSCL/P的关联均无统计学意义。表 2列出了P < 0.05的SNP位点和单体型。
表2.
806个中国NSCL/P核心家系传递不平衡检验结果
The selected single SNP and haplotype association results with nominal significance based on TDT in 806 Chinese case-parents trios
| Type | Gene | Haplotype | SNP ID | P value |
| SNP, single nucleotide polymorphism; TDT, transmission disequilibrium test. | ||||
| SNP | PTCH1 | - | rs4448343 | 0.023 |
| Haplotype | PTCH1 | AG | rs10512249-rs4448343 | 0.025 |
| Haplotype | SHH | GC | rs1461208-rs7786445 | 0.026 |
| Haplotype | PTCH1 | GA | rs10512249-rs4448343 | 0.031 |
| Haplotype | PTCH1 | CGA | rs16909865-rs10512249-rs4448343 | 0.036 |
| Haplotype | SHH | GCG | rs1461208-rs7786445-rs12698335 | 0.036 |
| Haplotype | SHH | GGC | rs288756-rs288758-rs1151790 | 0.047 |
2.2. PoO效应
单个SNP位点的PoO效应分析中未发现任何SNP具有潜在的亲源效应。表 3显示了单体型PoO分析结果中RRR的P值小于0.05的单体型。单体型分析中,6个单体型PoO检验P < 0.05:以2个SNPs为一个单体型,2个单体型P < 0.05(rs288765-rs1233563和rs12537550-rs11765352);以3个SNPs为一个单体型,4个单体型P < 0.05(rs872723-rs288765-rs1233563、rs288765-rs1233563-rs288756、rs6459952-rs12537550-rs11765352、rs12537550-rs11765352-rs6971211)。经过多重检验校正,所有结果均无统计学意义。
表3.
806个中国NSCL/P核心家系PoO效应结果
The results of PoO effects in 806 Chinese case-parents trios
| Gene | SNP ID |
RRm
(95% CI) |
RRm P value |
RRf (95% CI) |
RRf P value |
RRR (95% CI) |
RRR P value |
| SNP, single nucleotide polymorphism; TDT, transmission disequilibrium test; RRR, ratio of relative risks; PoO, parent-of-origin effects. | |||||||
| SHH | rs288765-rs1233563 | 0.43 (0.15-1.29) |
0.134 | 1.04 (0.41-2.75) |
0.919 | 0.41 (0.20-0.86) |
0.018 |
| SHH | rs12537550-rs11765352 | 1.22 (0.87-1.71) |
0.239 | 0.90 (0.64-0.24) |
0.518 | 1.36 (1.01-1.82) |
0.036 |
| SHH | rs872723-rs288765-rs1233563 | 0.54 (0.23-1.29) |
0.161 | 1.33 (0.66-2.69) |
0.427 | 0.41 (0.20-0.86) |
0.017 |
| SHH | rs288765-rs1233563-rs288756 | 0.58 (0.28-1.20) |
0.153 | 1.29 (0.72-2.31) |
0.386 | 0.45 (0.21-0.95) |
0.035 |
| SHH | rs6459952-rs12537550-rs11765352 | 1.16 (0.84-1.61) |
0.366 | 0.81 (0.59-1.11) |
0.192 | 1.44 (1.06-1.95) |
0.021 |
| SHH | rs12537550-rs11765352-rs6971211 | 1.13 (0.83-1.56) |
0.433 | 0.81 (0.59-1.11) |
0.180 | 1.40 (1.04-1.90) |
0.025 |
3. 讨论
本研究选择HH通路的4个基因(PTCH1、PTCH2、SHH、SMO)在806个中国人群NSCL/P核心家系中,针对单个SNP和单体型分别进行关联分析以及PoO效应探索。HH信号通路与胚胎发育密切相关,尤其在颌面部形态形成中发挥重要作用[15],其中Ptch1蛋白在HH通路中具有重要功能,当Hh蛋白未与Ptch1结合时,HH通路受到抑制;当两者结合后,解除对Smo的抑制,HH通路激活,开始转录活动[16]。唇腭裂发病受遗传因素影响,其发病呈现家族聚集性。传统双生子研究和家系研究均表明遗传因素在其发病中有重要的作用[17]。双生子研究发现同卵双胞胎的患病一致率远远高于异卵双胞胎,CL/P分别为50%和8%,CP则分别为33%和7%[18]。近年来开展的GWAS发现了多个致病基因或候选基因[6, 10-11, 19-20]。本研究纳入的4个基因中,既往研究发现PTCH1基因突变导致基底细胞癌综合征(basal cell nevus syndrome, BCNS,OMIM 109400),该病属于孟德尔遗传病,病人可伴有唇腭裂、眼畸形等出生缺陷[21]。动物研究发现PTCH1基因缺失会导致鼠颅面部缺陷,包括腭发育不全、继发腭裂[22]。Yu等[10]在中国人群进行的GWAS(genome-wide association studies)研究发现,PTCH1基因的rs10512248位点与NSCLP密切相关。Xiao等[23]利用NSCL/P的目标区域测序数据探索可能存在的基因-基因交互作用,研究发现rs3761910(ARHGAP29)与rs2149722(PTCH1)交互作用在亚洲人群中达到显著水平。研究者在巴西人群中发现SHH基因是NSCLP的遗传危险因素[24]。现有研究发现SMO与多种癌症相关。目前研究尚未发现SMO与唇腭裂的发生相关。当人群中NSCL/P的患病率为0.12%时,本研究纳入806个核心家系有80%的把握度,可以发现单个位点与NSCL/P的真实关联(双侧检验α=0.05,MAF=0.2,RR>1.3)。本研究未能发现HH通路相关基因与NSCL/P的关联,未来可通过扩大样本量进一步的探索。
PoO效应被认为是遗传因素可能的其他生物学机制之一,已有一些研究探索PoO效应在NSCL/P发病中的作用。2005年,研究者发现子代胱硫醚β-合酶基因(CBS)的c.844ins68位点来自母亲时,与该位点遗传自父亲相比,子代NSCL/P的发病风险增加18.7倍[25]。2008年Reutter等[26]利用204个中欧NSCL/P核心家系探索TGFB3在NSCL/P发病风险中的作用。研究发现子代的rs2300607来自母亲与来自父亲相比,其发病风险降低(IM=0.38,95%CI 0.17~0.86)(IM=1表示不存在亲源效应)。Sull等[27-29]纳入297个核心家系的样本,分别探索Runt相关转录因子2基因(runt-related transcription factor 2 gene,RUNX2)、转录生长因子α基因(transforming growth factor alpha,TGFA)、配对框转录因子基因(paired box transcript factor genes,PAX3, PAX6,PAX7,PAX9)的PoO效应,发现RUNX2的3个SNPs、TGFA的两个SNPs、PAX7的两个SNPs与PAX3的4个SNPs结果具有统计学意义。2010年,Suazo等[30]通过150个智利NSCL/P核心家系探索MSX1可能的PoO效应在唇腭裂发病中的作用, 发现rs12532位点的A等位基因来自父亲时子代患病风险增加2.08倍(95%CI: 1.10~4.02),MSX1通过基因印迹影响NSCL/P的发病风险。Shi等[31]纳入2 000多个非综合型唇腭裂核心家系探索亲源效应,研究没有发现达到GWAS显著水平的SNP位点。Garg等[32]在2 500个核心家系探索PoO效应,研究提示PoO效应与唇腭裂病因相关。目前,探索HH通路基因相关亲源效应对NSCL/P发病风险影响的研究较罕见。本研究没有发现HH通路基因与NSCL/P的关联,可能的原因是位点数目多,使得检验次数增加,SNP位点间具有相关性,而Bonferroni校正法较为保守。本研究发现SHH基因可通过单体型表现出PoO效应,但经多重检验校正后没有统计学意义。
本研究采用核心家系设计,核心家系包括患儿及其父母,亦可称为病例-双亲(case-parent trios)设计,采用该设计类型可探索亲源效应,这一优势是普通病例对照研究所不具备的。由于需要招募患儿父母为研究对象,更适用于探索复杂疾病特别是生命早期的疾病比如NSCL/P的病因, 这种设计还可以控制人群分层造成的混杂。本研究采用单位点以及单体型进行TDT检验和探索PoO效应,相对于单位点分析,单体型会产生更多杂合的个体,从而提供更多的信息并增加把握度[33]。本研究虽未发现具有PoO效应的基因,但未来可在特定环境因素暴露条件下或者在更大样本量的人群中进行进一步探索。本研究采用了中国人群NSCL/P的GWAS数据,值得注意的是GWAS研究只关注MAF>0.01的常见遗传变异位点,因此今后可在中国唇腭裂人群中进行测序研究,探索罕见遗传变异及其他遗传变异不同作用方式对发病风险的影响,为NSCL/P的发病机制研究提供更多的线索。
综上所述,本研究利用来自“唇腭裂基因和交互作用的国际合作研究”项目在中国地区募集的806个NSCL/P核心家系数据,从单位点以及单体型进行TDT检验和PoO效应探索,深入探索HH通路相关基因在中国人群NSCL/P发生风险中的作用,经过Bonferroni多重检验校正后,未能发现达到统计学显著性水平的结果。本研究未能在研究人群中发现显著的亲源效应,但不能据此认为PoO效应对NSCL/P发病没有影响。与患儿基因主效应相比,PoO效应属于更高阶的效应,因此需要更大的样本量,本研究的样本量可能不足以发现PoO效应;相关基因也可能在特定环境因素下才表现出PoO效应,或者PoO与环境因素交互作用影响子代发病风险。未来可采取扩大样本量、综合分析PoO与环境因素的作用来进一步探索PoO效应在发病中的作用。
Funding Statement
国家自然科学基金(81102178, 81573225)、北京市自然科学基金(7172115)和北京大学医学交叉研究基金(BMU2017MX018)
Supported by the National Natural Science Foundation of China (81102178, 81573225), Beijing Municipal Natural Science Foundation (7172115), and Peking University Health Science Center Interdisciplinary Research Fund (BMU2017MX018)
References
- 1.Wang M, Yuan Y, Wang Z, et al. Prevalence of orofacial clefts among live births in China: a systematic review and meta-analysis. Birth Defects Res. 2017;109(13):1011–1019. doi: 10.1002/bdr2.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harville EW, Wilcox AJ, Lie RT, et al. Cleft lip and palate versus cleft lip only: are they distinct defects. Am J Epidemiol. 2005;162(5):448–453. doi: 10.1093/aje/kwi214. [DOI] [PubMed] [Google Scholar]
- 3.Leslie EJ, Marazita ML. Genetics of cleft lip and cleft palate. Am J Med Genet C Semin Med Genet. 2013;163c(4):246–258. doi: 10.1002/ajmg.c.31381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang R, Bush JO, Lidral AC. Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn. 2006;235(5):1152–1166. doi: 10.1002/dvdy.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mossey PA, Little J, Munger RG, et al. Cleft lip and palate. Lancet. 2009;374(9703):1773–1785. doi: 10.1016/S0140-6736(09)60695-4. [DOI] [PubMed] [Google Scholar]
- 6.Grant SF, Wang K, Zhang H, et al. A genome-wide association study identifies a locus for nonsyndromic cleft lip with or without cleft palate on 8q24. J Pediatr. 2009;155(6):909–913. doi: 10.1016/j.jpeds.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Beaty TH, Marazita ML, Leslie EJ. Genetic factors influencing risk to orofacial clefts: today's challenges and tomorrow's opportunities. F1000Res. 2016;5:2800. doi: 10.12688/f1000research.9503.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilmatre A, Sharp AJ. Parent of origin effects. Clin Genet. 2012;81(3):201–209. doi: 10.1111/j.1399-0004.2011.01790.x. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Zuo X, He M, et al. Genome-wide analyses of non-syndromic cleft lip with palate identify 14 novel loci and genetic heterogeneity. Nat Commun. 2017;8:14364. doi: 10.1038/ncomms14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaty TH, Murray JC, Marazita ML, et al. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010;42(6):525–529. doi: 10.1038/ng.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gjessing HK, Lie RT. Case-parent triads: estimating single- and double-dose effects of fetal and maternal disease gene haplotypes. Ann Hum Genet. 2006;70(Pt 3):382–396. doi: 10.1111/j.1529-8817.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 13.Gjerdevik M, Haaland OA, Romanowska J, et al. Parent-of-origin-environment interactions in case-parent triads with or without independent controls. Ann Hum Genet. 2018;82(2):60–73. doi: 10.1111/ahg.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg CR. Methods for detection of parent-of-origin effects in genetic studies of case-parents triads. Am J Hum Genet. 1999;65(1):229–235. doi: 10.1086/302466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 16.Taipale J, Cooper MK, Maiti T, et al. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418(6900):892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 17.Wantia N, Rettinger G. The current understanding of cleft lip malformations. Facial Plast Surg. 2002;18(3):147–153. doi: 10.1055/s-2002-33061. [DOI] [PubMed] [Google Scholar]
- 18.Grosen D, Bille C, Petersen I, et al. Risk of oral clefts in twins. Epidemiology. 2011;22(3):313–319. doi: 10.1097/EDE.0b013e3182125f9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangold E, Ludwig KU, Birnbaum S, et al. Genome-wide association study identifies two susceptibility loci for nonsyndromic cleft lip with or without cleft palate. Nat Genet. 2010;42(1):24–26. doi: 10.1038/ng.506. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Huang Y, Yin A, et al. Genome-wide association study identifies a new susceptibility locus for cleft lip with or without a cleft palate. Nat Commun. 2015;6:6414. doi: 10.1038/ncomms7414. [DOI] [PubMed] [Google Scholar]
- 21.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3(1):32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metzis V, Courtney AD, Kerr MC, et al. Patched1 is required in neural crest cells for the prevention of orofacial clefts. Hum Mol Genet. 2013;22(24):5026–5035. doi: 10.1093/hmg/ddt353. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Y, Taub MA, Ruczinski I, et al. Evidence for SNP-SNP interaction identified through targeted sequencing of cleft case-parent trios. Genet Epidemiol. 2017;41(3):244–250. doi: 10.1002/gepi.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Araujo TK, Secolin R, Felix TM, et al. A multicentric association study between 39 genes and nonsyndromic cleft lip and palate in a Brazilian population. J Craniomaxillofac Surg. 2016;44(1):16–20. doi: 10.1016/j.jcms.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Rubini M, Brusati R, Garattini G, et al. Cystathionine beta-synthase c.844ins68 gene variant and non-syndromic cleft lip and palate. Am J Med Genet A. 2005;136a(4):368–372. doi: 10.1002/ajmg.a.30812. [DOI] [PubMed] [Google Scholar]
- 26.Reutter H, Birnbaum S, Mende M, et al. TGFB3 displays parent-of-origin effects among central Europeans with nonsyndromic cleft lip and palate. J Hum Genet. 2008;53(7):656–661. doi: 10.1007/s10038-008-0296-9. [DOI] [PubMed] [Google Scholar]
- 27.Sull JW, Liang KY, Hetmanski JB, et al. Differential parental transmission of markers in RUNX2 among cleft case-parent trios from four populations. Genet Epidemiol. 2008;32(6):505–512. doi: 10.1002/gepi.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sull JW, Liang KY, Hetmanski JB, et al. Maternal transmission effects of the PAX genes among cleft case-parent trios from four populations. Eur J Hum Genet. 2009;17(6):831–839. doi: 10.1038/ejhg.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sull JW, Liang KY, Hetmanski JB, et al. Evidence that TGFA influences risk to cleft lip with/without cleft palate through unconventional genetic mechanisms. Hum Genet. 2009;126(3):385–394. doi: 10.1007/s00439-009-0680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suazo J, Santos JL, Jara L, et al. Parent-of-origin effects for MSX1 in a Chilean population with nonsyndromic cleft lip/palate. Am J Med Genet A. 2010;152a(8):2011–2016. doi: 10.1002/ajmg.a.33528. [DOI] [PubMed] [Google Scholar]
- 31.Shi M, Murray JC, Marazita ML, et al. Genome wide study of maternal and parent-of-origin effects on the etiology of orofacial clefts. Am J Med Genet A. 2012;158a(4):784–794. doi: 10.1002/ajmg.a.35257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garg P, Ludwig KU, Bohmer AC, et al. Genome-wide analysis of parent-of-origin effects in non-syndromic orofacial clefts. Eur J Hum Genet. 2014;22(6):822–830. doi: 10.1038/ejhg.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris RW, Kaplan NL. On the advantage of haplotype analysis in the presence of multiple disease susceptibility alleles. Genet Epidemiol. 2002;23(3):221–233. doi: 10.1002/gepi.10200. [DOI] [PubMed] [Google Scholar]


