Introduction
Percutaneous closure of the left atrial appendage (LAA) is becoming more popular in patients suffering from atrial fibrillation (AF) with a high risk of stroke and bleeding. This is currently indicated as a class 2b recommendation in the 2016 ESC/EACTS guidelines.1 Trials have shown noninferiority with respect to ischemic stroke reduction for percutaneous closure compared to warfarin with additional reduced risk of major bleeding, hemorrhagic stroke, and mortality.2 However, percutaneous catheter-based closure is considered to be unsafe in LAA containing clot. Although thoracoscopic clipping of the LAA is commonly performed in arrhythmia surgery with good results,3 little is known about a thoracoscopic approach for patients with thrombus inside the LAA. Avoiding unnecessary manipulation of the LAA seems mandatory to avoid clot embolization. We describe 4 cases in which we used an open-end V-shaped clip to close the LAA containing thrombus. This V-shaped clip differs from the standard clip, since it is not a cage that has to be manipulated across the LAA. The V-shaped clip can be positioned directly at the LAA base without manipulating the appendage and it allows for a gradual closure of the LAA from tip to heel, preventing thrombus dislodgement.
Key Teaching Points.
-
•
A clot-containing left atrial appendage poses a treatment challenge owing to risk of thrombus dislodgement during any procedure.
-
•
A patient population with atrial fibrillation and embolic events despite adequate anticoagulation or with anticoagulation contraindications and with clot-containing left atrial appendages had no treatment options until recently.
-
•
Thoracoscopic clipping of the left atrial appendage with a V-shaped clip in a no-touch manner may be a promising alternative for patients with a clot-containing appendage in which oral anticoagulation therapy is contraindicated or is insufficient.
Case Series
Four patients (aged 73, 81, 67, and 62 years) were operated according to this technique between October 2019 and March 2020.
The first patient is known to have permanent atrial fibrillation (CHA₂DS₂-VASc 4) since 2006 for which she is treated with apixaban. In 2017 she had a myocardial infarction—without coronary disease—which was possibly due to an embolic event, and in 2019 she had a transient ischemic attack. Recent transesophageal echocardiography showed a large thrombus in the LAA.
The second patient had an aortic valve replacement in 2013 and is known to have permanent atrial fibrillation (CHA₂DS₂-VASc 4), for which he is treated with apixaban and rate control. In 2018 he had a transient ischemic attack and an acute ischemic event of the right arm due to a thrombus in the axillary artery, for which embolectomy was performed. In 2019 he had a second transient ischemic attack and later a minor stroke with vertigo and visual impairment. A large thrombus in the LAA was seen on echocardiography in 2018 and was initially treated conservatively.
The third patient had a coronary artery bypass graft in 2014 and developed paroxysmal atrial fibrillation (CHA₂DS₂-VASc 6) afterwards. Despite using rivaroxaban, he had 2 transient ischemic attacks. Recently he had several gastrointestinal bleeding episodes owing to a bowel angiodysplasia. He was planned for catheter pulmonary vein isolation and closure of the LAA. However, during the procedure LAA thrombus was observed and the procedure was aborted and surgery was planned.
In these 3 patients, preoperative echocardiography revealed LAA clots that were located close by but not inside the orifice, which is an important prerequisite for thoracoscopic clipping.
The fourth patient has permanent AF since 2008 and developed a hemorrhagic stroke in 2018 with cerebral small vessel disease (CHA₂DS₂-VASc 3). He underwent a percutaneous closure of the left appendage with a Watchman device in 2019. However, the device was dislocated and did not close off the appendage completely.
Technique
Patient positioning and thoracoport introduction on the left side were done as described previously.3 A transesophageal echocardiography was performed, confirming the presence of thrombus in the LAA. The pericardium was opened from the left pulmonary artery towards the left inferior pulmonary vein just anterior from the lung hilum, posterior from the phrenic nerve.
The base of the LAA was sized using a sizer (Selection Guide; Atricure, Inc, Mason, OH). Then, the open-ended V-shaped clip (Atriclip Pro-V Device; Atricure, Inc) was introduced, opened, and positioned at the base of the LAA without further manipulation.
After echocardiographic and thoracoscopic confirmation of complete inclusion of the base of the LAA, the clip was closed (Figures 1 and 2).
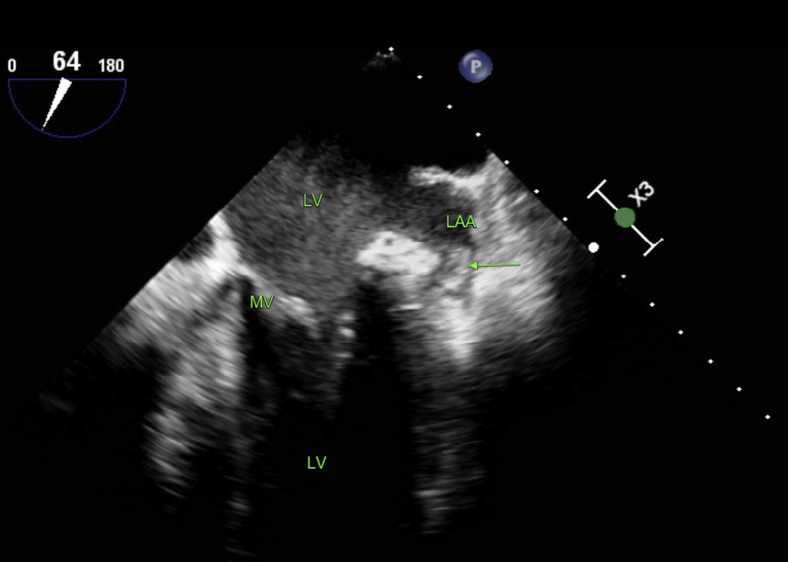
Figure 1.
Preoperative transesophageal echocardiography image of the clot-containing left appendage (patient 1). The arrow indicates the clot. LA = left atrium; LAA = left atrial appendage; LV = left ventricle; MV = mitral valve.
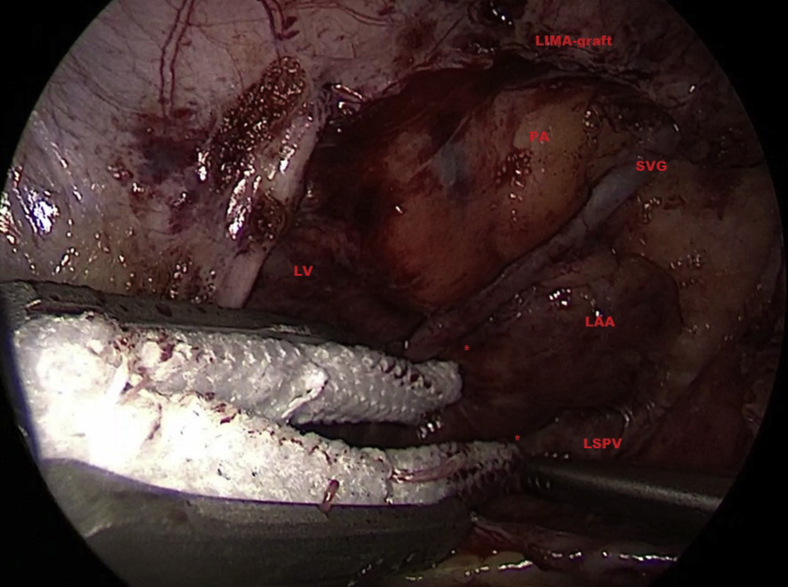
Figure 2.
Intraoperative view of patient 3. Owing to adhesions and to minimize manipulation, a tunnel was created around the base and the appendage was not released in its entirety. The 2 asterisks indicate the tunnel dissected at the left atrial appendage (LAA) base at the anterior and posterior side for positioning of the 2 jaws of the clip. LIMA-graft = left internal mammary graft; LV = left ventricle; LSPV = left superior pulmonary vein; PA = pulmonary artery; SVG = saphenous vein graft.
Finally, the clip was released after a waiting interval of 30 seconds to rule out any signs of ischemia. The pericardium was left open and a pleural drain was introduced.
All patients were extubated in the operating room and the chest tube was removed after 1 hour.
Operating time was 20 and 36 minutes in the patients without prior surgery (56 minutes and 120 minutes total operating room and anesthesia time) and 60 and 90 minutes, respectively, in the redo cases (116 and 180 minutes total operating room and anesthesia time).
The postoperative course was uncomplicated and patients were discharged after 2 (patients 1, 3, and 4) and 3 days (patient 2). Patient 4, however, developed a left phrenic nerve paresis postoperatively.
At 6 months follow-up, patients 1 and 2 recovered well and were free from stroke or other neurologic events.
Patients 3 and 4 recovered well and were free from any neurologic events at 3 months.
All patients received a computed tomography scan postoperatively that revealed complete closure of the left atrial appendage.
Discussion
This report describes a thoracoscopic approach for closure of the LAA containing clots in patients that were not suitable for percutaneous closure for this reason.
Based on this small case series with limited follow-up, thoracoscopic LAA clipping seems a viable option for troubleshooting in patients with a clot, even in patients with prior cardiac surgery or a Watchman device in situ. This may avoid more extensive surgery, including sternotomy or thoracotomy. A larger, prospective trial further evaluating safety and effectiveness of this technique, as well as long-term follow-up, is warranted.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: Drs Fleerakkers and Hofman do not have any conflict of interest.
Dr Van Putte is consultant with Atricure Europe BV. Dr Boersma is consultant with Boston Scientific.
References
- 1.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 2.Reddy V, Doshi SK, Kar S, et al. 5-Year outcomes after left-atrial appendage closure: From the PREVAIL and PROTECT-AF Trials. J Am Coll Cardiol 2017;19;70:2964–2975. [DOI] [PubMed]
- 3.Van Laar C., Verberkmoes N.J., van Es H.W. Thoracoscopic left atrial appendage clipping: A multicenter cohort analysis. JACC Clin Electrophysiol. 2018;4:893–901. doi: 10.1016/j.jacep.2018.03.009. [DOI] [PubMed] [Google Scholar]