Introduction
Catheter ablation is an acceptable treatment strategy for medically refractory atrial tachycardia (AT); however, only a few studies have reported on ablation for persistent left superior vena cava (PLSVC)-related AT.1 The arrhythmogenicity of the PLSVC is well known2; however, little is known regarding the electrophysiological characteristics of the epicardial fiber connecting the PLSVC to the left superior pulmonary vein (LSPV) ridge in the left atrium (LA).
This study reports a case of PLSVC-related macroreentrant AT that reveals novel electrophysiological characteristics of the epicardial connection between a PLSVC and the LA.
Case report
A 53-year-old man was admitted to our hospital and scheduled for catheter ablation for the treatment of the recurrence of symptomatic drug therapy–resistant postoperative AT. AT developed as a complication following mitral valve surgery and cryo-maze procedures 3 years prior to admission.3 Prior to ablation, computed tomography imaging revealed a PLSVC. In the first and second ablation sessions, electrophysiological mapping was performed using a 3-dimensional mapping system (RHYTHMIA; Boston Scientific, Natick, MA) and an Orion multipolar basket catheter (Boston Scientific). A 6F 8-pole or 4-pole 3-site mapping catheter (BeeAT; Japan Lifeline Co. Ltd., Tokyo, Japan) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording action potentials, and internal cardioversion. If AT persisted, the voltage characterization and the activation wavefronts of the atrium were assessed. The entrainment maneuver was performed to confirm the AT circuit.4 The ablation endpoint in this study was similar to that reported in previous studies.5,6 Through an Agilis sheath (St. Jude Medical, St. Paul, MN), radiofrequency currents were delivered with 4-mm irrigated tip ablation catheters of types in the first session (INTELLANAV) and second session (INTELLANAV MIFI; Boston Scientific). Radiofrequency energy was delivered at 20–30 W for a duration of 30 seconds at each point or when the fluctuation (rise and drop) in the impedance was regulated within 20 Ω. The irrigated electrode temperature was maintained at <40°C.
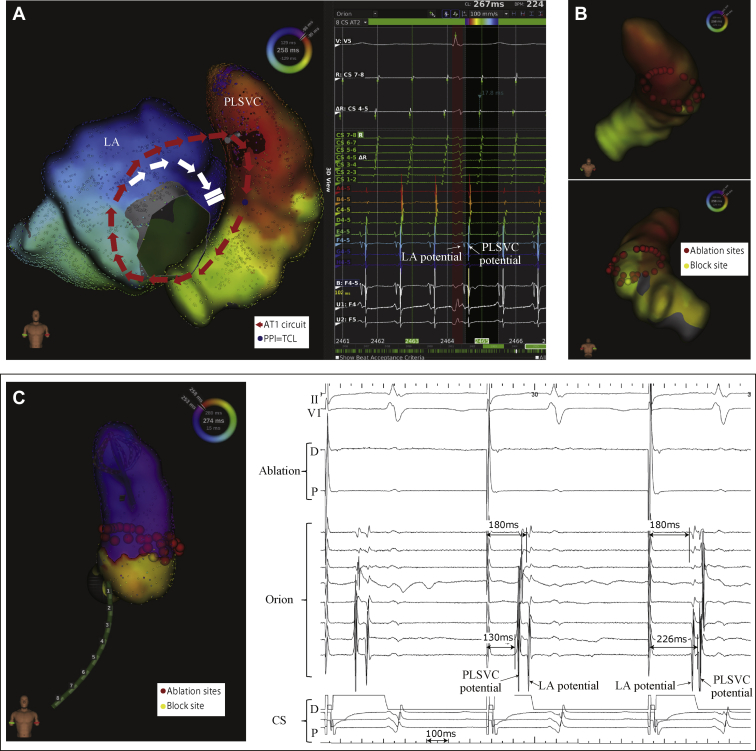
In the first ablation session, the initial tachycardia (AT1, cycle length [CL] = 265 ms) was induced via atrial burst pacing. In the activation pattern of AT1, the tachycardia circuit ascended the anterior LA and propagated in the 2-o’clock direction of the mitral annulus towards the PLSVC, descending from the PLSVC to the coronary sinus, and subsequently entering the LA (Figure 1A). AT1 was terminated via full circumferential ablation at the PLSVC, and a unidirectional block line was formed (Figure 1B and C). Pacing within the PLSVC could not be captured in the myocardium; hence, a bidirectional block could not be confirmed. The ablation session was considered completed when AT could no longer be induced after a waiting time of more than 30 minutes.
Figure 1.
A: Activation mapping of initial atrial tachycardia (AT1) circuit. The red arrows show the AT1 circuit and the white arrows go toward the dead end. The blue points indicate where the postpacing intervals had a tachycardia cycle length of +20 ms or less. B: The red points indicate ablation points and the yellow point shows where AT1 was terminated. C: Catheter position during circumferential ablation. The internal electrocardiogram of the unidirectional block line was completed. LAP = left atrial potential; PLSVC = persistent left superior vena cava; PPI = postpacing interval; TCL = tachycardia cycle length.
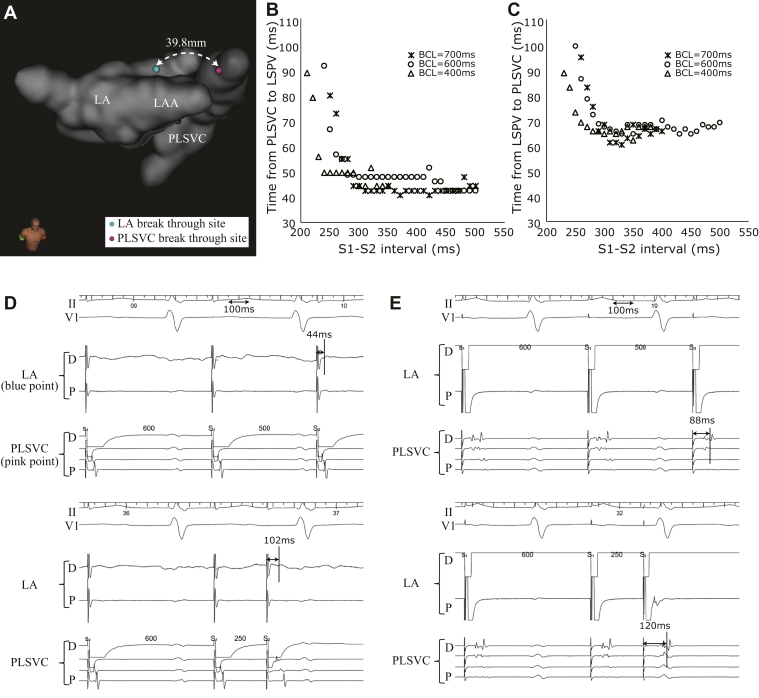
A second ablation session was performed following the recurrence of tachycardia 2 months after the first ablation session. The second AT (AT2, CL = 290 ms) showed an activation pattern different from that of AT1; here, the AT2 circuit ascended the PLSVC through the recurrent circumferential ablation line, propagating to the LA roof and descending the LA anterior wall, connecting to the coronary sinus (Figure 2A). After terminating the AT2 via overdrive pacing, we performed an electrophysiological study to determine the electrophysiological characteristics of the connecting fibers between the LA roof and PLSVC. The distance between the LA breakthrough site (LSPV ridge) during AT and PLSVC breakthrough site (during pacing from the LA) was 39.8 mm (Figure 3A). A catheter was placed at the breakthrough site of the LA, with another one placed at the equivalent PLSVC site. The decremental conduction properties of the connecting fibers were investigated via an atrial extrastimulus technique to clarify the association with the maintenance of the AT. In this technique, the baseline CLs of 700 ms, 600 ms, and 400 ms were used. The atrial effective refractory period was evaluated by shortening each extrastimulus by 10 ms after each test (for each base CL). It was observed that shorter coupling intervals caused a progressive delay in the connecting fibers between the LA roof and PLSVC. In addition, pacing from both the LA roof and PLSVC sites revealed the decremental conduction characteristics of the connecting fibers (Figure 3B–E). The attachment site of the LA and PLSVC connection was far above the circumferential site ablated during the first session, and also different from that of the ablation site during the maze procedure or surgical site; therefore, these decremental properties were not iatrogenic.
Figure 2.
A: Activation mapping of second atrial tachycardia (AT2) circuit. The red arrows show the AT2 circuit and the white arrows go toward the dead end. The blue points indicate where the postpacing intervals had a tachycardia cycle length of +20 ms or less. B: The application points (red points) illustrate where tachycardia was terminated. The bidirectional block line was completed. The internal electrocardiogram reveals the local ablated potential of termination.
Figure 3.
A: The distance from the left atrium (LA) breakthrough site to the persistent left superior vena cava (PLSVC) breakthrough site. LAA = left atrial appendage. B, C: The charts for the extrastimulus pacing assessment showing the results for each breakthrough point. Asterisks, circles, and triangles indicate basic cycle length (BCL) of 700 ms, 600 ms, and 400 ms, respectively. The horizontal line depicts the shortened pacing interval. The vertical line in panel B illustrates the conduction time from the pacing site to local potential at the left superior pulmonary vein (LSPV) ridge; the vertical line in panel C shows the conduction time from the pacing site to the local potential at the PLSVC. D, E: Internal electrocardiogram showing decremental conduction properties.
Following the induction of AT2, AT2 was terminated by ablating the earliest propagation site (inside the PLSVC) during LA roof pacing, which is the supposed exit from the PLSVC to the LA (Figure 2B). Consequently, a bidirectional block was achieved between the LA and PLSVC, and inability of AT induction was the endpoint of this ablation.
Discussion
This case report describes a patient who presented with macroreentrant AT associated with a 39.8-mm epicardial connection between the LA roof and a PLSVC. A precise electrophysiological analysis revealed that this connection exhibited bidirectional decremental properties. Although this connection did not account for the majority of the tachycardia CL, the conduction delay was supposedly essential for the incidence of the reentrant tachycardia.
The prevalence rate of PLSVC ranges between 0.3% and 0.5% in the general population.2,7 Additionally, PLSVC has unexpected epicardial connections with the LA. Hsu and colleagues8 and Maruyama and colleagues7 have reported on the existence of multiple fibers connecting a PLSVC or the ligament of Marshall to the LA. Furthermore, such connections have been known to be associated with AT,1,9 and the structure of a PLSVC may serve as a substrate for atrial fibrillation. Similarly, Peltier and colleagues10 have reported that PLSVC fibers may exhibit automaticity or the ability to generate reentry circuits. Moreover, Hsu and colleagues8 have reported on the relationship between the LSPV ridge and high PLSVC levels and between the anterior aspect of the PLSVC and mid-PLSVC levels. In addition, electrical modification of PLSVC has been confirmed to cause arrhythmogenic substrate modification.2 In this study, PLSVC was not isolated, as this was a case of AT and AT does not continue once the bidirectional block is completed. The reentry circuits were formed in this case because PLSVC constituted a fiber that was widely electrically isolated from the LA; therefore, it was reasonable to ablate the PLSVC from within because the left atrial appendage may be isolated owing to catheter ablation after maze procedure. Two techniques were performed to terminate AT. The first technique involved a circumferential ablation inside the PLSVC to achieve a block; however, AT recurrence was observed afterwards. This strategy was initially selected because multiple connections have been reported between PLSVC and the LA; in addition, ablating 1 site may not be ideal for terminating AT, as it may change the circuit via another connection. On the contrary, the second technique was performed to treat AT2. This involved the ablation of the earliest propagation site inside the PLSVC during LA roof pacing. This site is the supposed exit from the PLSVC to the epicardial fiber connected to the LSPV ridge during AT2. During AT2, the latest activation site inside the PLSVC was above the circumferential ablation site, which was eventually a bystander for the AT2 circuit. Based on our observations, once an LA-PLSVC macroreentrant tachycardia is confirmed, we recommend terminating AT, performing pacing at the LA roof, and detecting the PLSVC breakthrough site, as most sites inside the PLSVC may be outside of the circuit. The LA breakthrough site can equally serve as an alternative ablation site.
Conclusions
Currently, there has been no research regarding the conduction properties of the epicardial connection between the PLSVC and LA. Here, we present novel findings regarding the decremental conduction properties of such connections, knowledge of which can facilitate the understanding of the potential mechanism of reentrant ATs by experts in the field. Furthermore, pacing studies are necessary to detect ideal ablation sites for the treatment of macroreentrant tachycardias, which involve epicardial connections.
Key Teaching Points.
-
•
This is the first case report describing bidirectional persistent left superior vena cava (PLSVC)-related macroreentrant atrial tachycardia (AT) in a patient.
-
•
The connection between the PLSVC and left atrium showed decremental conduction properties.
-
•
The optimum ablation site for PLSVC-related macroreentrant AT can either be the connecting site of the PLSVC and left atrium or the circumferential ablation site of the PLSVC.
Acknowledgment
The editors thank Editage for English-language editing.
Footnotes
Funding Sources: This work was partly supported by the Intramural Research Fund 17 (Kusano) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center. Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Hasegawa K., Miyazaki S., Kaseno K., Tada H. Persistent left superior vena cava-related atrial tachycardia: A variant of ridge-related re-entry. JACC Clin Electrophysiol. 2018;4:1644–1646. doi: 10.1016/j.jacep.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Tyrak K.W., Holda J., Holda M.K., Koziej M., Piatek K., Klimek-Piotrowska W. Persistent left superior vena cava. Cardiovasc J Afr. 2017;28:e1–e4. doi: 10.5830/CVJA-2016-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakuta T., Fukushima S., Minami K. Contemporary outcomes of the concomitant CryoMaze procedure. Interact Cardiovasc Thorac Surg. 2019;29:28–34. doi: 10.1093/icvts/ivz029. [DOI] [PubMed] [Google Scholar]
- 4.Saoudi N., Anselme F., Poty H., Cribier A., Castellanos A. Entrainment of supraventricular tachycardias: a review. Pacing Clin Electrophysiol. 1998;21:2105–2125. doi: 10.1111/j.1540-8159.1998.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y.J., Tai C.T., Kao T. Electrophysiological characteristics and catheter ablation in patients with paroxysmal right atrial fibrillation. Circulation. 2005;112:1692–1700. doi: 10.1161/CIRCULATIONAHA.104.512731. [DOI] [PubMed] [Google Scholar]
- 6.Jais P., Hocini M., O'Neill M.D. How to perform linear lesions. Heart Rhythm. 2007;4:803–809. doi: 10.1016/j.hrthm.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama M., Ino T., Miyamoto S., Tadera T., Atarashi H., Kishida H. Characteristics of the electrical activity within the persistent left superior vena cava: comparative view with reference to the ligament of Marshall. J Electrocardiol. 2003;36:53–57. doi: 10.1054/jelc.2003.50004. [DOI] [PubMed] [Google Scholar]
- 8.Hsu L.F., Jais P., Keane D. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828–832. doi: 10.1161/01.CIR.0000116753.56467.BC. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura I., Fukamizu S., Arai M. Characteristics of Marshall bundle-related atrial tachycardias using an ultrahigh-resolution mapping system. J Interv Card Electrophysiol. 2019;55:161–169. doi: 10.1007/s10840-019-00544-9. [DOI] [PubMed] [Google Scholar]
- 10.Peltier J., Destrieux C., Desme J., Renard C., Remond A., Velut S. The persistent left superior vena cava: anatomical study, pathogenesis and clinical considerations. Surg Radiol Anat. 2006;28:206–210. doi: 10.1007/s00276-005-0067-7. [DOI] [PubMed] [Google Scholar]