Abstract
Recent studies have shown that metabolic tumor volume (MTV) by positron emission tomography/computed tomography (PET/CT) is an important prognostic parameter in patients with non‐Hodgkin's lymphoma. However, it is unknown whether doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) alone in early stage Hodgkin's lymphoma would lead to similar disease control as combined modality therapy (CMT) using MTV by PET/CT. One hundred and twenty‐seven patients with early stage Hodgkin's lymphoma who underwent PET/CT at diagnosis were enrolled. The MTV was delineated on PET/CT by the area ≥SUV max, 2.5 (standardized uptake value [SUV]). Sixty‐six patients received six cycles of ABVD only. The other 61 patients received CMT (involved‐field radiotherapy after 4–6 cycles of ABVD). The calculated MTV cut‐off value was 198 cm3. Clinical outcomes were compared according to several prognostic factors (i.e. age ≥50 years, male, performance status ≥2, stage II, B symptoms, ≥4 involved sites, extranodal site, large mediastinal mass, CMT, elevated erythrocyte sedimentation rate and high MTV). Older age (progression‐free survival [PFS], P = 0.003; overall survival [OS], P = 0.007), B symptoms (PFS, P = 0.006; OS, P = 0.036) and high MTV (PFS, P = 0.008; OS, P = 0.007) were significant independent prognostic factors. Survival of two high MTV groups treated with ABVD only and CMT were lower than the low MTV groups (PFS, P < 0.012; OS, P < 0.045). ABVD alone was sufficient to control disease in those with low MTV status. However, survival was poor, even if the CMT was assigned a high MTV status. The MTV would be helpful for deciding the therapeutic modality in patients with early stage Hodgkin's lymphoma.
Previously, extensive radiation therapy was the first therapeutic option to treat early stage Hodgkin's lymphoma (HL). However, patients remained at risk of death due to late radiation‐induced adverse effects including secondary cancer.1, 2, 3, 4 Several clinical studies have shown that better disease control rates occur in patients with early stage HL treated with combined modality treatment (CMT) compared with radiotherapy alone.5, 6 These results allowed for reduced numbers of chemotherapy cycles or low‐dose involved‐field radiotherapy (IFRT) for patients with early stage HL. Several recent studies have shown that chemotherapy alone could be an available treatment option for some populations of patients with early stage HL.7, 8 However, the massive pathological lesion in early stage HL is still a difficult problem to successfully treat with chemotherapy alone.
18F‐Fluoro‐deoxyglucose positron emission tomography (18F‐FDG‐PET) is a valuable functional imaging modality in patients with malignant lymphoma.9, 10, 11 This modality provides useful guidance for managing HL at several points in staging, monitoring or response during and after treatment.9, 10, 11, 12, 13, 14, 15 Recent studies have shown that metabolic tumor volume (MTV) as the tumor burden on positron emission tomography/computed tomography (PET/CT) is a prognostic factor in some subtypes of non‐Hodgkin's lymphoma.16, 17, 18, 19 However, it is not well established whether the quantitative tumor burden detected using PET/CT is a predictive factor for clinical outcome or an important indicator for establishing therapeutic planning in early stage HL.
The objective of the present study was to investigate whether MTV by PET/CT is a clinical parameter predicting survival in patients with early stage HL.
Patients and Methods
Patients
Newly diagnosed patients at five medical centers (Pusan National University Hospital, Chonnam National University Hospital, Kyungpook National University Hospital, Busan Paik Hospital and Gachon University Gil Medical Center) from 2006 to 2011 with clinical stage I and II HL who had one or more clinical risk factors were enrolled. All patients underwent conventional staging procedures including a physical examination, complete blood cell counts and blood chemistry, CT scan (neck, chest and abdominal‐pelvic area), bilateral bone marrow biopsy and a 18F‐FDG‐PET/CT scan. Patients at an advanced stage, those with other malignant diseases, HIV‐positive status or those who received autologous stem cell transplantation after relapse were not included. Patients with an acute or chronic inflammatory condition or disease, active lung diseases or chronic liver disease were also excluded. Approval for the retrospective review of these records was obtained from the Institutional Review Boards of all participating medical centers.
Treatment schedule and response criteria
The doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) regimen consisted of standard doses of drugs along with routine antiemetic and steroid prophylaxis. The regimen consisted of 25 mg/m2 doxorubicin intravenously (i.v.), 10 mg/m2 bleomycin i.v., 6 mg/m2 vinblastine i.v. and 375 mg/m2 dacarbazine i.v. Each cycle was administered on days 1 and 15 and repeated every 4 weeks for a total of six cycles.
Radiation was scheduled to begin 3 weeks after completion of chemotherapy and was in the form of IFRT. Involved‐field radiotherapy was administered to all lymph node (LN) regions involved initially. Patients received radiation doses of 30 Gy over 3–3.5 weeks. Additional radiotherapy (RT) of 10 Gy was applied during the fourth week to areas of initial bulky disease. The radiotherapy was performed as described previously.20
The response to treatment was evaluated after the third and sixth cycles, following Cotswold's criteria.21 A response in patients who also received radiotherapy was assessed 2 months later and was documented based on the clinical situation and the results of the test and imaging investigation that were abnormal at presentation.
Measurement of quantitative parameters using 18F‐FDG PET/CT
The patients were studied after fasting for 6 h. At the time of FDG injection, all patients had blood glucose levels <160 mg/dL. Whole body emission scans (8–9 bed position for 3 min for each bed position) were acquired, beginning 60 min after an intravenous injection of FDG (range of dose, 222–370 MBq). Based on dual‐slice helical CT and full‐ring PET tomography, dual‐modality PET/CT was performed on a Biograph instrument (Siemens Medical Solution, Hoffman Estates, IL, USA). The FDG‐PET images were evaluated for regions of focally increased tracer uptake. In the lymphoma lesions of the FDG tracer uptake, SUV (standardized uptake value) ≥2.5 as a contouring border was considered to represent lymphoma, as suggested by Freudenberg et al.22 The CT images were used for PET attenuation correction. Imaging reconstruction of corrected emission data was performed after Fourier transform with an AWOSEM algorithm (two iterations, eight subsets, 5 mm Gaussian filter).
The PET/CT criteria for the pathological lymphoma‐involved site, including the nodal and extra‐nodal (EN) sites, was that the lesion exceeded 1.0 cm by CT scan and FDG uptake of the lesions was SUV ≥2.5 as a contouring border by the PET image. The MTV was defined as the sum of the overall pathological involved site, including the positive LN and EN sites. The FDG‐PET images were acquired using dedicated PET/CT scanners (Gemini, Philips; Biograph 40, Siemens) according to which is consistent with a major guideline for standard oncological PET imaging.23 The PET images were interpreted by nuclear physicians at each institution. The data were then reviewed by a nuclear medicine expert at Pusan National University Hospital.
Prognostic factor assessment
Risk factors included those according to European Organization for Research and Treatment of Cancer (EORTC) criteria, such as age ≥50 years, large mediastinal mass (≥1/3 of maximal thoracic diameter or maximum diameter ≥10 cm), elevated erythrocyte sedimentation rate (≥50 mm/h without B symptoms or ≥30 mm/h with B symptoms), B symptoms, four or more nodal sites involved24 and other available prognostic factors such as sex, stage II, Eastern Cooperative Oncology Group performance status ≥ grade 2, EN site involvement, CMT strategy compared with chemotherapy only and MTV by PET/CT.
Statistical analyses
Receiver operating characteristic (ROC) curves were prepared to estimate the accuracy of predicting the ideal MTV cut‐off value. Estimates of sensitivity and specificity were based on the cut‐off value. Overall survival (OS) and progression‐free survival (PFS) were measured from the date of diagnosis and were estimated according to the Kaplan–Meier method. Comparisons between the variables of interest were performed using the log‐rank test. A survival analysis was performed using Cox regression to determine whether there was a difference in survival between the treatment groups. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were determined for all survival end‐points. Statistical analysis was carried out using spss software version 18.0 (SPSS Inc., Chicago, IL, USA). A P‐value < 0.05 was considered significant.
Results
The baseline characteristics of the 127 patients are shown in Table 1. Treatment schedules were as follows: 66 patients received IFRT after 4–6 cycles of the ABVD regimen. The remaining 61 patients received only six cycles of the ABVD regimen due to some cardiac or pulmonary problems or refusal of the patients to radiotherapy. The median age was 42 years (range, 18–78 years) and the male : female ratio was 1.44:1. The nodular sclerosis subtype was the predominant histology (55 patients), with 41 patients presenting with mixed cellularity, 20 with lymphocyte‐rich and 11 with lymphocyte‐depleted subtypes. Twenty‐seven patients were stage I (21.3%) and the other 100 patients were stage II (78.7%). Seventy‐eight (61.4%) patients had more than three involved sites and 19 (14.9%) patients had mediastinal disease. Twenty‐four (18.9%) patients had B symptoms.
Table 1.
Baseline patient characteristics
| Total | CMT | ABVD only | |
|---|---|---|---|
| No. patients (%) | 127 | 61 (48.0) | 66 (52.0) |
| Median age, (range) (years) | 42 (18–78) | 34 (19–76) | 48 (18–78) |
| Sex, n (%) | |||
| Male | 75 (59.1) | 31 (24.4) | 44 (34.7) |
| Female | 52 (40.9) | 30 (23.6) | 22 (17.3) |
| Histology, n (%) | |||
| Nodular sclerosis | 55 (43.3) | 28 (22.0) | 27 (21.3) |
| Lymphocyte rich | 20 (15.7) | 8 (6.2) | 12 (9.5) |
| Lymphocyte depleted | 11 (8.7) | 6 (4.8) | 5 (3.9) |
| Mixed cellularity | 41 (32.3) | 19 (15.0) | 22 (17.3) |
| Stage, n (%) | |||
| I | 27 (21.3) | 13 (10.3) | 14 (11.0) |
| II | 100 (78.7) | 48 (37.8) | 52 (40.9) |
| ECOG PS ≥2 | 11 (8.7) | 5 (3.9) | 6 (4.8) |
| B symptoms, n (%) | 24 (18.9) | 11 (8.7) | 13 (10.3) |
| ESR, n (%) | |||
| ≥50 mm/L | 31 (24.4) | 13 (10.3) | 18 (14.1) |
| No. involved sites (%) | |||
| 1–2 | 49 (38.6) | 25 (19.7) | 24 (18.9) |
| ≥3 | 78 (61.4) | 36 (28.3) | 42 (33.1) |
| EN site involvement, n (%) | 30 (23.6) | 15 (11.8) | 15 (11.8) |
| Mediastinum | 19 (14.9) | 9 (7.0) | 10 (7.9) |
| Other | 11 (8.7) | 6 (4.8) | 5 (3.9) |
| Bulky disease, n (%) | 27 (21.3) | 16 (12.6) | 11 (8.7) |
ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; CMT, combined modality treatment; ECOG, Eastern Cooperative Oncology Group; EN, extra‐nodal site; ESR, erythrocyte sedimentation rate; PS, performance status.
Assessment of MTV as a quantitative tumor burden by PET/CT
The median value of the initial MTV as a tumor burden was 142.6 cm3 (range, 6.1–587.2 cm3). A ROC was used to measure the optimal MTV cut‐off value to distinguish the low MTV from the high MTV group. The ideal value was 198.0 cm3 and the estimated area under the MTV ROC curve was 0.849. The ideal value provided sensitivity of 93.3% and specificity of 62.5%. The value had more predictive power compared with the median value of MTV (cut‐off value, HR = 7.480, P = 0.041; median value, HR = 1.847, P = 0.684, data not shown).
Clinical impact assessment of prognostic factors on survival
The median follow‐up time was 45.8 months (range, 13.0–78.5 months). The PFS and OS in all patients was 85.8% and 88.2%, respectively. The PFS and OS in the group treated with CMT were not significantly higher than those who received ABVD only (PFS, 91.8% in the CMT group vs 80.3% in the ABVD only group, P = 0.056; OS, 91.8% in the CMT group vs 84.8% in the ABVD only group, P = 0.151; Fig. 1a,b). Secondary cancers were detected in five patients in the CMT group, but not in the ABVD only group. Three of the five patients were dead at the last follow up. Although patients had secondary cancer in the follow‐up time, the malignancies did not affect the prognosis of patients during follow up. Instead, the cause of progression and death was HL.
Figure 1.
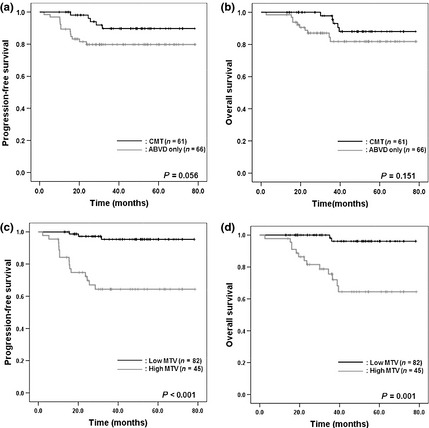
Comparisons of survival according to treatment strategy or metabolic tumor burden using 18F‐fluoro‐deoxyglucose positron emission tomography (18F‐FDG‐PET) in patients with early stage Hodgkin's lymphoma (HL). In a median follow‐up time of 45.8 months, progression‐free survival (PFS) and overall survival (OS) in the combined modality treatment (CMT) group were not significantly different with the doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) only group (PFS, 91.8% in the CMT group vs 80.3% in the ABVD only group, P = 0.056 [a]; OS, 91.8% in the CMT group vs 84.8% in the ABVD only group, P = 0.151, [b]). The PFS and OS were higher in the low metabolic tumor volume (MTV) group compared with that in the high MTV group (PFS, 96.3% in the low MTV group vs 66.7% in the high MTV group, P < 0.001 [c]; OS, 97.6% in the low MTV group vs 71.1% in the high MTV group, P = 0.001 [d]).
Variables of older age (≥50 years), the presence of B symptoms, large mediastinal disease and high MTV status (≥198.0 cm3) were associated with poor PFS and OS (Table 2). The PFS and OS were 66.7% and 71.1% for the high MTV group and 96.3% and 97.6% for the low MTV group respectively (PFS, P < 0.001; P = 0.001, Fig. 1c,d). In the multivariate analysis, older age (PFS, HR = 5.305, P = 0.002; OS, HR = 5.128, P = 0.004), the presence of B symptoms (PFS, HR = 3.556, P = 0.012; OS, HR = 2.682, P = 0.041) and high MTV status (PFS, HR = 13.008, P < 0.001; OS, HR = 15.831; P = 0.001) were independently associated with PFS and OS (Table 3).
Table 2.
Univariate analysis for prognostic factors of survival in patients
| Prognostic factor | Progression‐free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age ≥50 years | 2.884 (1.117–7.446) | 0.029 | 2.898 (1.030–8.153) | 0.044 |
| Male | 0.904 (0.357–2.292) | 0.904 | 0.596 (0.216–1.645) | 0.318 |
| ECOG PS ≥2 | 1.423 (0.327–6.188) | 0.638 | 1.689 (0.381–7.494) | 0.490 |
| Stage II | 2.054 (0.472–8.937) | 0.337 | 3.560 (0.468–27.081) | 0.220 |
| B symptoms | 5.676 (2.450–15.815) | <0.001 | 5.676 (2.052–15.699) | 0.001 |
| ≥4 involved sites | 3.298 (0.954–11.397) | 0.059 | 5.553 (0.730–42.245) | 0.098 |
| EN site involvement | 1.762 (0.661–4.698) | 0.258 | 2.581 (0.916–7.273) | 0.073 |
| Large mediastinal mass | 4.944 (1.850–13.215) | 0.001 | 4.959 (1.683–14.617) | 0.004 |
| CMT | 0.380 (0.135–1.066) | 0.066 | 0.463 (0.158–1.360) | 0.161 |
| Elevated ESR | 1.524 (0.601–3.862) | 0.374 | 1.834 (0.653–5.155) | 0.250 |
| MTV ≥198.0 cm3 | 10.707 (3.098–37.002) | <0.001 | 13.201 (2.975–58.579) | 0.001 |
CI, confidence interval; CMT, combined modality therapy; ECOG, Eastern Cooperative Oncology Group; EN, extra‐nodal; ESR, erythrocyte sedimentation rate; HR, hazards ratio; MTV, metabolic tumor volume; PS, performance status.
Table 3.
Multivariate analysis for prognostic factors of survival in patients
| Prognostic factors | Progression‐free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age ≥50 years | 5.305 (1.890–14.887) | 0.002 | 5.128 (1.682–15.634) | 0.004 |
| B symptoms | 3.556 (1.319–9.591) | 0.012 | 2.682 (0.889–8.091) | 0.042 |
| Large mediastinal mass | 1.486 (0.492–4.488) | 0.483 | 1.464 (0.432–4.959) | 0.541 |
| MTV ≥198.0 cm3 | 13.008 (3.441–49.174) | <0.001 | 15.831 (3.301–75.926) | 0.001 |
CI, confidence interval; HR, hazards ratio; MTV, metabolic tumor volume.
Survival pattern according to treatment strategy and quantitative tumor burden
To examine whether the clinical results of each treatment strategy would be affected by the initial tumor burden, subgroups were created and analyzed according to treatment strategy (CMT vs ABVD only) and MTV (low MTV vs high MTV status).
The PFS in the low MTV group received ABVD only was not different to those in low MTV group who received CMT (97.4% in the low MTV group with CMT vs 95.5% in the low MTV group with ABVD only, P = 0.667, Fig. 2a). The PFS in the low MTV group that received ABVD only was higher than the high MTV group that received CMT (82.6% in the high MTV group with CMT, P = 0.012). Furthermore, the PFS in the high MTV group that received CMT was higher than the high MTV group that received ABVD only (50.0% in the high MTV group with ABVD only, P = 0.014).
Figure 2.
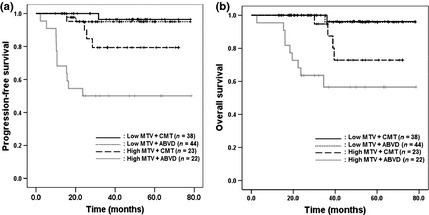
Comparisons of survival according to treatment strategy and metabolic tumor volume (MTV) using 18F‐fluoro‐deoxyglucose positron emission tomography (18F‐FDG‐PET) in patients with early stage Hodgkin's lymphoma (HL). Progression‐free survival (PFS) and overall survival (OS) were not different in the low MTV group regardless of the treatment strategy (PFS, P = 0.667 [a]; OS, P = 0.911 [b]). The PFS in the low MTV group was higher than that in the high MTV group treated with combined modality therapy (CMT) (P = 0.012) and OS in the low MTV group was also higher than that in the high MTV group treated with CMT (P = 0.045). The PFS in the high MTV group treated with CMT was higher than that in the group treated with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) alone (P = 0.014). However, the difference in OS between the two groups was not significant (P = 0.055).
The OS in the low MTV group that received ABVD only was similar to those in the low MTV group that received CMT (97.7% in the low MTV group with CMT vs 97.4% in the low MTV group with ABVD only, P = 0.911, Fig. 2b). Furthermore, the OS in the low MTV group that received ABVD only was higher than the high MTV group that received CMT (82.6% in the high MTV group with CMT, P = 0.045). However, the difference of OS between the high MTV group that received CMT and the high MTV group with ABVD only was not different (50.0% in the high MTV group with ABVD only, P = 0.055).
Discussion
The standard of care for patients with early stage HL is chemotherapy followed by IFRT. The National Comprehensive Cancer Network (NCCN) guideline for favorable early stage HL lists chemotherapy alone as an option only for highly selected patients for whom radiotherapy is contraindicated.25 However, the detection of serious complications such as cardiopulmonary disease and second malignancy in long‐term survivors treated with radiotherapy has prompted reconsideration of the role for CMT. Some investigators have advocated the option of chemotherapy alone and several study groups tested the hypothesis that chemotherapy alone could provide equivalent disease control to that achieved with CMT.26, 27, 28, 29 A clinical study from the Memorial Sloan–Kettering Cancer Center showed similar freedom from progression and overall prognosis between AVBD alone and a CMT group in stages I, II and IIIA without bulky disease.7 A non‐randomized study from Spain also demonstrated similar results between patients with non‐bulky early stage HL treated with CT alone and those with CMT.8 Coincident with these studies, our results showed that survival in the ABVD alone group was not different from that in the CMT group, although a small population with bulky disease was included.
The CMT is still a reasonable standard therapeutic option in patients with early stage HL, particularly those with bulky disease. This means that radiotherapy to bulky disease in combination with chemotherapy would be needed to reduce the risk of recurrence. However, if the overall pathological lesion is not bulky disease and there is a high tumor burden status due to non‐bulky multifocal mass lesions, the selection of treatment modality is complicated. Previous studies have demonstrated that total tumor burden measured using a CT scan can be a predictive tool for evaluating the curative potential of a treatment combination.30, 31 According to those studies, the treatment strategy might be decided dependent on the total tumor burden as well as the presence of bulky disease.
The ability of 18F‐FDG PET to distinguish viable tumors and necrosis or fibrosis in residual masses provides an advantage over conventional imaging using CT or magnetic resonance imaging.32, 33, 34 18F‐FDG PET/CT is now strongly recommended by the International Harmonization Project in Lymphoma for staging and reassessment of FDG‐avid potentially curable lymphomas.35 However, almost all evaluations using 18F‐FDG PET/CT in HL were qualitative.
Several clinical studies that focused on quantitative tumor burden using 18F‐FDG PET/CT found that high tumor burden status regardless of a bulky lesion is an important prognostic factor in patients with non‐HL.16, 17, 18, 19 In the present study, a high MTV status by 18F‐FDG PET/CT was also associated with poor prognosis in early stage HL. Moreover, compared with unfavorable prognostic factors reflecting the quantitative manifestation of a mass lesion such as large mediastinal disease, high MTV status by 18F‐FDG PET/CT had more potent predictive power in the clinical outcome. Therefore, the risk evaluation would be determined according to the assessment of 3‐D quantitative burden rather than simple cross‐sectional parameters.
The treatment strategy could be determined according to MTV status. No differences in the clinical outcomes of patients with low MTV status and who underwent CMT and ABVD were observed. Therefore, ABVD alone would be available to be performed in low tumor burden status in the early stage, because the standard treatment is clarified as CMT, but radiotherapy‐induced complications is still a concern.
However, a high MTV status requires a more intensive treatment strategy and ABVD alone is not a sufficient treatment option for disease control. Furthermore, even if CMT was assigned to patients with high MTV status, the clinical outcome was poorer than that of patients with low MTV status. Because a large radiation dose was identified as one relavant risk factor to secondary malignancy or cardiopulmonary disease, CMT such as ABVD followed by IFRT is recommended as the standard of treatment in patients with early stage HL. However, our results suggest that high MTV status remains an unfavorable prognostic factor and that more higher intensity therapeutic options such as an increasing dose of radiation therapy compared with IFRT or development of more potent chemotherapy regimen compared with ABVD are needed.
In conclusion, assessing the total tumor burden by 18F‐FDG PET/CT was valuable for predicting the prognosis in patients with early stage HL. A well‐designed prospective study will provide further information regarding confirmation of our results.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
The present study was supported by grants from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050) and the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A070001).
(Cancer Sci 2013; 104: 1656–1661)
References
- 1. Boivin JF, Hutchison GB, Zauber AG et al Incidence of second cancers in patients treated for Hodgkin's disease. J Natl Cancer Inst 1995; 87: 732–41. [DOI] [PubMed] [Google Scholar]
- 2. van Leeuwen FE, Klokman WJ, Veer MB et al Long‐term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol 2000; 18: 487–97. [DOI] [PubMed] [Google Scholar]
- 3. Ng AK, Bernardo MP, Weller E et al Long‐term survival and competing causes of death in patients with early‐stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol 2002; 20: 2101–8. [DOI] [PubMed] [Google Scholar]
- 4. Franklin J, Pluetschow A, Paus M et al Second malignancy risk associated with treatment of Hodgkin's lymphoma: meta‐analysis of the randomised trials. Ann Oncol 2006; 17: 1749–60. [DOI] [PubMed] [Google Scholar]
- 5. Noordijk EM, Carde P, Dupouy N et al Combined‐modality therapy for clinical stage I or II Hodgkin's lymphoma: long‐term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006; 24: 3128–35. [DOI] [PubMed] [Google Scholar]
- 6. Engert A, Franklin J, Eich HT et al Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. J Clin Oncol 2007; 25: 3495–502. [DOI] [PubMed] [Google Scholar]
- 7. Straus DJ, Portlock CS, Qin J et al Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood 2004; 104: 3483–9. [DOI] [PubMed] [Google Scholar]
- 8. Rueda Domínguez A, Márquez A, Gumá J et al Treatment of stage I and II Hodgkin's lymphoma with ABVD chemotherapy: results after 7 years of a prospective study. Ann Oncol 2004; 15: 1798–804. [DOI] [PubMed] [Google Scholar]
- 9. Gallamini A, Rigacci L, Merli F et al The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin's disease. Haematologica 2006; 91: 475–81. [PubMed] [Google Scholar]
- 10. Hutchings M, Loft A, Hansen M et al FDG‐PET after two cycles of chemotherapy predicts treatment failure and progression‐free survival in Hodgkin lymphoma. Blood 2006; 107: 52–9. [DOI] [PubMed] [Google Scholar]
- 11. Gallamini A, Hutchings M, Rigacci L et al Early interim 2‐[18F] fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: a report from a joint Italian–Danish study. J Clin Oncol 2007; 25: 3746–52. [DOI] [PubMed] [Google Scholar]
- 12. Wirth A, Seymour JF, Hicks RJ et al Fluorine‐18 fluorodeoxyglucose positron emission tomography, gallium‐67 scintigraphy, and conventional staging for Hodgkin's disease and non‐Hodgkin's lymphoma. Am J Med 2002; 112: 262–8. [DOI] [PubMed] [Google Scholar]
- 13. Munker R, Glass J, Griffeth LK et al Contribution of PET imaging to the initial staging and prognosis of patients with Hodgkin's disease. Ann Oncol 2004; 15: 1699–704. [DOI] [PubMed] [Google Scholar]
- 14. Weihrauch MR, Re D, Bischoff S et al Whole‐body positron emission tomography using 18F‐fluorodeoxyglucose for initial staging of patients with Hodgkin's disease. Ann Hematol 2002; 81: 20–5. [DOI] [PubMed] [Google Scholar]
- 15. Hutchings M, Mikhaeel NG, Fields PA et al Prognostic value of interim FDG‐PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 2005; 16: 1160–8. [DOI] [PubMed] [Google Scholar]
- 16. Song MK, Chung JS, Shin HJ et al Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol 2012; 91: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song MK, Chung JS, Shin HJ et al Prognostic value of metabolic tumor volume on PET / CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci 2012; 103: 477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song MK, Chung JS, Shin HJ et al Clinical value of metabolic tumor volume by PET/CT in extranodal natural killer/T cell lymphoma. Leuk Res 2013; 37: 58–63. [DOI] [PubMed] [Google Scholar]
- 19. Oh MY, Chung JS, Song MK et al Prognostic value of Waldeyer's ring involvement of diffuse large B‐cell lymphoma treated with R‐CHOP. Int J Hematol 2013; 97: 397–402. [DOI] [PubMed] [Google Scholar]
- 20. Engert A, Schiller P, Josting A et al Involved‐field radiotherapy is equally effective and less toxic compared with extended‐field radiotherapy after four cycles of chemotherapy in patients with early‐stage unfavorable Hodgkin's lymphoma. J Clin Oncol 2003; 19: 3601–8. [DOI] [PubMed] [Google Scholar]
- 21. Lister TA, Crowther D, Sutcliffe SB et al Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: cotswolds meeting. J Clin Oncol 1989; 7: 1630–6. [DOI] [PubMed] [Google Scholar]
- 22. Freudenberg LS, Antoch G, Schütt P et al FDG‐PET/CT in re‐staging of patients with lymphoma. Eur J Nucl Med Mol Imaging 2004; 31: 325–9. [DOI] [PubMed] [Google Scholar]
- 23. Boellaard R, O'Doherty MJ, Weber WA et al FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010; 37: 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin's lymphoma. Eur J Haematol Suppl 2005; 66: 135–40. [DOI] [PubMed] [Google Scholar]
- 25. Hoppe RT, Advani RH, Ai WZ et al National Comprehensive Cancer Network. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012; 10: 589–97. [DOI] [PubMed] [Google Scholar]
- 26. DeVita VT Jr. Hodgkin's disease‐clinical trials and travails. N Engl J Med 2003; 348: 2375–6. [DOI] [PubMed] [Google Scholar]
- 27. Longo DL. Radiation therapy in the treatment of Hodgkin's disease–do you see what I see? J Natl Cancer Inst 2003; 95: 928–9. [DOI] [PubMed] [Google Scholar]
- 28. Longo DL. Radiation therapy in Hodgkin disease: why risk a Pyrrhic victory? J Natl Cancer Inst 2005; 97: 1394–5. [DOI] [PubMed] [Google Scholar]
- 29. Canellos GP. Chemotherapy alone for early Hodgkin's lymphoma: an emerging option. J Clin Oncol 2005; 23: 4574–6. [DOI] [PubMed] [Google Scholar]
- 30. Gobbi PG, Ghirardelli ML, Solcia M et al Image‐aided estimate of tumor burden in Hodgkin's disease: evidence of its primary prognostic importance. J Clin Oncol 2001; 19: 1388–94. [DOI] [PubMed] [Google Scholar]
- 31. Gobbi PG, Broglia C, Di Giulio G et al The clinical value of tumor burden at diagnosis in Hodgkin lymphoma. Cancer 2004; 101: 1824–34. [DOI] [PubMed] [Google Scholar]
- 32. Jerusalem G, Beguin Y, Fassotte MF et al Whole‐body positron emission tomography using 18F‐fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non‐Hodgkin's lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood 1999; 94: 429–33. [PubMed] [Google Scholar]
- 33. Jerusalem G, Warland V, Najjar F et al Whole‐body 18F‐FDG PET for the evaluation of patients with Hodgkin's disease and non‐Hodgkin's lymphoma. Nucl Med Commun 1999; 20: 13–20. [DOI] [PubMed] [Google Scholar]
- 34. Stumpe KD, Urbinelli M, Steinert HC et al Whole‐body positron emission tomography using fluorodeoxyglucose for staging of lymphoma: effectiveness and comparison with computed tomography. Eur J Nucl Med 1998; 25: 721–8. [DOI] [PubMed] [Google Scholar]
- 35. Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am 2007; 21: 841–54. [DOI] [PubMed] [Google Scholar]


